Abstract
Context: Estrogen and its metabolites play a critical role in the pathophysiology of the endometrium. The bioavailability of estrogen and estrogen metabolites in endometrial tissues depends on the expression of enzymes involved in estrogen biosynthesis and metabolism. Substantial evidence indicates that estrogen-dependent endometrial disorders are also associated with proinflammatory milieu. However, the mechanism whereby inflammation contributes to these conditions is not known.
Objective: The objective of the study was to investigate the effect of TNF-α on estrogen metabolism and the expression of estrogen-metabolizing genes in human endometrial glandular epithelial cells (EM1).
Design: EM1 were treated with 17β-estradiol (E2) with or without TNF-α. Capillary liquid chromatography-tandem mass spectrometry analysis was used for quantitative measurement of estrogens and estrogen metabolites. Western blot analysis, reporter gene assay, and real-time RT-PCR were used to assess the expression of estrogen-metabolizing genes.
Results: TNF-α treatment significantly increased the level of total estrogen and estrogen metabolites and significantly increased the rate of conversion of estrone (E1) into E2. TNF-α also enhanced the oxidative metabolism of estrogen into catecholestrogens with concomitant inhibition of their conversion into methoxyestrogens. Gene expression analysis revealed that TNF-α induced the expression of genes involved in E2 biosynthesis (steroidogenic factor-1 and aromatase) and activation (17β- hydroxysteroid dehydrogenase type 1 and cytochrome P-450, 1B1) with simultaneous repression of genes involved in estrogen inactivation (17β-hydroxysteroid dehydrogenase type 2; catechol O-methyltransferase; and nicotinamide adenine dinucleotide phosphate-quinone oxidoreductase 1).
Conclusion: TNF-α increases the local estrogen biosynthesis in human endometrial glandular cells and directs estrogen metabolism into more hormonally active and carcinogenic metabolites. These effects may impact many physiological and pathological processes that occur within the endometrium.
TNF-α increases the local estrogen biosynthesis in endometrial glandular cells and directs estrogen metabolism into more hormonally active and carcinogenic metabolites.
Estrogen mediates important physiological and pathological responses in the endometrium (1,2,3). In addition to its physiological function, estrogen is involved in many pathological conditions of endometrium, such as endometrial hyperplasia (4), polyp formation (4), abnormal uterine bleeding, endometriosis, and adenocarcinoma (5). The physiological and pathological effects of estrogens are dependent on the local estrogen biosynthesis, intracellular metabolism of estrogens, and the microenvironment in the target tissues (6). Indeed, a substantial percentage of the estrogens in women (75% premenopausal and almost 100% of postmenopausal women) are synthesized locally within the target tissues (7), and the serum levels of estrogens do not necessarily represent their tissue concentration, suggesting an in situ regulation of estrogen availability (8). The importance of the local estrogen biosynthesis in endometrial tissues lies in the fact that this intracrine estrogen exerts maximal biological effects with minimal concentrations (9). In fact, estrogen levels in endometrial tissues can be 1 order of magnitude greater than those present in the circulation due to in situ biosynthesis (10).
In addition, estrogen metabolism and the profile of estrogen metabolites have a profound consequence on the biological effects of estrogen. Indeed, there is evidence that estrogen metabolites can have biological properties, even at very low concentrations, which can exceed manyfold times those of their parent substance (6,11). Many studies have suggested that estrogen metabolites formed in target tissues may have unique effects and function as local mediators of estrogen activity. Although certain estrogen metabolites exert hormonal effects in target tissues by interaction with the classical estrogen receptor, other metabolites appear to elicit unique biological responses not associated with activation of this receptor. Thus, estrogenic effects have to be understood as the net effect of the corresponding individual metabolite pattern (11). It has been suggested that many estradiol effects may not be caused by estradiol per se but may result from the formation of active estrogen metabolite(s) that function as local mediators or may activate their own unique receptors or effectors (12). 17β-Estradiol (E2) is metabolized into a variety of compounds that are different in their hormonal and carcinogenic potentials. The 4-hydroxyestradiol and 16α-hydroxyestrone are tumor promoting (13), whereas the 2-hydroxylation pathway has been demonstrated to have weak estrogenic or even antiestrogenic effects as well as tumor and aromatase inhibition properties (12). 2-Hydroxylation of E2 followed by methylation results in the formation of 2-methoxyestradiol (2-ME2), an anticancer and antiangiogenic agent in a number of cell culture, animal, and human clinical studies (14). Recently we demonstrated that E2 and 4-hydroxyestradiol (4-OHE2) induce oxidative stress, microsatellite instabilities, and neoplastic transformation of human endometrial glandular epithelial cells, whereas 2-hydroxyestradiol (2-OHE2) does not cause cellular transformation or genomic instabilities (15). Thus, the profile of estrogen metabolites and predisposition of the individual metabolite pattern might be crucial for many physiological and pathological conditions in the endometrium.
Local estrogen biosynthesis and metabolism are greatly affected by the microenvironment in the target tissues. Experimental and clinical data suggest that inflammatory mediators modify the physiological and pathological effects of estrogen human uterine endometrium (16). In fact, many estrogen-regulated physiological and pathological processes in endometrium, such as endometrial remodeling (17), implantation (18), and endometrial cancer (19), are associated with inflammatory mediator TNF-α. The expression of TNF-α in different types of human endometrial cells has been extensively studied, and different types of endometrial cells, including the epithelial cells, have been found to express TNF-α and TNF receptors (16).
However, the precise mechanism whereby inflammation affects the estrogen-regulated physiological and pathological process in endometrium is completely obscured. The current study was undertaken to investigate the effect of TNF-α, a hallmark of inflammation, on estrogen signaling and estrogen metabolism in human endometrial glandular epithelial cells.
Materials and Methods
Cell lines and cell culture
Immortalized, nonmalignant human endometrial glandular cells (EM1) were a generous gift from Dr. Satoru Kyo (Department of Obstetrics and Gynecology, Kanazawa University, Kanazawa, Japan). These cells retain the normal functions and characteristics of the primary cells, contain no chromosomal abnormalities or only nonclonal aberrations, retain responsiveness to sex steroid hormones, exhibit glandular structure on three-dimensional culture, and lack transformed phenotypes on soft agar or in nude mice. The cells also express TNF-α receptors and secrete TNF-α in the growth media (20).
EM1 was maintained in DMEM/F12 (1:1) supplemented with 10% fetal bovine serum and ITS (BD Biosciences, Bedford, MA) in an atmosphere of 5% CO2 at 37 C, as described previously (15). To study the effect of TNF-α on the profile of estrogen metabolism, the cells were grown in serum-free, phenol red-free DMEM/F12 (1:1) media for 48 h. The cells were then treated with 10 nm of E2 with or without TNF-α (5 ng/ml) for 48 h. The media were collected for estrogen metabolite measurements. To study the effect of TNF-α on the expression of estrogen-metabolizing genes, the cells were grown in regular growth media and treated with different concentrations of TNF-α (1–5 ng/ml), and total RNA or total protein extraction was performed after 24 or 72 h, respectively.
Estrogen and estrogen metabolite analysis
Estrogen and estrogen metabolites in cell media were analyzed using capillary liquid chromatography-tandem mass spectrometry as previously described (21,22). A 400-μl aliquot of cell medium was used for the analysis.
Measurement of cellular estrogenic milieu
The effect of TNF-α on the intracellular estrogenic milieu and estrogen signaling in EM1 was determined using an estrogen-responsive reporter in the adenoviral vector (Ad-ERE-luc), as described previously (15). Briefly, the cells were transfected with Ad-ERE-luc (50 PFU/cell). The next day, the media were replaced with fresh media containing E2 (10 nm) or E2 (10 nm) plus increasing concentrations of TNF-α (1, 2, or 5 ng/ml). Forty-eight hours later, luciferase activities were determined using a luciferase enzyme assay system. The luciferase activity was normalized against protein concentrations.
Plasmids construct and reporter gene assay
Luciferase reporter plasmids containing the CYP1A1 promoter region (23) was generously provided by Dr. Simon D. Spivack (Laboratory of Human Toxicology and Molecular Epidemiology, New York State Department of Health, Albany, NY). The luciferase reporter plasmid containing the CYP1B1 promoter (24) was a generous gift from Dr. Barbara C. Spink (Wadsworth Center, New York State Department of Health, Albany, NY). Reporter plasmids containing the 17β-hydroxysteroid dehydrogenase type 2 (HSD17B2) (25) and steroidogenic factor-1 (SF-1) promoter regions (26) were the generous gift from Dr. Serdar E. Bulun (Feinberg School of Medicine, Northwestern University, Chicago, IL). Nicotinamide adenine dinucleotide phosphate-quinone oxidoreductase 1 (NQO1)-luciferase reporter vector was constructed according to the method described by Dhakshinamoorthy and Porter (27). The proximal (COMTP1-Luc) and distal COMT promoters (COMTP2-Luc) were constructed as previously described (28). To test the effect of TNF-α on steroidogenic-enzyme gene promoter’s activation, different clones of EM1 stably expressing different luciferase-reporter genes were generated. To test the effect of TNF-α on the steroidogeneic enzyme genes promoter activation, the selected colonies were treated with different concentrations of TNF-α for 48 h, and then the cell extracts were prepared using the luciferase cell lysis reagent (Promega, Madison, WI) (15).
Reverse transcription and real-time quantitative PCR
Total RNA isolation and reverse transcription was performed as described previously (28). Quantitative real-time RT-PCR was carried out using an Applied Biosystems 7500 real-time RT-PCR system (Applied Biosystems, Foster City, CA) using TaqMan gene expression assays (Applied Biosystems) for SF-1 (assay ID Hs01018737_m1), aromatase (assay ID Hs00903413_m1), 17α-hydroxysteroid dehydrogenase type 1 (HSD17B1; assay ID Hs00907287_g1), HSD17B2 (assay ID Hs00157993_m1), cytochrome P450 1A1 (assay ID Hs01054797_g1), cytochrome P450 1B1 (assay ID Hs02382916_s1), catechol O-methyltransferase (COMT; assay ID Hs00241349_m1), NQO1 (assay ID (Hs00168547_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (assay ID Hs99999905_m1). The amount of RT-PCR product for the gene of interest was normalized to the quantity of GAPDH in the same sample. Each assay was performed in triplicate.
Immunoblotting
Immunblotting was performed as described previously (15). Antisera used were rabbit polyclonal IgGs raised against SF-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); mouse antihuman aromatase (AbD Serotec, Raleigh, NC); HSD17B2 (29) (a generous gift from Dr. Yves Tremblay, Hospitalier Universitaire de Québec, Québec, Canada); COMT (Chemicon International Inc., Temecula, CA); or NQO1 (Novus Biologicals, Inc, Littleton, CO). Monoclonal mouse antibody specific for β-actin (Sigma-Aldrich Co., St. Louis, MO) was used to confirm equal loading.
Statistical analysis
The data are presented as mean ± se from three experiments with similar results. Group differences were analyzed using one-way ANOVA followed by Tukey test as a post-ANOVA multiple comparison test; P < 0.05 was considered statistically significant.
Results
TNF-α increased total estrogens and alters the profile of estrogen metabolites in EM1 growth media
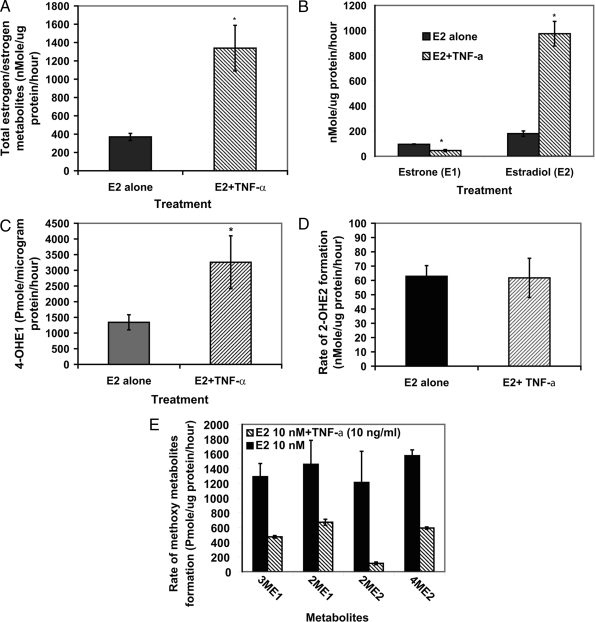
To investigate the effects of the TNF-α on estrogen metabolism in EM1, cells were treated with E2 (10 nm) with or without TNF-α (5 ng/ml) for 48 h, and the estrogens/estrogen metabolites were measured in culture media using liquid chromatography-tandem mass spectrometry. Our data clearly demonstrated that TNF-α treatment resulted in a dramatic increase in total estrogens (estradiol and its metabolites) in the growth media. As shown in Fig. 1A, the rate of total estrogens formation was 4-fold higher in the media from EM1 treated with E2 plus TNF-α compared with media from cells treated with E2 alone (1339 ± 249 vs. 370 ± 38.5 nmol/μg protein per hour, respectively). Our data also indicated that TNF-α altered the relative abundance of estrone (E1)/E2 in the growth media. As indicated in Fig. 1B, the rate of E1 formation in growth media from cells treated with E2 alone or E2 plus TNF-α was 95 ± 3 and 46 ± 9, nmol/μg protein per hour, respectively (26.3 ± 2.5 and 3.8 ± 1.3%, respectively, from total estrogen metabolites). In contrast, the rate of E2 formation was 180 ± 21 and 975 ± 98 nmol/μg protein per hour, respectively (48.7 ± 2.5 and 75.4 ± 8% from total estrogen/estrogen metabolites, respectively).
Figure 1.
Profile of different estrogen metabolites in the culture media from endometrial cells treated with E2 (10 nm) with or without with TNF-α (5 ng/ml). The cells were grown in serum-free media and treated for 48 h. The media were collected to quantify different estrogen/estrogen metabolites by HPLC-electrospray ionization-tandem mass spectrometry2. The data were normalized against the protein concentration. Data are the means ± sem of three replicates. *, Significantly different (P < 0.05) compared with cells treated with E2 alone. 3-ME1, 3-methoxyestrone; 2-ME1, 2-methoxyestrone; 4-ME2, 4-methoxyestradiol.
4-Hydroxyestrone (4-OHE1) formation was significantly increased by TNF-α. As shown in Fig. 1C, the rate of 4-OHE1 formation was 1342 ± 240 and 3258 ± 843 pmol/μg protein per hour in growth media from cells treated with E2 alone or E2 plus TNF-α, respectively. On the other hand, the rate of formation of 2-OHE2 was 63 ± 7 and 62 ± 14 nmol/μg protein per hour, respectively (Fig. 1D). The other estrogen metabolites affected by TNF-α were methoxyestrogens. In growth media obtained from cells treated with E2 (10 nm) alone, the rate of formation of 3-methoxyestrone, 2-methoxyestrone, 2-ME2, and 4-methoxyestradiol was 1290 ± 178, 1458 ± 325, 1212 ± 422, and 1574 ± 79 pmol/μg protein per hour, respectively (Fig. 1E). In media obtained from cells treated with E2 plus TNF-α, the rate of formation of these metabolites was significantly reduced to 473 ± 17, 671 ± 41, 113 ± 19, and 593 ± 15 pmol/μg protein per hour, respectively.
TNF-α enhanced the reporter gene activity induced by E2 from a consensus estrogen response element (ERE) in EM1
Because the estrogen metabolites data indicated that TNF-α increased the total estrogen/estrogen metabolites and enhanced the disposition of more hormonally active estrogen metabolites, the effect of TNF-α on estrogenic milieu in EM1 was assessed. To do this, the E2-induced activation of Ad-ERE-luc reporter in EM1 after treatment with E2 (10 nm) alone or in combination with increasing concentrations of TNF-α was measured. Our data demonstrated that E2-induced luciferase activity was significantly enhanced by cotreatment with TNF-α. As indicated in Fig. 2, TNF-α (1, 2, or 5 ng/ml) increased the E2-induced Ad-ERE-luc reporter activity by 50, 90, and 380%, respectively (P < 0.001), compared with E2 treatment alone (Fig. 2).
Figure 2.
Effects of E2 with or without TNF-α on transcription activation of Ad-ERE-luc construct. EM1 were transfected with 50 PFU/cells of Ad-ERE-luc. The cells were treated with E2 (10 nm) with or without increasing concentrations of TNF-α (1, 2, or 5 ng/ml) for 48 h. Results are presented as percentage from the E2-treated cells.
TNF-α up-regulated SF-1 and aromatase (CYP19) expression in EM1
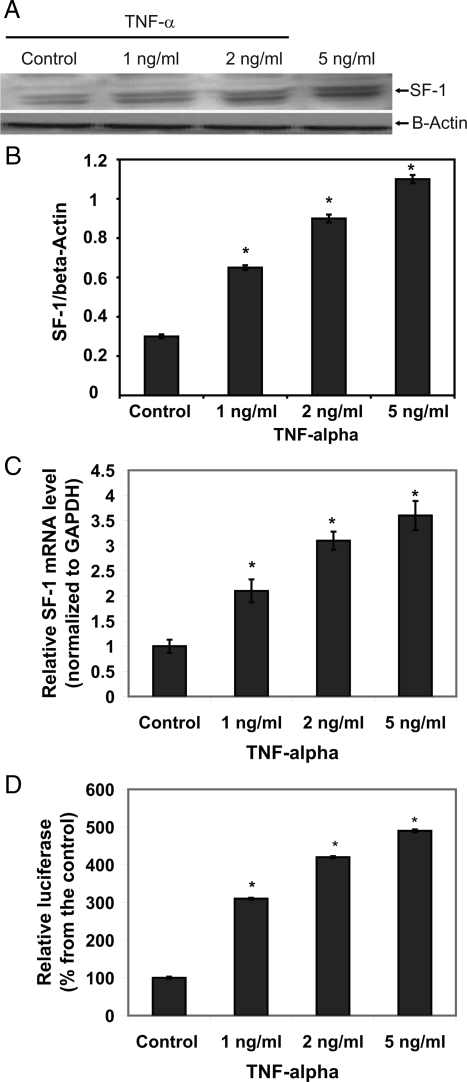
To understand how TNF-α enhanced estrogenic activity and increased the total amount of estrogens in EM1 culture media, the effect of TNF-α on SF-1 and CYP19 gene expression was investigated. As indicated in Fig. 3, A and B, TNF-α increased SF-1 protein expression in a concentration-dependent manner. To assess whether the effect of TNF-α on SF-1 protein expression occurs at the transcriptional level, RT-PCR and luciferase reporter assays were performed. Consistent with the immunoblotting results, TNF-α (1, 2, or 5 ng/ml) increased SF-1 mRNA levels by 2.1 ± 0.23-, 3.1 ± 0.18-, and 3.6 ± 0.29-fold, respectively, compared with the control (Fig. 3C). Similarly, the results of the reporter assays indicated that TNF-α (1, 2, or 5 ng/ml) increased SF-1 promoter activity by 210 ± 2.5, 320 ± 2.8, and 390 ± 3.7%, respectively (Fig. 3D). These data confirmed that TNF-α induces SF-1 gene transactivation.
Figure 3.
Effect of TNF-α on SF-1expression in EM1. A, Immunoblotting for SF-1. SF-1 protein levels were determined 72 h after treatment with different concentrations of TNF-α (1, 2, or 5 ng/ml−1). B, The bar graph of densitometry analysis of immunoblots. Intensities of the bands are expressed as multiples of protein levels in control cells. Data are the mean values ± sem of three separate immunoblots. C, Real-time RT-PCR analyses. The cells were treated with TNF-α (2 ng, or 5 ng/ml−1) for 24 h followed by total RNA isolation from the cells, and the SF-1 mRNA expression was determined by real-time RT-PCR. The threshold cycle value of SF-1 was normalized based on that of GAPDH. D, SF-1 reporter gene assays. The cells were stably transfected with pGL3-SF1-1588, which contains the human SF-1 promoter from −1588 to +165 bp, and treated with TNF-α (1, 2, or 5 ng/ml−1) for 48 h. Data represent the mean ± sem from three separate experiments. *, P < 0.01, significant compared with vehicle-treated control.
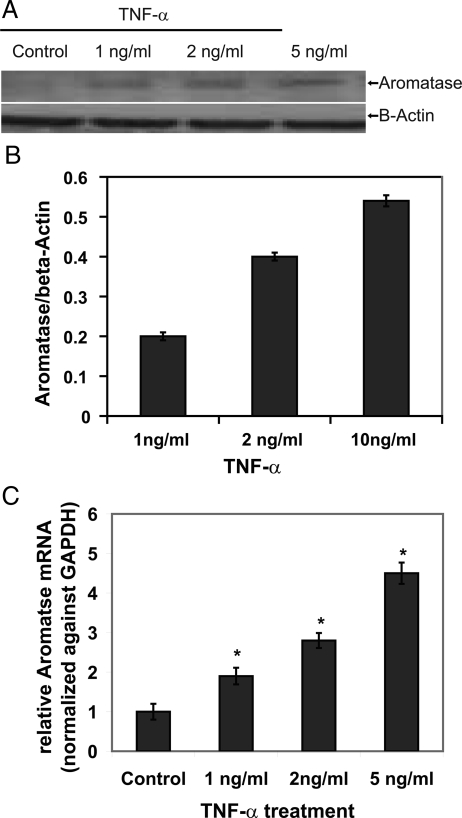
Because CYP19 is a rate-limiting enzyme in estrogen biosynthesis, the effect of TNF-α CYP19 expression in EM1 was measured. CYP19 was not detected at the protein level in control EM1, as shown in Fig. 4, A and B. However, CYP19 protein expression was induced by TNF-α in a concentration-dependent manner. Similarly, RT-PCR confirmed that TNF-α (1, 2, or 5 ng/ml) increased the CYP19 mRNA expression by 1.9 ± 0.21-, 2.8 ± 0.19-, and 4.5 ± 0.27-fold, respectively (Fig. 4C).
Figure 4.
Effect of TNF-α on aromatase (CYP19) mRNA expression in endometrial cells. Endometrial cells were treated with TNF-α. A, Immunoblotting for aromatase. Aromatase protein levels were determined 72 h after treatment with different concentrations of TNF-α (1, 2, or 5 ng ml−1). The relative aromatase levels were measured by scanning densitometry. B, Bar graph of densitometry analysis of immunoblots. Intensities of the bands are expressed as multiples of protein levels in control cells. The data are mean values ± sem of three separate immunoblots. C, Real-time RT-PCR analyses. The cells were treated with TNF-α (1, 2, or 5 ng/ml−1) for 12 h followed by total RNA isolation from the cells, and the aromatase mRNA expression was determined by real-time RT-PCR. The threshold cycle value for aromatase gene was normalized to the threshold cycle value of GAPDH. The results are expressed as mean ± sem of independent experiments.
Distinctive effect of TNF-α on HSD17B1 and HSD17B2 expression
To understand how TNF-α alters the rate of E1 and E2 formation, the effect of TNF-α on HSD17B1 and HSD17B2 expression in EM1 was evaluated. The data show that the addition of TNF-α down-regulated HSD17B2 expression, whereas it increased HSD17B1 expression in EM1. As demonstrated in Fig. 5, A and B, TNF-α down-regulates HSD17B2 protein expression in a concentration-dependent fashion. This finding is confirmed by real-time quantitative RT-PCR (Fig. 5C) and reporter gene assays (Fig. 5D). In contrast, TNF-α up-regulates HSD17B1 expression in EM1. As shown in Fig. 5E, TNF-α induced a concentration-dependent increase in HSD17B1 mRNA expression. Thus, the observed effect of TNF-α on E2 accumulation is ascribed to the distinctive mutual effect of TNF-α on HSD17B1 and HSD17B 2.
Figure 5.
Effects of TNF-α on HSD17B2 and HSD17B1 expression in endometrial cells. Endometrial cells were treated with increased concentrations of TNF-α. A, Immunoblotting for HSD17B2. HSD17B2 protein levels were determined 72 h after treatment with different concentrations of TNF-α (1, 2, or 5 ng/ml). B, Bar graph of densitometry analysis of immunoblots. Intensities of the bands are expressed as multiples of protein levels in control cells. The mean values ± sem of three separate immunoblots. C, Real-time RT-PCR analyses of HSD17B2 transcript. The cells were treated with TNF-α (1, 2, or 5 ng ml−1) for 12 h followed by total RNA isolation from the cells, and the HSD17B2 mRNA expression was determined by real-time RT-PCR. The threshold cycle value HSD17B2 gene was normalized to the threshold cycle value of GAPDH. D, HSD17B2 reporter gene assays. The cells were stably transfected with pGL3 HSD17B2 reporter, which contains the human HSD17B2 promoter from −1588 to +165 bp, and treated with TNF-α (1, 2, or 5 ng ml−1) for 48 h. D, Real-time RT-PCR analyses of HSD17B1 transcript in EM1 treated with TNF-α (1, 2, or 5 ng ml−1) for 12 h followed by total RNA isolation from the cells. The HSD17B1 mRNA expression was determined by real-time RT-PCR. The threshold cycle value HSD17B1gene was normalized to the threshold cycle value of GAPDH. The results are expressed as mean ± sem of independent experiments.
Effect of TNF-α on CYP1B1, CYP1A1, COMT, and NQO1 expression in EM1
As demonstrated previously in Fig. 1, TNF-α significantly increased the rate of catecholestrogens formation with a concomitant decrease in the rate of methoxyestrogens formation in EM1. Thus, the effect of TNF-α on the expression of genes involved in formation and detoxification of these metabolites was investigated. The expression of CYP1B1 was scarce in the control EM1 as shown in Fig. 6A and 6B; however, CYP1B1 expression was induced by adding TNF-α to the cells. Consistent with the Western blotting results, real time RT-PCR and reporter gene assays confirmed that TNF-α induced CYP1B1 expression in a concentration-dependent fashion (Figs. 6C and 6D). In contrast to its effect on CYP1B1, TNF-α did not affect the CYP1A1 expression in EM1. As depicted in supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org, CYP1A1 protein expression was barely detected in EM1, even after treatment with TNF-α. Real time RT-PCR and reporter assays revealed that CYP1A1 expression in EM1 cells was not affected by TNF-α treatment (supplemental Fig. 1, B and C).
Figure 6.
Effect of TNF-α on CYP1B1 expression in endometrial cells. A, Western blot analysis of CYP1B1in EM1 treated with increasing concentrations of TNF-α (1, 2, or 5 ng/ml) for 72 h. B, Bar graph of densitometry analysis of immunoblots. Intensities of the bands are expressed as multiples of protein levels in control cells. The mean values ± sem of three separate immunoblots. C, Real-time RT-PCR analyses. The cells were treated with TNF-α (1, 2, or 5 ng ml−1) for 12 h followed by total RNA isolation from the cells, and the CYP1B1 mRNA expression was determined by real-time RT-PCR. The threshold cycle value for CYP1B1 gene was normalized to the threshold cycle value of GAPDH. D, CYP1B1 reporter gene assays. The cells were stably transfected with Cyp1B1 reporter vector, which contains the human CYP1B1 promoter from −1588 to +165 bp, and treated with TNF-α (1, 2, or 5 ng /ml−1) for 48 h. Data represent the mean ± sem from three separate experiments. *, P < 0.01, significant compared with vehicle-treated control.
In endometrial tissues, COMT is the main enzyme involved in the detoxification of catecholestrogens through their conversion to methoxy derivatives. We investigated the effect of TNF-α on COMT expression in EM1. Our data demonstrated that TNF-α down-regulated the COMT protein expression in a concentration-dependent fashion (supplemental Fig. 2A). The effect of TNF-α on COMT expression occurs at the transcriptional level as indicated by a significant decrease in COMT mRNA expression and the results of the reporter gene assay (supplemental Fig. 2, B and C).
NQO1 has the ability to catalyze the two-electron reduction of quinones to hydroquinones, thereby preventing further oxidation of catecholestrogen to toxic semiquinone radicals and reactive oxygen species, the toxic metabolites involved in estrogen carcinogenicity. Thus, we studied the effect of TNF-α on NQO1 in EM1. Similar to its effect on COMT, TNF-α down-regulated NQO1 expression in EM1 (supplemental Fig. 3, A–C).
Discussion
TNF-α is a pluripotent, multifunctional, proinflammatory cytokine, which has been found throughout the menstrual cycle and the first trimester of pregnancy (30), and its level rises at menopause (12). Different types of endometrial cells, including the epithelial cells, express TNF-α and TNF receptors (16), suggesting that TNF-α may have a key role in regulating endometrial responses to hormonal variation and may be implicated in many physiological and pathological functions of endometrium (15,31). However, the effect of TNF-α on endometrium has not been studied beyond the inflammation process. In addition, studying cross talk between estrogen and TNF-a revealed that estrogen exerts antiinflammatory action and antagonistic effects on TNF-α (32), but it is not known what the reciprocal effect of TNF-α is on estrogen metabolism/signaling. This functional interaction between estrogen/estrogen receptor and nuclear factor-κB (NF-κB) systems encouraged us to examine the effect of TNF-α on estrogen metabolism and homeostasis in endometrial cells. Understanding the effect of TNF-α on local estrogen biosynthesis and metabolism in endometrial cells is paramount to comprehending many physiological and pathological phenomena in the endometrium. Our study demonstrated for the first time that TNF-α enhances the intracellular estrogenic milieu in EM1 by stimulating the de novo synthesis of estrogen. In addition, TNF-α alters the profile of estrogen/estrogen metabolites by directing estrogen metabolism into more hormonally active estrogen metabolites.
The effect of TNF-α on estrogen metabolism and homeostasis is mediated by the effect of TNF-α on the coordinated expression of the enzymes that are involved in estrogen biosynthesis and metabolism. The significance of these findings lies in the fact that intracrine estrogens exert maximal biological effects at very low concentrations and have profound impacts in estrogen-regulated physiological and pathological processes in endometrium (9). Our findings are in accordance with previous studies that indicate TNF-α regulates steroidogenesis in several cell lines in a tissue-specific fashion (33). It inhibits testicular steroidogenesis, whereas it stimulates this process in human adrenocortical cell lines (34,35). It is well established that the enzymes involved in estrogen biosynthesis and metabolism are expressed in endometrial tissues (36). Our data show that TNF-α stimulates the expression of orphan nuclear receptor SF-1, a transcription factor that activates the promoters of critical genes in estrogen biosynthesis. This finding is consistent with many reports that TNF-α regulates the expression of many orphan nuclear receptors (34). Similarly, TNF-α induces the expression of CYP19 in EM1. These finding are consistent with the previous reports that TNF-α can regulate the expression of CYP19 in various tissues (33). The effect of TNF-α on SF-1 and CYP19 expression in EM1 might explain the significant increase in total estrogens/estrogen metabolites in EM1 growth media.
Our data also clearly demonstrate that TNF-α enhances the accumulation of estrogen as E2, whereas it decreases E1 formation. This shift in E2/E1 dispositions is consistent with the finding that TNF-α differentially regulates the expression of HSD17B2 and HSD17B1, which regulate the interconversion E2 to E1. Thus, the increase in total estrogens and enhanced accumulation of estrogen as E2 explain the ERE-luciferase activity in EM1 treated with TNF-α. Considering the fact that estrogens possess antiinflammatory and antagonizing effects on TNF-α, it is rational to speculate that the TNF-α-induced effect on estrogen homeostasis and signaling may represent a negative feedback loop to ameliorate the effect of TNF-α and may impact many physiological and pathological processes in the endometrium.
In addition to its effect on enhancing local estrogen formation, our data indicate that TNF-α alters subsequent estrogen metabolism pathways. TNF-α stimulates the expression CYP1B1 with simultaneous repression of COMT and NQO1. This effect resulted in a significant increase in the rate of formation of the carcinogenic estrogen metabolite 4-OHE1 with a concomitant significant decrease in the formation of the anticarcinogenic, antiangiogenic estrogen metabolite 2-ME2. Ultimately, this effect of TNF-α would create an environment more conducive for endometrial cancer development. Recently we reported that 4-OHE2 can transform EM1 into neoplastic phenotype, an effect that is augmented by down-regulation of COMT inhibition (15). Thus, this finding provides a mechanistic explanation for the causal link between inflammation and the initiation, promotion, and progression of endometrial cancer (19).
The observed effects of TNF-α on steroidogenic genes in EM1 could be mediated by the direct effect of TNF-α on NF-κB response elements in the promoter regions of steroidogenic genes. Indeed, we have identified multiple NF-κB response elements in the promoter regions of HSD17B2 and COMT (data not shown). Alternatively, TNF-α can regulate steroidogenic genes indirectly by its effect on the transactivation of orphan nuclear receptors, which regulate the expression of steroidogenic enzyme genes (34). TNF-α-induced steroidogenesis and increased intracellular estrogenic effects in EM1 may have physiological and pathological significance as an endocrine and/or paracrine/autocrine regulator of endometrial function. Indeed, many inflammatory proliferative disorders of endometrium are associated with enhanced expression of enzymes involved in estrogen biosynthesis and local estrogen production, such as endometriosis, adenomyosis, and endometrial cancers (19,37,38), which are associated with endocrine milieu of estrogen predominance, and multiple lines of evidence suggest that inflammation plays a critical role in the pathogenesis of these disorders (38).
Accordingly, several reports indicate that the inflammatory disorders of endometrium, such as endometriosis, endometrial hyperplasia, and endometrial cancer, express higher level of steroidogenic enzymes such as SF-1, CYP19, HSD17B1, and lower level of HSD17B2 (26,39,40), a profile that is similar to the effect of TNF-α in EM1.
In conclusion, TNF-α increases the local estrogen biosynthesis in human endometrial glandular epithelial cells and directs estrogen metabolism to produce more hormonally active and carcinogenic metabolites. This effect may represent a negative feedback loop to ameliorate the effects of TNF-α and may impact many physiological and pathological processes in the endometrium. The effect of TNF-α on estrogen homeostasis and metabolism in endometrial cells makes this cytokine a potentially important auto- and paracrine modulator of endometrial steroidogenesis in various pathophysiological conditions. More work remains to be carried out for a better understanding of the role of this cytokine as part of the complex network of cytokines in the endometrium.
Supplementary Material
Footnotes
This work was supported by University of Texas Medical Branch National Institute of Environmental Health Sciences Center Pilot Project E506676 (to S.A.S.) and federal funds from the National Cancer Institute, National Institutes of Health, under Contract N01-CO-12400 (to T.D.V.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Disclosure Summary: The authors have nothing to declare.
First Published Online October 28, 2008
Abbreviations: COMT, Catechol O-methyltransferase; CYP19, aromatase; E1, estrone; E2, 17β-estradiol; EM1, endometrial glandular cells; ERE, estrogen response element; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSD17B1, 17α-hydroxysteroid dehydrogenase type 1; HSD17B2, 17β-hydroxysteroid dehydrogenase type 2; 2-ME2, 2-methoxyestradiol; NF-κB, nuclear factor-κB; NQO1, nicotinamide adenine dinucleotide phosphate-quinone oxidoreductase 1; 4-OHE1, 4-hydroxyestrone; 2-OHE2, 2-hydroxyestradiol; 4-OHE2, 4–2-hydroxyestradiol; SF-1, steroidogenic factor-1.
References
- Slayden OD, Brenner RM 2004 Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol 67:393–409 [DOI] [PubMed] [Google Scholar]
- Groothuis PG, Dassen HHNM, Romano A, Punyadeera C 2007 Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update 13:405–417 [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RPA, Morani A, Omoto Y, Warner M, Gustafsson JA 2006 Role of estrogen receptor β in uterine stroma and epithelium: insights from estrogen receptor β(−/−) mice. Proc Natl Acad Sci USA 103:18350–18355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis PG, Koutlaki NG, Skaphida PG, Galazios GC, Tsikouras PN, Liberis VA 2000 Endometrial polyps: prevalence, detection, and malignant potential in women with abnormal uterine bleeding. Eur J Gynaecol Oncol 21:180–183 [PubMed] [Google Scholar]
- Parazzini F, Lavecchia C, Bocciolone L, Franceschi S 1991 The epidemiology of endometrial cancer. Gynecol Oncol 41:1–16 [DOI] [PubMed] [Google Scholar]
- Straub RH 2007 The complex role of estrogens in inflammation. Endocr Rev 28:521–574 [DOI] [PubMed] [Google Scholar]
- Foster PA 2008 Steroid metabolism in breast cancer. Minerva Endocrinol 33:27–37 [PubMed] [Google Scholar]
- Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C 1996 Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab 81:1460–1464 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Moriya T, Ishida T, Ohuchi N, Sasano H 2003 Intracrine mechanism of estrogen synthesis in breast cancer. Biomed Pharmacother 57:460–462 [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE 2007 Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab 92:3261–3267 [DOI] [PubMed] [Google Scholar]
- Mueck AO, Seeger H 2007 Breast cancer: are oestrogen metabolites carcinogenic? Maturitas 57:42–46 [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, Pfohl M, Schatz H 2002 Changes in proinflammatory cytokine activity after menopause. Endocr Rev 23:90–119 [DOI] [PubMed] [Google Scholar]
- Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH 2006 17β-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J 20:1622–1634 [DOI] [PubMed] [Google Scholar]
- Mooberry SL 2003 Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Update 6:355–361 [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S, Satyaswaroop PG, von Wolff M, Strowitzki T 1999 Regulation of TNF-α mRNA expression in endometrial cells by TNF-α and by oestrogen withdrawal. Mol Hum Reprod 5:1141–1149 [DOI] [PubMed] [Google Scholar]
- Hunt JS, Chen HL, Hu XL, Tabibzadeh S 1992 Tumor necrosis factor-α messenger ribonucleic acid and protein in human endometrium. Biol Reprod 47:141–147 [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Zhang J, Brasted M 2002 Leukocyte networks and human endometrial remodelling. J Reprod Immunol 57:95–108 [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Mazor M 2004 Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril 82:799–804 [DOI] [PubMed] [Google Scholar]
- Margolis KL, Rodabough RJ, Thomson CA, Lopez AM, McTiernan A 2007 Prospective study of leukocyte count as a predictor of incident breast, colorectal, endometrial, and lung cancer and mortality in postmenopausal women. Arch Intern Med 167:1837–1844 [DOI] [PubMed] [Google Scholar]
- Kyo S, Nakamura M, Kiyono T, Maida Y, Kanaya T, Tanaka M, Yatabe N, Inoue M 2003 Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. Am J Pathol 163:2259–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG 2007 Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem 79:7813–7821 [DOI] [PubMed] [Google Scholar]
- Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG 2005 Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem 77:6646–6654 [DOI] [PubMed] [Google Scholar]
- Han WG, Pentecost BT, Spivack SD 2003 Functional evaluation of novel single nucleotide polymorphisms and haplotypes in the promoter regions of CYP1B1 and CYP1A1 genes. Mol Carcinog 37:158–169 [DOI] [PubMed] [Google Scholar]
- Spink BC, Pang S, Pentecost BT, Spink DC 2002 Induction of cytochrome P450 1B1 in MDA-MB-231 human breast cancer cells by non-ortho-substituted polychlorinated biphenyls. Toxicol In Vitro 16:695–704 [DOI] [PubMed] [Google Scholar]
- Yang SJ, Fang ZJ, Gurates B, Tamura M, Miller J, Ferrer K, Bulun SE 2001 Stromal PRs mediate induction of 17β-hydroxysteroid dehydrogenase type 2 expression in human endometrial epithelium: a paracrine mechanism for inactivation of E2. Mol Endocrinol 15:2093–2105 [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, Kim JJ, Yaegashi N, Bulun SE 2008 Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol 22:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakshinamoorthy S, Porter AG 2004 Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J Biol Chem 279:20096–20107 [DOI] [PubMed] [Google Scholar]
- Salama SA, Jamaluddin M, Kumar R, Hassan MH, Al-Hendy A 2007 Progesterone regulates catechol-O-methyl transferase gene expression in breast cancer cells: distinct effect of progesterone receptor isoforms. J Steroid Biochem Mol Biol 107:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet R, Simard M, Plante J, Laberge P, Tremblay Y 2007 Human type 2 17β-hydroxysteroid dehydrogenase mRNA and protein distribution in placental villi at mid and term pregnancy. Reprod Biol Endocrinol 5:30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HL, Yang YP, Hu XL, Yelavarthi KK, Fishback JL, Hunt JS 1991 Tumor necrosis factor α mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol 139:327–335 [PMC free article] [PubMed] [Google Scholar]
- Kharfi A, Labelle Y, Mailloux J, Akoum A 2003 Deficient expression of tumor necrosis factor receptor type 2 in the endometrium of women with endometriosis. Am J Reprod Immunol 50:33–40 [DOI] [PubMed] [Google Scholar]
- Harnish DC 2006 Estrogen receptor ligands in the control of pathogenic inflammation. Curr Opin Invest Drugs 7:997–1001 [PubMed] [Google Scholar]
- Herrmann M, Scholmerich J, Straub RH 2002 Influence of Cytokines and growth factors on distinct steroidogenic enzymes in vitro—a short tabular data collection. Ann NY Acad Sci 966:166–186 [DOI] [PubMed] [Google Scholar]
- Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, Mellon SH, Lee K 2004 Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor α. Mol Cell Biol 24:2593–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova IV, Kuulasmaa T, Jaaskelainen J, Voutilainen R 2007 Tumor necrosis factor-α regulates steroidogenesis, apoptosis, and cell viability in the human adrenocortical cell line NCI-H295R. Endocrinology 148:386–392 [DOI] [PubMed] [Google Scholar]
- Pal L, Niklaus AL, Kim M, Pollack S, Santoro N 2008 Heterogeneity in endometrial expression of aromatase in polyp-bearing uteri. Hum Reprod 23:80–84 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Gurates B, Fang ZJ, Tamura M, Sebastian S, Zhou HF, Amin S, Yang SJ 2002 Mechanisms of excessive estrogen formation in endometriosis. J Reprod Immunol 55:21–33 [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR 2008 Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 149:1190–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE 1998 Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J Clin Endocrinal Metab 83:4474–4480 [DOI] [PubMed] [Google Scholar]
- Sasano H, Suzuki T, Harada N 1998 From endocrinology to intracrinology. Endocr Pathol 9:9–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.