Abstract
Context: Vitamin D insufficiency has now reached epidemic proportions and has been linked to low bone mineral density, increased risk of fracture, and obesity in adults. However, this relationship has not been well characterized in young adults.
Objective: The objective of the study was to examine the relationship between serum 25-hydroxyvitamin D (25OHD), anthropometric measures, body fat (BF), and bone structure at the time of peak bone mass.
Design: This was a cross-sectional study.
Outcome Measures and Subjects: Anthropometric measures, serum 25OHD radioimmunoassay values, and computed tomography and dual-energy x-ray absorptiometry values of BF and bone structure in 90 postpubertal females, aged 16–22 yr, residing in California were measured.
Results: Approximately 59% of subjects were 25OHD insufficient (≤29 ng/ml), and 41% were sufficient (≥30 ng/ml). Strong negative relationships were present between serum 25OHD and computed tomography measures of visceral and sc fat and dual-energy x-ray absorptiometry values of BF. In addition, weight, body mass, and imaging measures of adiposity at all sites were significantly lower in women with normal serum 25OHD concentrations than women with insufficient levels. In contrast, no relationship was observed between circulating 25OHD concentrations and measures of bone mineral density at any site. Unexpectedly, there was a positive correlation between 25OHD levels and height.
Conclusions: We found that vitamin D insufficiency is associated with increased BF and decreased height but not changes in peak bone mass.
Vitamin D insufficiency in young women is associated with increased body fat and decreased height.
Vitamin D, a key regulator of bone metabolism, is thought to play an important role in adipogenesis and the prevention of a variety of diseases, including osteoporosis, cancer, diabetes, and immune disorders (1,2,3). It is derived from skin exposure to sunlight (vitamin D3) or food supplements (vitamins D2 or D3) and undergoes successive hydroxylations in the liver and kidneys to give rise to its active metabolite 1α,25 dihydroxyvitamin D (4). The vitamin D receptor is widely distributed in various tissues including bone and fat and triggers most of the action of vitamin D (5). There are, however, significant discrepancies in the results of previous studies assessing the relation between vitamin D, bone health, and adiposity.
Whereas several studies in adults have shown that vitamin D increases bone mineral density (BMD) (6), prevents osteoporotic fractures (7,8), and reduces the risk of falling in the elderly (9), other studies in adolescents and young adults have yielded conflicting results; some, but not all, found an association between vitamin D and bone mass (10,11,12,13,14,15). Moreover, a recent study in adults aged 30–79 yr suggests the relation between 25-hydroxyvitamin D (25OHD) levels and BMD is present in the white population but not African-Americans or subjects of Hispanic ethnicity (16).
Obesity has now reached epidemic proportions, and the combined percentage of overweight and obese individuals in the United States is staggering, approaching 32% in children and adolescents and 66% in young adults (17). Although vitamin D insufficiency is prevalent in this population, especially in low socioeconomic groups (18,19), limited information regarding the relationship between weight and vitamin D levels is available. Several studies have shown adult obesity to be inversely correlated with 25OHD levels (20,21,22,23,24,25,26), and it has been suggested that adipogenesis may be inhibited by 1α,25 dihydroxyvitamin D (27). Even obese adults who take supplemental vitamin D2 and are exposed to UV light have 25OHD levels substantially lower than nonobese matched controls (24).
Discrepancies in the results from previous studies may, in part, be related to the use of dual-energy x-ray absorptiometry (DXA) to obtain bone and/or fat measures because this projection technique cannot correct for the influence of other soft tissues in the region of interest (28). In this investigation, to account for the influence of soft tissues on DXA bone measurements, we examined the relations between vitamin D, bone health, and adiposity by using both DXA and computed tomography (CT). Additionally, the confounding effects of growth and development, aging, and gender on the relations between fat mass, bone mass, and vitamin D were controlled by including only sexually and skeletally mature young females aged 16–22 yr.
Subjects and Methods
Study subjects
This study was approved by the institutional review board at our institution, and informed consent was obtained from all parents and/or subjects. An initial interview was conducted to describe the purpose and aims of the study and the tests to be performed. Candidates for this study were excluded if they had a diagnosis of any underlying disease or chronic illness, if they had been ill for longer than 2 wk during the previous 6 months, if they had been admitted to the hospital at any time during the previous 3 yr, or if they were taking any medications including oral contraceptives. Candidates who were pregnant, had ever been pregnant, or with absence of menses for more than 4 consecutive months were also excluded from the study. To decrease the seasonal variability in biochemical determinations, all appointments were scheduled between May and October. In addition, all subjects had normal kidney function and normal liver function tests, and there was no evidence of liver abnormalities detected by CT.
All potential participants underwent a general physical examination, including assessments of the degree of sexual development, and a radiographic examination of the left hand and wrist. Only those who had reached sexual maturity, defined as Tanner V of breast development (29), and skeletal maturity, defined as epiphyseal closure in the phalanges and metacarpals using the radiographic atlas of Greulich and Pyle (30), were included in the study. Measurements of weight were obtained to the nearest 0.1 kg, using the Scale-Tronix (Scale-Tronix, Inc, Wheaton, IL), and measurements of height were obtained to the nearest 0.1 cm, using the Harpenden stadiometer (Holtain Ltd., Crymmych, Wales, UK). Body mass index (BMI) was calculated as weight (kilograms) divided by height squared (square meters); for the purpose of this study, subjects were divided into a lean group (BMI < 25 kg/m2) and an overweight group (BMI ≥ 25 kg/m2). Using this approach, 90 female subjects were enrolled in this study and underwent imaging determinations of bone and adipose tissue and biochemical measurements of calcium-regulating hormones.
Bone and fat measurements
DXA and CT determinations of bone and fat were performed on the same day by the same technologist. Using a Hologic QDR4500 DXA scanner (Hologic, Inc., Bedford, MA), the bone mineral content (BMC; grams) and the BMD (grams per square centimeter) were measured for the total body and for the axial and appendicular skeleton independently. In addition, the total, subtotal (excluding the head), arms, trunk, and leg fat mass (kilograms) were determined. The coefficients of variation (CVs) for repeated DXA measurements of BMC, BMD, and fat mass at the various locations examined have been reported to range from 0.7 to 4.1%, and the radiation exposure is negligible (31).
For CT determinations, a Hilite Advantage scanner (General Electric Healthcare, Milwaukee, WI) with a standardized reference phantom for simultaneous calibration was used. In the axial skeleton, values for cancellous bone density (milligrams per cubic centimeter) and the cross-sectional area (CSA; square centimeters) were measured at the midportion of the first three lumbar vertebral bodies, and in the appendicular skeleton, the CSA (square centimeters), cortical bone area (square centimeters), and cortical bone density (milligrams per /cubic centimeter) at the midshafts of the femurs were obtained; CVs for these bone measurements in young adults were previously reported between 0.6 and 1.5% (32). Additionally, from the same cross-sectional abdominal images measurements of the visceral fat (square centimeters) and sc fat (square centimeters) were obtained. For the purpose of this study, sc fat was defined as the amount of adipose tissue located between the skin and the rectus muscles of the abdomen, the external oblique muscles, the broadest muscles of the back, and the erector muscles of the spine at the level of the umbilicus. Visceral fat was defined as the intraabdominal adipose tissue surrounded by the rectus muscles of the abdomen, the external oblique muscles, the lumbar quadrate muscle, the psoas muscles, and the lumbar spine at the same level. The CV for repeated measures of visceral and sc fat has been reported to range from 1.5 to 3.5% (33). The time to complete the CT scans was approximately 10 min and the effective radiation dose was approximately 0.1 mSv (34).
Biochemical determinations
Serum levels of 25OHD were assayed using a RIA as described by Hollis et al. (35). The lower limit of detection was 5 ng/ml (12.5 nmol/liter). Goat anti-25OHD was a gift from Dr. Bruce Hollis (Medical University of South Carolina, Charleston, SC). 125I-25-hydroxy-vitamin D3 and donkey antigoat secondary antibody were purchased from Diasorin (Stillwater, MN). This assay recognizes equally serum 25-hydroxy-vitamin D2 and serum 25-hydroxy-vitamin D3 and shows no bias when compared with HPLC (36). Calculated assay precision for within-assay variation averages 6% and for interassay 16%. For the purpose of this study and according to the current consensus, subjects were divided into a 25OHD sufficient, or normal, group (≥30 ng/ml) and an insufficient group (≤29 ng/ml). Intact PTH (1–84) was measured with an electrochemiluminescent assay (37). The sensitivity of the assay is 1.2 pg/ml (0.127 pmol/liter) and intra- and interassay variations are 1.9–4 and 2.6–6.5%, respectively. To minimize interassay variability, all samples were analyzed simultaneously.
Statistical analysis
A sample size of 90 subjects allows the determination of correlations greater than r = .28 with 80% power. Statistical analysis was carried out using Statview (version 5.0.1; SAS Institute Inc., Cary, NC). Data were analyzed using simple linear regression analysis, multiple regression analysis, and unpaired t tests. All values are expressed as mean ± sd.
Results
Relation between 25OHD and subject characteristics
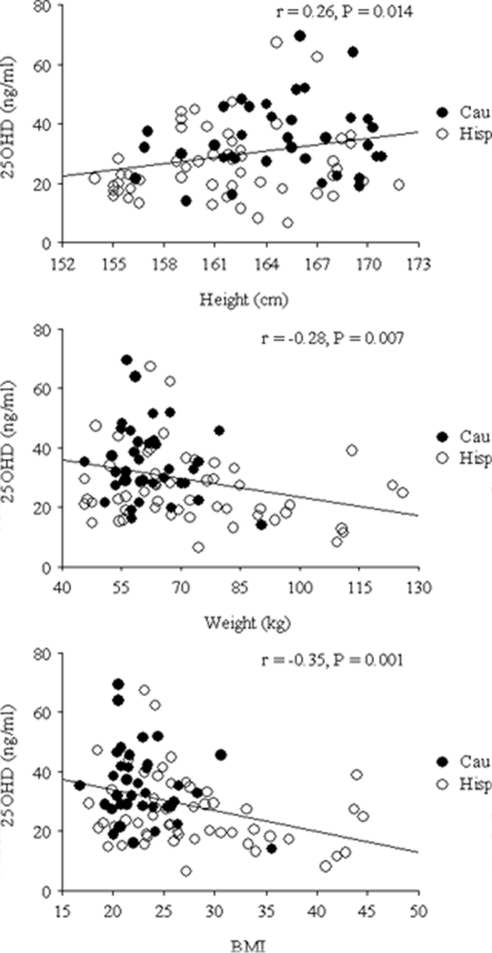
The age, anthropometric characteristics, and ethnic background of the women studied are described in Table 1. Weight and BMI were significantly higher, whereas height was significantly lower, in Hispanics than Caucasians. When all subjects were taken together, a significant positive correlation was found between height and 25OHD (Fig. 1). In contrast, significant negative correlations were observed between 25OHD, weight, and BMI (Fig. 1). Multiple regression analysis showed that the negative relation between 25OHD and weight and the positive relation between 250HD and height persisted, even after accounting for differences in ethnic background.
Table 1.
Age and anthropometric characteristics of 90 women separated by ethnic background
| All (n = 90) | Hispanic (n = 53) | Caucasian (n = 37) | P | |
|---|---|---|---|---|
| Age (yr) | 19.4 ± 1.5 (16.3–22.8) | 19.6 ± 1.4 (17.0–22.8) | 19.1 ± 1.6 (16.3–22.2) | 0.100 |
| Weight (kg) | 68.3 ± 17.5 (45.5–126.0) | 72.7 ± 20.5 (45.5–126.0) | 61.9 ± 8.8 (45.6–90.3) | 0.003 |
| Height (cm) | 162.9 ± 4.7 (153.9–171.8) | 161.6 ± 4.7 (153.9–171.8) | 164.8 ± 4.1 (156.3–170.8) | 0.001 |
| BMI | 25.7 ± 6.3 (16.7–44.5) | 27.7 ± 7.1 (17.6–44.5) | 22.8 ± 3.5 (16.7–35.6) | <0.001 |
Values are expressed as mean ± sd and (range). P values indicate results of unpaired t tests between ethnic backgrounds.
Figure 1.
Relation between vitamin D concentrations and height, weight, and BMI. Cau, Caucasian; Hisp, Hispanics.
Table 2 shows the mean values for 25OHD concentrations in lean (BMI < 25 kg/m2) and overweight (BMI ≥25 kg/m2) subjects. Whereas mean serum values were significantly lower in Hispanics than Caucasians, ethnic differences in 25OHD concentrations did not persist after adjusting for BMI (Table 2).
Table 2.
25OHD concentrations (nanograms per milliliter) of 90 women separated by ethnicity and body mass
| 25OHD (ng/ml)
|
|||
|---|---|---|---|
| All (n = 90) | Hispanic (n = 53) | Caucasian (n = 37) | |
| All BMI (n = 90) | 30.1 ± 13.0 (6.7–69.6) | 26.6 ± 12.3a,b (6.7–67.3) | 35.1 ± 12.4 (14.2–69.6) |
| Lean (BMI < 25 kg/m2) (n = 51) | 34.3 ± 13.8c (15.2–69.6) | 31.2 ± 14.6 (15.2–67.3) | 36.6 ± 12.9 (16.1–69.6) |
| Overweight (BMI ≥ 25 kg/m2) (n = 39) | 24.6 ± 9.5 (6.7–46.0) | 23.3 ± 9.3 (6.7–44.9) | 29.7 ± 9.3 (14.2–46.0) |
Values are expressed as mean ± sd and (range).
Indicates a significant difference between Hispanics and Caucasians (P = 0.002).
ANOVA analysis indicates no statistical difference between Hispanics and Caucasians when adjusted for BMI (P = 0.09).
Indicates a significant difference between lean and overweight subjects (P < 0.001).
Thirty-seven women (41%) had normal 25OHD concentrations (≥30 ng/ml), whereas 53 women (59%) had insufficient 25OHD concentrations (≤29 ng/ml); of the insufficient group, 24 (45%) had values 20 ng/ml or less. Compared with women with normal 25OHD values, vitamin D-insufficient subjects were of identical age but were significantly shorter and heavier and had greater BMI (Table 3). When the sufficient group was analyzed independently, no associations were present between vitamin D and any anthropometric measures. In contrast, there were significant negative correlations between 25OHD and both weight and BMI (r = −0.28 and −0.33, respectively; P = 0.045 and 0.015, respectively) in the insufficient group.
Table 3.
25OHD values, age, and anthropometric characteristics of 90 women separated by 25OHD concentration groups
| All (n = 90) | Sufficient (n = 37) | Insufficient (n = 53) | P values | |
|---|---|---|---|---|
| 25OHD (ng/ml) | 30.1 ± 13.0 (6.7–69.6) | 42.4 ± 10.1 (30.0–69.6) | 21.5 ± 5.9 (6.7–29.6) | <0.001 |
| Age (yr) | 19.4 ± 1.5 (16.3–22.8) | 19.2 ± 1.6 (16.3–22.8) | 19.5 ± 1.4 (17.0–22.87) | 0.408 |
| Weight (kg) | 68.3 ± 17.5 (45.5–126.0) | 63.9 ± 11.9 (45.6–113.0) | 71.3 ± 20.0 (45.5–126.0) | 0.046 |
| Height (cm) | 162.9 ± 4.7 (153.9–171.8) | 164.1 ± 3.9 (156.8–170.3) | 162.1 ± 5.1 (153.9–171.8) | 0.048 |
| BMI (kg/m2) | 25.7 ± 6.3 (16.7–44.5) | 23.7 ± 4.6 (16.7–43.9) | 27.1 ± 7.1 (17.6–44.5) | 0.014 |
Values are expressed as mean ± sd and (range). P values indicate results of unpaired t test between 25OHD concentration groups.
A significant inverse correlation was found between 25OHD and PTH (r = −0.27; P = 0.01), and PTH values were higher in the insufficient than the sufficient group (2.28 ± 0.88 and 1.92 ± 0.90, respectively; P = 0.025).
Relation between 25OHD and imaging measures of body fat and bone
CT measures for sc and visceral fat and DXA measurements for whole-body fat, truncal fat, and upper and lower extremity fat were significantly lower in women with normal 25OHD concentrations than women with insufficient 25OHD (Table 4). In contrast, there were no differences in CT or DXA values for bone in the axial and appendicular skeleton between women with sufficient and those with insufficient 25OHD concentrations (Table 4).
Table 4.
CT and DXA fat and bone measurements in 90 women separated by 25OHD concentration groups
| All (n = 90) | Sufficient (n = 37) | Insufficient (n = 53) | P | |
|---|---|---|---|---|
| Fat phenotypes | ||||
| CT | ||||
| Subcutaneous (cm2) | 252.8 ± 152.7 | 203.3 ± 98.9 | 288.1 ± 174.0 | 0.029 |
| Visceral (cm2) | 36.46 ± 42.89 | 24.74 ± 33.88 | 44.81 ± 46.83 | 0.009 |
| DXA | ||||
| Total (kg) | 24.81 ± 11.79 | 21.59 ± 7.67 | 27.10 ± 13.62 | 0.029 |
| Trunk (kg) | 11.29 ± 5.76 | 9.35 ± 3.82 | 12.69 ± 5.61 | 0.006 |
| Arms (kg) | 1.64 ± 1.02 | 1.34 ± 0.61 | 1.85 ± 1.19 | 0.019 |
| Legs (kg) | 4.75 ± 1.91 | 4.33 ± 1.38 | 5.05 ± 2.17 | 0.077 |
| Bone phenotypes | ||||
| CT | ||||
| Vertebral BD (mg/cm3) | 299.1 ± 43.5 | 294.4 ± 37.3 | 302.5 ± 47.5 | 0.392 |
| Vertebral CSA (cm2) | 8.78 ± 1.32 | 8.73 ± 1.21 | 8.83 ± 1.40 | 0.723 |
| Femoral CBD (mg/cm3) | 1234 ± 36 | 1236 ± 37 | 1233 ± 37 | 0.763 |
| Femoral CBA (cm2) | 4.23 ± 0.53 | 4.24 ± 0.39 | 4.23 ± 0.61 | 0.955 |
| Femoral CSA (cm2) | 5.11 ± 0.72 | 5.07 ± 0.57 | 5.14 ± 0.81 | 0.649 |
| DXA | ||||
| Total BMC (g) | 2105 ± 298 | 2110 ± 272 | 2101 ± 317 | 0.886 |
| Total BMD (g/cm2) | 1.11 ± 0.07 | 1.11 ± 0.08 | 1.11 ± 0.07 | 0.919 |
| Trunk BMC (g) | 168.0 ± 26.9 | 161.8 ± 19.3 | 172.5 ± 30.6 | 0.771 |
| Trunk BMD (g/cm2) | 0.88 ± 0.07 | 0.88 ± 0.07 | 0.88 ± 0.08 | 0.884 |
| Hip BMC (g) | 39.56 ± 6.16 | 40.19 ± 4.95 | 39.12 ± 6.90 | 0.421 |
| Hip BMD (g/cm2) | 1.05 ± 0.11 | 1.05 ± 0.08 | 1.04 ± 0.13 | 0.663 |
| Arm BMC (g) | 138.8 ± 23.9 | 137.8 ± 18.0 | 139.5 ± 27.4 | 0.933 |
| Arm BMD (g/cm2) | 0.73 ± 0.06 | 0.74 ± 0.06 | 0.73 ± 0.06 | 0.446 |
| Leg BMC (g) | 389.9 ± 62.7 | 392.8 ± 50.4 | 387.8 ± 70.5 | 0.715 |
| Leg BMD (g/cm2) | 1.17 ± 0.09 | 1.17 ± 0.08 | 1.16 ± 0.10 | 0.586 |
P-values indicate results of unpaired t test between 250HD concentration groups. CBD, Cortical bone density; BD, bone density.
Regardless of imaging technique, strong negative correlations were observed between all measures of body adiposity and 25OHD at all sites (Table 5). These associations were present when all women were considered together and when 25OHD-insufficient subjects were analyzed independently (Table 5); this relation was not present in women with sufficient 25OHD. In contrast, regardless of whether all subjects were taken together or were separated by 25OHD concentration group, no significant association was found between 25OHD levels and any DXA or CT bone phenotypes (data not shown).
Table 5.
Relations between 25OHD concentrations and imaging measures of fat and bone in 90 women
| Fat phenotypes | All (n = 90)
|
Sufficient (n = 37)
|
Insufficient (n = 53)
|
|||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| CT | ||||||
| sc | −0.36 | <0.001 | −0.19 | 0.261 | −0.32 | 0.019 |
| Visceral | −0.28 | 0.007 | −0.05 | 0.769 | −0.30 | 0.031 |
| DXA | ||||||
| Total | −0.32 | 0.002 | −0.18 | 0.303 | −0.32 | 0.022 |
| Trunk | −0.37 | <0.001 | −0.16 | 0.333 | −0.35 | 0.011 |
| Arms | −0.29 | 0.006 | −0.16 | 0.204 | −0.33 | 0.025 |
| Legs | −0.33 | 0.001 | −0.22 | 0.342 | −0.31 | 0.016 |
Discussion
We found a strong inverse correlation between weight and body mass and circulating vitamin D and that young women with vitamin D insufficiency were significantly heavier and had greater body mass than women with normal levels. Additionally, the results of this study showed significant reciprocal relations between 25OHD and CT measures for sc and visceral fat and DXA measures of adiposity for the whole body, trunk, and extremities. The high prevalence of vitamin D insufficiency in this young population living in a sun-rich area is surprising and likely multifactorial. A recent report indicates that vitamin D insufficiency is common in children aged 6–21 yr living in the northeastern United States and is associated with season, ethnicity/black race, age, and vitamin D intake (18), but similar observations have not yet been reported in California. Whereas vitamin D insufficiency was more common in Hispanics than Caucasians in our study cohort, this difference did not persist after adjusting for BMI, indicating that the predominant risk factor was body fat rather than any variability in skin color attributed to ethnicity.
In view of the prevalence of both vitamin D insufficiency and obesity in children and adolescents, it is possible that vitamin D status is an independent predictor of weight gain. Several studies in the adult population suggest that obesity is associated with vitamin D insufficiency (20,21,22,23,24,26,38), and one indicates that low vitamin D intake is an independent predictor of obesity (25). Another investigation in postmenopausal women receiving calcium plus vitamin D reported a small effect on weight gain prevention compared with placebo (39). Indeed, vitamin D has been shown to lower leptin concentrations and may therefore contribute to the maintenance of body mass (40). On the other hand, body fat may also contribute to low circulating vitamin D levels by trapping vitamin D in fat tissues (24). Thus, obesity may, in part, be a direct consequence of vitamin D insufficiency and/or may result in vitamin D insufficiency. It is noteworthy that vitamin D insufficiency has been implicated in numerous health conditions including osteoporosis, cancer, diabetes, and rheumatoid arthritis (1,2,41) and that increased body fat is also strongly associated with greater risk of diabetes and cancer (42). Consequently, vitamin D insufficiency may play an important role in the development of these various clinical conditions either directly or indirectly.
In addition to weight and body mass, we specifically determined fat content and fat distribution using DXA and CT. Previously, using bioelectrical impedance analysis in a large group of women of all ages, indirect measures of the percentage of body fat were found to be inversely related to circulating 25OHD; an association that was particularly noticeable in white females aged 12–49 yr (38). Another study using DXA also found a negative correlation between 25OHD and percentage of body fat, but not BMI, in healthy adult women (22). The current study extends these findings to a young population of white females and indicates a strong inverse correlation between body fat and 25OHD using total-body measurements by DXA and site-specific measurements by CT. Our data indicate that 25OHD is inversely correlated with not only total body fat but also specific measures of visceral fat and sc fat, suggesting that this relationship is independent of the site of fat accumulation.
Unexpectedly, there was a positive correlation between circulating 25OHD and height in the population studied. Whereas vitamin D is key to skeletal development and its deficiency may result in short stature associated with rickets (15), none of the subjects in this study had any clinical or radiological evidence of rickets. A significant decrease in height was previously reported in adolescent girls aged 13–17 yr who had vitamin D deficiency without any clinical evidence of rickets (10). Further studies are needed to determine the possible role of vitamin D in longitudinal bone growth in the absence of clinical evidence of rickets.
An intriguing result of this study was the absence of a correlation between vitamin D status and bone determinations, regardless of site or whether assessed by DXA or CT. Previous investigations in adults indicated that vitamin D supplementation improved BMD and reduced the risk of osteoporosis and fractures (6,7,8,14). However, studies in adolescent females yielded discrepant results; some reported an association between low bone mass and vitamin D insufficiency and low vitamin D intake (12,13), whereas others, like ours, found no such relation (10,11). Although our population was comprised of Hispanics and Caucasians, this study was not powered to analyze Caucasians and Hispanics separately, and the possibility of ethnic variability in the response to vitamin D exposure cannot be excluded. Similarly, our findings in females do not exclude the notion that vitamin D influences bone mass in adolescent and young adult males, as previously reported (43,44). Despite these limitations, the results of the current study support the hypothesis that the negative effect of vitamin D insufficiency on bone mass may not be present in healthy young adults around the time that bone mass reaches its peak.
The use of two techniques for the accurate and independent assessment of the relations of vitamin D to bone and fat tissue, the use of the same technologist to obtain all CT and DXA measures and the rigorous assessment of the sexual and skeletal development, is a major strength of this study. Previous studies on the effects of vitamin D insufficiency on bone were mostly conducted using DXA, a technique that is low in cost, has minimal radiation exposure, and is readily accessible and easy to use.
Although DXA values are influenced by changes in body configuration (28,45,46) and inherently underestimate bone acquisition in short and/or overweight individuals (47), it should be noted that despite these limitations, our findings were similar, regardless of technique.
In conclusion, our study indicates that vitamin D insufficiency is extremely common in young women living in a sun-rich area of the United States. It also supports the hypotheses that either vitamin D insufficiency is a risk factor for increased body fat or increased body fat is a risk factor for vitamin D insufficiency. The positive association between height and vitamin D status is unexplained and intriguing and warrants further investigation. Our data, however, do not support a role for vitamin D in regulating bone mass acquisition around the time it reaches its peak.
Footnotes
This work was supported by the Department of the Army (DAMD17-01-1-0817), the National Institutes of Health (1R01 AR052744-01), and Natural Sciences and Engineering Research Council and Dimensional Fund Advisors Canada.
Disclosure Summary: R.K., P.P.C., T.R., and V.G. have nothing to declare.
First Published Online November 4, 2008
Abbreviations: BMC, Bone mineral content; BMD, bone mineral density; BMI, body mass index; CSA, cross-sectional area; CT, computed tomography; CV, coefficient of variation; DXA, dual-energy x-ray absorptiometry; 25OHD, 25-hydroxyvitamin D.
References
- Davis CD, Dwyer JT 2007 The “sunshine vitamin”: benefits beyond bone? J Natl Cancer Inst 99:1563–1565 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Badenhoop K 2005 Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab 16:261–266 [DOI] [PubMed] [Google Scholar]
- Kremer R, Rabbini SA 2005 Vitamin D and vitamin D analogs in cancer progression. In: Singh G, Rabbani SA, eds. Bone Metasis experimental and clinical therapeutics. 1st ed. Totowa, NJ: Humana Press; 29–57 [Google Scholar]
- Holick MF 2003 Vitamin D: a millennium perspective. J Cell Biochem 88:296–307 [DOI] [PubMed] [Google Scholar]
- Issa LL, Leong GM, Eisman JA 1998 Molecular mechanism of vitamin D receptor action. Inflamm Res 47:451–475 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B 2004 Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634–639 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B 2005 Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–2264 [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ 1992 Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327:1637–1642 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB 2004 Effect of Vitamin D on falls: a meta-analysis. JAMA 291:1999–2006 [DOI] [PubMed] [Google Scholar]
- Hatun S, Islam O, Cizmecioglu F, Kara B, Babaoglu K, Berk F, Gokalp AS 2005 Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr 135:218–222 [DOI] [PubMed] [Google Scholar]
- Kristinsson JO, Valdimarsson O, Sigurdsson G, Franzson L, Olafsson I, Steingrimsdottir L 1998 Serum 25-hydroxyvitamin D levels and bone mineral density in 16–20 year-old girls: lack of association. J Intern Med 243: 381–388 [DOI] [PubMed] [Google Scholar]
- Outila TA, Karkkainen MU, Lamberg-Allardt CJ 2001 Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr 74:206–210 [DOI] [PubMed] [Google Scholar]
- Lehtonen-Veromaa MK, Mottonen TT, Nuotio IO, Irjala KM, Leino AE, Viikari JS 2002 Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr 76:1446–1453 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B 2006 Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28 [DOI] [PubMed] [Google Scholar]
- Pettifor JM 2005 Vitamin D deficiency and nutritional rickets in children. In: Feldman DPW, Glorieux FH, eds. Vitamin D. 2nd ed. Boston: Elsevier Academic Press; 1065–1084 [Google Scholar]
- Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF 2008 Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab 93:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM 2004 Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291:2847–2850 [DOI] [PubMed] [Google Scholar]
- Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS 2007 Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 86:150–158 [DOI] [PubMed] [Google Scholar]
- Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR 2002 Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777 [DOI] [PubMed] [Google Scholar]
- Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR 1981 Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr 34:2359–2363 [DOI] [PubMed] [Google Scholar]
- Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S 1985 Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest 76:370–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunabh S, Pollack S, Yeh J, Aloia JF 2003 Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 88:157–161 [DOI] [PubMed] [Google Scholar]
- Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA 2004 The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab 89:1196–1199 [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF 2000 Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693 [DOI] [PubMed] [Google Scholar]
- Kamycheva E, Joakimsen RM, Jorde R 2003 Intakes of calcium and vitamin d predict body mass index in the population of Northern Norway. J Nutr 133:102–106 [DOI] [PubMed] [Google Scholar]
- Buffington C, Walker B, Cowan Jr GS, Scruggs D 1993 Vitamin D deficiency in the morbidly obese. Obes Surg 3:421–424 [DOI] [PubMed] [Google Scholar]
- Kong J, Li YC 2006 Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab 290:E916–E924 [DOI] [PubMed] [Google Scholar]
- Hangartner TN, Johnston CC 1990 Influence of fat on bone measurements with dual-energy absorptiometry. Bone Miner 9:71–81 [DOI] [PubMed] [Google Scholar]
- Tanner J 1987 Physical growth and development. 2nd ed. Edinburgh, Scotland: Churchill Livinston, Textbook of Pediatrics [Google Scholar]
- Greulich WW, Pyle SI 1959 Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press [Google Scholar]
- Margulies L, Horlick M, Thornton JC, Wang J, Ioannidou E, Heymsfield SB 2005 Reproducibility of pediatric whole body bone and body composition measures by dual-energy X-ray absorptiometry using the GE Lunar Prodigy. J Clin Densitom 8:298–304 [DOI] [PubMed] [Google Scholar]
- Hangartner TN, Gilsanz V 1996 Evaluation of cortical bone by computed tomography. J Bone Miner Res 11:1518–1525 [DOI] [PubMed] [Google Scholar]
- Arfai K, Pitukcheewanont PD, Goran MI, Tavare CJ, Heller L, Gilsanz V 2002 Bone, muscle, and fat: sex-related differences in prepubertal children. Radiology 224:338–344 [DOI] [PubMed] [Google Scholar]
- Kalender WA 1992 Effective dose values in bone mineral measurements by photon absorptiometry and computed tomography. Osteoporos Int 2:82–87 [DOI] [PubMed] [Google Scholar]
- Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL 1993 Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 39:529–533 [PubMed] [Google Scholar]
- Carter GD, Carter R, Jones J, Berry J 2004 How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem 50:2195–2197 [DOI] [PubMed] [Google Scholar]
- Seehofer D, Rayes N, Ulrich F, Muller C, Lang M, Neuhaus P, Steinmuller T 2001 Intraoperative measurement of intact parathyroid hormone in renal hyperparathyroidism by an inexpensive routine assay. Langenbecks Arch Surg 386:440–443 [DOI] [PubMed] [Google Scholar]
- Looker AC 2005 Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab 90:635–640 [DOI] [PubMed] [Google Scholar]
- Caan B, Neuhouser M, Aragaki A, Lewis CB, Jackson R, LeBoff MS, Margolis KL, Powell L, Uwaifo G, Whitlock E, Wylie-Rosett J, LaCroix A 2007 Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Intern Med 167:893–902 [DOI] [PubMed] [Google Scholar]
- Menendez C, Lage M, Peino R, Baldelli R, Concheiro P, Dieguez C, Casanueva FF 2001 Retinoic acid and vitamin D(3) powerfully inhibit in vitro leptin secretion by human adipose tissue. J Endocrinol 170:425–431 [DOI] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Ferrell RE 2000 Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev 22:203–217 [DOI] [PubMed] [Google Scholar]
- Holick MF 2004 Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79:362–371 [DOI] [PubMed] [Google Scholar]
- Valimaki VV, Alfthan H, Lehmuskallio E, Loyttyniemi E, Sahi T, Stenman UH, Suominen H, Valimaki MJ 2004 Vitamin D status as a determinant of peak bone mass in young Finnish men. J Clin Endocrinol Metab 89:76–80 [DOI] [PubMed] [Google Scholar]
- Taha W, Chin D, Silverberg AI, Lashiker L, Khateeb N, Anhalt H 2001 Reduced spinal bone mineral density in adolescents of an Ultra-Orthodox Jewish community in Brooklyn. Pediatrics 107:E79 [DOI] [PubMed] [Google Scholar]
- Formica C, Loro ML, Gilsanz V, Seeman E 1995 Inhomogeneity in body fat distribution may result in inaccuracy in the measurement of vertebral bone mass. J Bone Miner Res 10:1504–1511 [DOI] [PubMed] [Google Scholar]
- Tothill P, Laskey MA, Orphanidou CI, van Wijk M 1999 Anomalies in dual energy X-ray absorptiometry measurements of total-body bone mineral during weight change using Lunar, Hologic and Norland instruments. Br J Radiol 72:661–669 [DOI] [PubMed] [Google Scholar]
- Fewtrell MS 2003 Bone densitometry in children assessed by dual x ray absorptiometry: uses and pitfalls. Arch Dis Child 88:795–798 [DOI] [PMC free article] [PubMed] [Google Scholar]