Abstract
Context: Peripubertal obesity (body mass index-for-age ≥ 95%) in girls is associated with hyperandrogenemia. LH likely contributes to this relationship, but overnight LH secretion in obese girls is poorly characterized.
Objective: The aim of the study was to evaluate LH pulse characteristics in obese girls throughout pubertal maturation.
Design: We conducted a cross-sectional analysis.
Setting: The study was performed in a general clinical research center.
Participants: Eight nonobese and five obese Tanner 1–2 girls participated, as well as 32 nonobese and 12 obese Tanner 3–5 girls.
Intervention: Blood samples were collected every 10 min overnight (from 1900 to 0700 h).
Main Outcome Measures: LH pulse frequency, amplitude, and mean LH were measured in three 4-h time blocks (block 1, 1900–2300 h; block 2, 2300–0300 h; and block 3, 0300–0700 h).
Results: Tanner stage 1–2 nonobese girls demonstrated nocturnal increases of LH frequency (P < 0.01, block 1 vs. 2) and mean LH (P < 0.05, block 1 vs. 2 and 3). Obese Tanner 1–2 girls had lower 12-h LH frequency and LH amplitude (P < 0.05 for both), with no overnight changes of LH pulse parameters. Compared to normal, LH frequency was elevated in Tanner 3–5 obese girls (P < 0.01 in all blocks), whereas LH amplitude was low (P < 0.05 in all blocks). Overnight increases of LH amplitude were observed in nonobese Tanner 3–5 girls (P < 0.0001), but not in obese Tanner 3–5 girls.
Conclusions: Obesity in prepubertal and early pubertal girls is associated with reduced LH secretion and reduced nocturnal changes of LH. In later pubertal girls, obesity is linked with reduced LH amplitude, but elevated LH frequency; the latter may reflect effects of hyperandrogenemia.
Obesity in pre- and early pubertal girls is associated with reduced LH secretion and dampened nocturnal changes of LH. In later pubertal girls, obesity is linked with reduced LH amplitude and elevated LH frequency, with the latter possibly reflecting hyperandrogenemia.
Orderly pulsatile secretion of GnRH is essential for pubertal development and normal reproductive function. Early puberty is marked by nocturnal (sleep-associated) increases of LH amplitude and LH (GnRH) pulse frequency, with reduction during the following day (1,2,3,4,5). Such diurnal variation of GnRH frequency may be important for normal pubertal secretion of LH and FSH because high and low GnRH pulse frequencies favor pituitary synthesis of LH and FSH, respectively (6). The mechanisms underlying diurnal changes of GnRH frequency remain unclear, but we have hypothesized that sex steroid feedback plays a key role (7).
Polycystic ovary syndrome (PCOS) and adolescent hyperandrogenemia (HA) are associated with elevated GnRH pulse frequency (8,9,10,11,12), which contributes to HA and oligoanovulation via LH excess and relative FSH deficiency (7). In adult PCOS (13,14) and in some adolescents with HA (15), rapid GnRH pulses are partly related to relative GnRH pulse generator resistance to negative feedback (i.e. reduced ability of progesterone (P) to suppress GnRH pulse frequency). This defect can be reversed in adult PCOS by androgen receptor blockade (16), suggesting that it is a result of HA itself.
Clinical manifestations of PCOS often begin during or shortly after puberty, and HA during adolescence can represent a precursor of adult PCOS (17,18). We have suggested that early pubertal HA interferes with diurnal GnRH frequency changes, leading to gonadotropin abnormalities that support a progression toward a classical PCOS phenotype (7). Study of this putative relationship in early puberty is difficult because HA generally does not manifest clinically (e.g. as hirsutism) until later puberty. However, we and others have recently described an association between HA and obesity in early pubertal girls (19,20,21). Although early data suggest that LH may play an important role in obesity-associated HA (20,21), these data originated from early morning blood samples. Thus, LH secretory dynamics in obese peripubertal girls remain unclear. We hypothesized that obese peripubertal girls—who are hyperandrogenemic in general—demonstrate elevated LH pulse frequency during daytime and nighttime. Such a relationship could reflect the effects of abnormal LH drive on androgen production, effects of HA on LH pulsatility, or both. Herein we describe overnight LH and sex steroid secretory dynamics in obese girls throughout pubertal development.
Subjects and Methods
Volunteers were recruited through advertisements and from Pediatric Clinics at the University of Virginia Health System (UVAHS). Subjects included 40 nonobese girls and 17 obese girls, ranging from pubertal stage 1 (prepubertal) to stage 5 and from ages 7 to 17 yr. Exact body mass index (BMI)-for-age percentiles were calculated using a SAS program that incorporates normative data from the National Health Examination and National Health and Nutrition Examination Surveys (22) (available at http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm). Subjects were classified as obese if their gender-specific BMI-for-age was 95th percentile or greater (23,24). Those with a BMI-for-age below the 95th percentile were classified as nonobese. Subject characteristics are shown in Table 1. Early morning data from some of these subjects were included in previous reports (20,21). None of the girls was anorectic or overly athletic. Only one girl could be considered underweight (BMI-for-age percentile 4.4), but this Tanner 2 girl had no evidence of hypothalamic-pituitary-ovarian disturbance (e.g. 7 LH pulses over 12 h with a normal pattern; average LH amplitude, 5.3 IU/liter; mean LH, 5.3 IU/liter; mean FSH, 4.8 IU/liter; and average estradiol (E2), 46 pg/ml). None of the girls was sexually precocious, and none had premature adrenarche. Seven girls (all Tanner 5 and obese) had abnormal menses (i.e. average intermenstrual length >45 d in girls at least 2 yr postmenarche) and hyperandrogenism [i.e. hirsutism and/or Tanner stage-specific HA (20)], and therefore met criteria for PCOS (25).
Table 1.
Baseline characteristics
| Nonobese | Obese | P value | |
|---|---|---|---|
| Tanner 1 | (n = 4) | (n = 2) | |
| BMI (kg/m2) | 15.9 ± 0.6 (16.1, 14.6–17.0) | 27.4 ± 0.4 (27.4, 26.9–27.8) | |
| BMI-for-age percentile | 45.1 ± 9.4 (44.9, 25.4–65.4) | 98.8 ± 0.6 (98.8, 98.2–99.4) | |
| Age (yr) | 8.5 ± 0.4 (8.6, 7.4–9.5) | 9.3 ± 1.2 (9.3, 8.1–10.4) | |
| Bone age (yr) | 7.3 ± 1.5 (7.3, 5.8–8.8) (n = 2) | 9.9 ± 1.1 (9.9, 8.8–11.0) | |
| Postmenarcheal | 0 of 4 (0%) | 0 of 2 (0%) | |
| Hirsutism | 0 of 4 (0%) | 0 of 2 (0%) | |
| Tanner 2 | (n = 4) | (n = 3) | |
| BMI (kg/m2) | 17.3 ± 1.6 (16.1, 15.1–21.8) | 30.2 ± 3.3 (30.5, 24.4–35.7) | |
| BMI-for-age percentile | 36.2 ± 19.5 (23.5, 5.1–92.8) | 99.1 ± 0.5 (99.3, 98.2–99.8) | |
| Age (yr) | 11.3 ± 0.8 (11.2, 9.9–13.1) | 9.1 ± 0.5 (8.7, 8.6–10.0) | |
| Bone age (yr) | 11.1 ± 0.3 (11.0, 10.5–12.0) | 11.0 ± 1.0 (11.0, 10.0–12.0) (n = 2) | |
| Postmenarcheal | 0 of 4 (0%) | 0 of 3 (0%) | |
| Hirsutism | 0 of 4 (0%) | 0 of 3 (0%) | |
| Tanner 1–2 | (n = 8) | (n = 5) | |
| Tanner stage | 1.5 ± 0.2 (1.5, 1–2) | 1.6 ± 0.2 (2.0, 1–2) | >0.1 |
| BMI (kg/m2) | 16.6 ± 0.8 (16.1, 14.6–21.8) | 29.1 ± 1.9 (27.8, 24.4–35.7) | 0.0016 |
| BMI-for-age percentile | 40.7 ± 10.2 (31.1, 5.1–92.8) | 99.0 ± 0.3 (99.3, 98.2–99.8) | 0.0016 |
| Age (yr) | 9.9 ± 0.7 (9.7, 7.4–13.1) | 9.2 ± 0.5 (8.7, 8.1–10.4) | >0.1 |
| Bone age (yr) | 9.9 ± 0.9 (10.8, 5.8–12.0) (n = 6) | 10.5 ± 0.7 (10.5, 8.8–12.0) (n = 4) | >0.1 |
| Postmenarcheal | 0 of 8 (0%) | 0 of 5 (0%) | NA |
| Hirsutism | 0 of 8 (0%) | 0 of 5 (0%) | NA |
| Race, ethnicity | 7 W, 1 NR; 1 of 8 Hispanic | 4 W, 1 B; 1 of 5 Hispanic | |
| Tanner 3 | (n = 5) | (n = 1) | |
| BMI (kg/m2) | 20.3 ± 1.0 (21.5, 17.2–23.1) | 32.5 | |
| BMI-for-age percentile | 66.9 ± 11.8 (83.3, 28.6–88.1) | 99.1 | |
| Age (yr) | 12.4 ± 0.5 (12.8, 10.8–13.5) | 11.8 | |
| Bone age (yr) | 12.6 ± 0.6 (12.5, 11.5–14.0) (n = 4) | 15.0 | |
| Postmenarcheal | 2 of 5 (40%) | no | |
| Gynecological age (yr)* | 1.0 ± 0.0 (1, 1–1) (n = 2) | ||
| Irregular menses* | 1 of 2 (50%) | ||
| Hirsutism | 0 of 5 (0%) | yes | |
| Tanner 4 | (n = 10) | (n = 2) | |
| BMI (kg/m2) | 20.5 ± 1.0 (20.8, 16.0–25.1) | 32.3 ± 0.7 (32.3, 31.6–32.9) | |
| BMI-for-age percentile | 59.6 ± 9.1 (67.2, 4.2–90.7) | 98.2 ± 0.5 (98.2, 97.6–98.7) | |
| Age (yr) | 13.8 ± 0.5 (13.9, 12.0–16.9) | 14.3 ± 0.8 (14.3, 13.6–15.1) | |
| Bone age (yr) | 14.5 ± 1.0 (15.0, 12.0–16.0) (n = 4) | ||
| Postmenarcheal | 8 of 10 (80%) | 2 of 2 (100%) | |
| Gynecological age (yr)* | 2.1 ± 0.6 (2, 0–4) (n = 8) | <1 yr | |
| Irregular menses* | 1 of 8 (13%) | 0 of 2 | |
| Hirsutism | 0 of 10 (0%) | 1 of 2 (50%) | |
| Tanner 5 | (n = 17) | (n = 9) | |
| BMI (kg/m2) | 23.0 ± 0.8 (24.0, 18.1–28.1) | 37.3 ± 2.4 (34.4, 28.2–49.2) | |
| BMI-for-age percentile | 71.0 ± 5.8 (80.8, 16.6–94.7) | 98.2 ± 0.4 (98.2, 96.3–99.8) | |
| Age (yr) | 15.1 ± 0.3 (15.3, 13.1–17.3) | 15.9 ± 0.5 (16.1, 13.4–17.7) | |
| Bone age (yr) | 17.3 ± 0.7 (18.0, 16.0–18.0) (n = 3) | 17.3 ± 0.5 (17.5, 16.0–18.0) (n = 4) | |
| Postmenarcheal | 15 of 17 (88%) | 8 of 9 (89%) | |
| Gynecological age (yr)* | 2.7 ± 0.3 (3, 0–5) (n = 15) | 3.0 ± 0.6 (2.5, 1–6) | |
| Irregular menses* | 3 of 15 (20%) | 7 of 8 (88%) | |
| Hirsutism | 3 of 17 (18%) | 9 of 9 (100%) | |
| (Continued) | |||
Table 1A.
Continued
| Nonobese | Obese | P value | |
|---|---|---|---|
| Tanner 3–5 | (n = 32) | (n = 12) | |
| Tanner stage | 4.4 ± 0.1 (5, 3–5) | 4.7 ± 0.2 (5, 3–5) | >0.1 |
| BMI (kg/m2) | 21.8 ± 0.6 (21.7, 16.0–28.1) | 36.1 ± 1.9 (33.1, 28.2–49.2) | <0.0001 |
| BMI-for-age percentile | 66.8 ± 4.5 (76.5, 4.2–94.7) | 98.3 ± 0.3 (98.4, 96.3–99.8) | <0.0001 |
| Age (yr) | 14.3 ± 0.3 (14.0, 10.8–17.3) | 15.3 ± 0.5 (15.9, 11.8–17.7) | 0.0968 |
| Bone age (yr) | 14.6 ± 0.7 (14.0, 11.5–18.0) (n = 11) | 16.8 ± 0.6 (17.0, 15.0–18.0) (n = 5) | 0.0949 |
| Postmenarcheal | 25 of 32 (78%) | 10 of 12 (83%) | >0.1 |
| Gynecological age (yr)* | 2.4 ± 0.3 (2, 0–5) (n = 25) | 2.4 ± 0.6 (2, 0–6) (n = 10) | >0.1 |
| Irregular menses* | 5 of 25 (20%) | 7 of 10 (70%) | 0.0146 |
| Hirsutism | 3 of 32 (9%) | 11 of 12 (92%) | <0.0001 |
| Race, ethnicity | 25 W, 6 B, 1 NR; 1 of 32 Hispanic | 10 W, 2 B; 0 of 12 Hispanic |
Continuous data are presented as mean ± sem (median, minimum–maximum). Asterisks denote data for postmenarcheal girls only. NA, Not applicable (i.e. all subjects in one category); W, white; B, black; NR, not reported. Of the 25 postmenarcheal nonobese girls, gynecological age was no greater than 2 yr in 13, and at least 4 yr in six. Of the 10 postmenarcheal obese girls, gynecological age was no greater than 2 yr in 8, and at least 4 yr in two. Gynecological age = number of full years (as a whole number) since menarche.
Study procedures
Study procedures were approved by the Institutional Review Board at UVAHS. Informed assent and consent were obtained from study participants and parents, respectively. Participants were taking no medications known to affect the reproductive axis, and none had used hormonal medications for 90 d before study.
Each volunteer underwent a detailed history and physical exam, including assessment of pubertal breast stage [using the Tanner scale (26)] via inspection and palpation. All subjects were screened for hormonal and health-related abnormalities with determinations of LH, FSH, P, E2, total testosterone (T), SHBG, 17-hydroxyprogesterone, dehydroepiandrosterone sulfate (DHEAS), β-human chorionic gonadotropin, TSH, prolactin, complete blood count, chemistry and liver panels. With the exception of androgens and insulin, all laboratory values were within normal limits. Twenty-six of 57 subjects had a bone age determination using standard techniques.
Subjects underwent an overnight sampling study in the General Clinical Research Center (GCRC), with evening sampling (i.e. before sleep) serving as a surrogate for daytime due to blood volume limitations. Premenarcheal girls were studied on a random day; regularly cycling subjects between cycle days 8 and 10; and oligomenorrheic subjects at least 60 d after menses. Subjects were admitted to the GCRC at 1700 h. A hematocrit and β-human chorionic gonadotropin were obtained to exclude anemia and pregnancy, respectively. A standard dinner was served at 1730 h. Beginning at 1900 h, blood for later hormone measurement was obtained through an indwelling iv heparin lock catheter over 12 h as follows: LH every 10 min; FSH, P, E2, T, and cortisol every 2 h. Subjects began fasting no later than 2200 h. Lights were extinguished at 2300 h to facilitate sleep, which was recorded every 10 min by trained observers. At 0700 h, blood was drawn for fasting insulin, SHBG, and DHEAS. Thereafter, breakfast was served, and volunteers were discharged on oral iron supplementation to help replenish iron stores.
Hormonal measurements
Blood was withdrawn into serum separator tubes and allowed to clot at room temperature before centrifugation. Serum was removed and stored at −20 C before analysis, which occurred within several days. Assays were performed by the Ligand Core Laboratory of the Center for Research in Reproduction at UVAHS. All samples from an individual subject were analyzed in duplicate in the same assay for each hormone. The pulse analysis program used both LH values for each time point; otherwise, the mean of the duplicates was used for data analysis. Samples with measured values below assay sensitivity were assigned the value of the assay’s sensitivity.
LH and FSH were measured by chemiluminescence [Diagnostic Products Corporation (DPC), Los Angeles, CA; sensitivities, 0.1 and 0.05 IU/liter; intraassay coefficients of variation (CVs), 1.9–3.2%; interassay CVs 4.8–6.6%]. Total T, E2, P, and cortisol were measured by RIA (DPC; sensitivities, 10 ng/dl, 10 pg/ml, 0.1 ng/ml, and 1 μg/dl, respectively; intraassay CVs, 3.5–6.8%; interassay CVs, 5.8–15.8%). Given minor cross-reaction of cortisol in the P assay, we employed a cortisol concentration-P measurement nomogram as previously described (20). This allowed adjustment of measured P concentrations for a small contribution of cortisol. SHBG and DHEAS were measured by chemiluminescence (DPC; sensitivities, 0.2 nmol/liter and 7 μg/dl; intraassay CVs, 2.0–6.2%; interassay CVs, 3.8–7.8%, respectively). Insulin was measured by chemiluminescence (DPC; sensitivity, 2.6 μIU/ml; intraassay CV, 3.0–4.0%; interassay CV, 8.3–8.8%) or RIA (Diagnostics Systems Laboratories, Inc., Webster, TX; sensitivity, 1.3 μIU/ml; intraassay CV, 7.5–8.5%; interassay CV, 9.5–21%).
Free T was calculated from total T and SHBG using the following equation: FT = ([T − (N)(FT)]/[(KT)(SHBG) − (KT)(T) + (N)(KT)(FT)])/3.467. In this equation, FT = free T (pg/ml); KT = association constant of SHBG for T (1.0 × 109); T = total T concentration (ng/dl); SHBG = SHBG concentration (nmol/liter); and N = (KA)(CA) + 1, where KA = association constant of albumin for T (3.6 × 104), and CA = concentration of albumin (assumed to be 4.3 g/dl) (27).
Formulas for converting conventional units to SI units are: free T (pg/ml) × 3.467 (pmol/liter); P × 3.18 (pmol/liter); E2 × 3.671 (pmol/liter); DHEAS × 0.002714 (μmol/liter); and insulin × 7.175 (pmol/liter).
Data analysis
LH pulses were identified using the computer algorithm Cluster 7 (28). Analysis parameters were a test nadir and peak size of 2 × 2 with a t-statistic of 2.45 for both upstroke and downstroke. Missing values represented less than 0.1% of the total and were ignored. If the amplitude of a detected LH pulse (by Cluster 7) was less than the range of intraassay variability for the LH chemiluminescence method, it was not considered a pulse in subsequent analysis, as previously described (29). Specifically, pulses with the following characteristics were excluded: peak less than 0.5 and amplitude less than 0.1; peak from 0.5 to 1 and amplitude less than 0.25; peak from 1 to 5 and amplitude less than 0.5; peak greater than 5 and amplitude less than 1.0 (all in units IU/liter).
Statistical methods
Data are presented as mean ± sem. We employed nonparametric statistical tests, which are based on ranks of observations and require no assumptions about the underlying distribution of data. All hypothesis tests were two-sided and conducted at the 0.05 level of significance.
The primary outcome of this study was LH pulse count (a measure of LH pulse frequency) over 12 h and across three 4-h time blocks: 1900–2300 h (i.e. before “lights out”; block 1); 2300–0300 h (block 2); and 0300–0700 h (block 3). Secondary outcomes included mean LH, LH amplitude, FSH, P, T, and E2 across the same time blocks and over 12 h. For formal statistical analysis, we combined Tanner 1 and 2 girls because nonobese Tanner 1 and 2 girls demonstrated nocturnal increases in LH frequency, and because obese girls in these stages had lower than normal LH frequency (Fig. 1). Likewise, we combined the Tanner 3, 4, and 5 girls because these girls demonstrated daytime LH frequency at least as high as that observed at nighttime, and because obese Tanner 3–5 girls generally had higher LH frequency compared with nonobese girls.
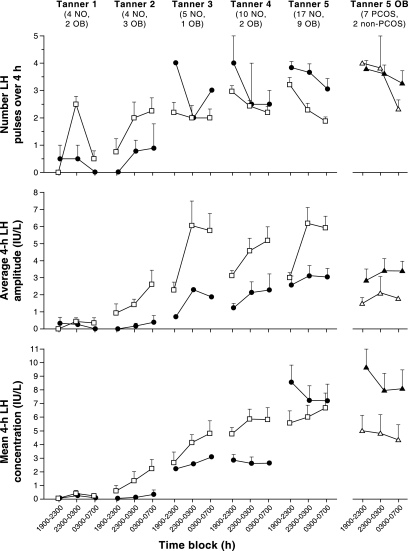
Figure 1.
Late evening and overnight LH characteristics in obese (solid circles) and nonobese (open squares) peripubertal girls stratified by Tanner breast stage. Data are presented as mean ± sem. The numbers of subjects in each group are shown below the Tanner stage labels. NO, Nonobese; OB, obese. For reference, data for obese Tanner 5 girls with PCOS (solid triangles) and without PCOS (open triangles) are shown separately in the last column.
To compare baseline characteristics between groups (i.e. obese vs. nonobese), Wilcoxon rank sum tests and Fisher’s exact tests were used for continuous and categorical variables, respectively. Wilcoxon rank sum tests were used to compare 12-h and 4-h values between obese and nonobese groups. When comparison involved at least 10 observations from each of the two samples, the method of normal approximation was employed; otherwise, exact tests were performed. Wilcoxon sign rank tests were used to test for day-night differences, with values during block 1 (a surrogate for daytime values) serving as the baseline.
Results
LH pulse frequency
LH pulse frequency increased overnight (i.e. block 1 vs. block 2) in nonobese Tanner 1 and 2 girls (Fig. 1). By block 3, LH frequency had decreased in nonobese Tanner 1 girls but remained elevated in nonobese Tanner 2 girls. In nonobese Tanner 3 girls, LH frequency was similar in all time blocks; and daytime LH frequency was higher than nighttime frequency in nonobese Tanner 4 and 5 girls. Thus, daytime frequency preferentially increased across normal puberty, whereas nighttime frequency (block 2) remained approximately constant (∼0.5 pulses per hour). In Tanner 1 and 2 obese girls, LH frequency was generally lower than that in nonobese Tanner 1 and 2 girls. Nighttime frequency did not increase in Tanner 1 obese girls, although there was a suggestion of such in Tanner 2 obese girls. Mean LH frequency in Tanner 3–5 obese girls was as high as or higher than that of corresponding nonobese girls.
In Tanner 1–2 nonobese girls, LH pulse frequency increased approximately 5.9-fold from blocks 1 to 2 (P = 0.0078; Fig. 2). LH frequency decreased by block 3 such that it was no longer statistically different from block 1 (P = 0.0625). In contrast, LH frequency did not clearly increase overnight in obese Tanner 1–2 girls (P ≥ 0.5; Fig. 2). In Tanner 3–5 nonobese girls, LH pulse frequency decreased by 23% from block 1 to 2 and 33% from block 1 to 3 (P < 0.0001 for both). In obese Tanner 3–5 girls, mean LH frequency decreased by 24% from block 1 to 3, but this did not reach statistical significance (P = 0.0596).
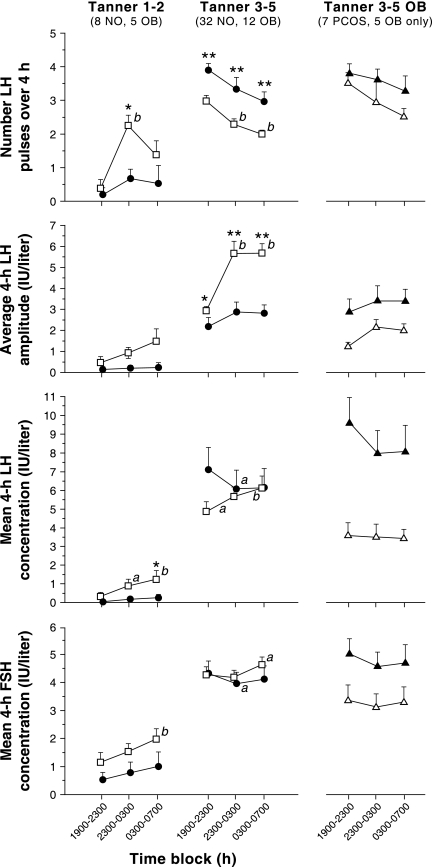
Figure 2.
Late evening and overnight gonadotropin characteristics in obese (solid circles) and nonobese (open squares) peripubertal girls stratified by Tanner breast stage grouping. Data are presented as mean ± sem. The numbers of subjects in each group are shown below the Tanner stage labels. NO, Nonobese; OB, obese. a, P < 0.05 vs. baseline (1900–2300 h); b, P < 0.01 vs. baseline (1900–2300 h); *, P < 0.05 vs. nonobese in the same time block; **, P < 0.01 vs. nonobese in the same time block. For reference, data for obese Tanner 5 girls with PCOS (solid triangles) and without PCOS (open triangles) are shown separately in the last column.
LH frequency over 12 h was 65% lower in obese Tanner 1–2 girls compared with nonobese Tanner 1–2 girls (P = 0.0241) (see Tables 2 and 3). Compared with nonobese Tanner 3–5 girls, LH frequency was higher in obese girls during all time blocks: 31% higher in block 1 (P = 0.0045), 45% higher in block 2 (P = 0.0088), and 49% higher in block 3 (P = 0.0026) (Fig. 2)
Table 2.
Mean hormonal values over 12 h of sampling (1900–0700 h) stratified by Tanner breast stage
| Nonobese | Obese | |
|---|---|---|
| Tanner 1 | (n = 4) | (n = 2) |
| LH pulses per 12 h | 3.0 ± 0.4 (3, 2–4) | 1.0 ± 1.0 (1, 0–2) |
| LH amplitude (IU/liter) | 0.4 ± 0.2 (0.3, 0.1–1.1) | 0.3 ± 0.3 (0.3, 0.0–0.6) |
| LH (IU/liter) | 0.2 ± 0.1 (0.1, 0.1–0.6) | 0.1 ± 0.06 (0.1, 0.1–0.2) |
| FSH (IU/liter) | 1.2 ± 0.3 (1.2, 0.5–1.8) | 0.9 ± 0.6 (0.9, 0.3–1.5) |
| Free T (pg/ml) | 0.58 ± 0.13 (0.53, 0.36–0.88) | 0.86 ± 0.12 (0.86, 0.74–0.98) |
| P (ng/ml) | 0.2 ± 0.04 (0.2, 0.1–0.3) | 0.2 ± 0.4 (0.2, 0.1–0.2) |
| E2 (pg/ml) | 29.7 ± 8.1 (29.5, 12.4–47.5) | 21.6 ± 0.1 (21.6, 21.5–21.7) |
| SHBG (nmol/liter)* | 58.8 ± 8.2 (60.9, 38.8–74.7) | 23.2 ± 0.7 (23.2, 22.5–23.8) |
| DHEAS (μg/dl)* | 26 ± 14 (21, 7–61) | 26 ± 17 (26, 9–43) |
| Fasting insulin (μIU/ml)* | 6.5 ± 2.6 (5.4, 1.7–13.7) | 34.9 ± 9.3 (34.9, 25.6–44.2) |
| Tanner 2 | (n = 4) | (n = 3) |
| LH pulses per 12 h | 5.0 ± 1.2 (5.5, 2–7) | 1.7 ± 1.2 (1.0, 0–4) |
| LH amplitude (IU/liter) | 1.9 ± 0.3 (1.8, 1.3–2.7) | 0.3 ± 0.3 (0.1, 0.0–0.9) |
| LH (IU/liter) | 1.4 ± 0.6 (1.2, 0.4–2.8) | 0.2 ± 0.1 (0.1, 0.1–0.5) |
| FSH (IU/liter) | 1.9 ± 0.6 (1.5, 1.1–3.5) | 0.7 ± 0.6 (0.3, 0.1–1.8) |
| Free T (pg/ml) | 0.85 ± 0.23 (0.76, 0.40–1.47) | 1.12 ± 0.35 (1.10, 0.52–1.75) |
| P (ng/ml) | 0.3 ± 0.03 (0.3, 0.2–0.3) | 0.3 ± 0.1 (0.2, 0.2–0.5) |
| E2 (pg/ml) | 38.2 ± 6.7 (36.9, 23.7–55.4) | 34.1 ± 5.4 (39.2, 23.3–39.7) |
| SHBG (nmol/liter)* | 58.1 ± 9.6 (50.9, 44.1–86.4) | 27.4 ± 9.4 (28.4, 10.7–43.2) |
| DHEAS (μg/dl)* | 45 ± 5 (46, 31–56) | 68 ± 16 (62, 45–98) |
| Fasting insulin (μIU/ml)* | 10.9 ± 2.8 (10.9, 5.5–16.4) | 11.7 ± 2.8 (12.4, 6.6–16.2) |
| Tanner 3 | (n = 5) | (n = 1) |
| LH pulses per 12 h | 6.2 ± 0.9 (6, 4–9) | 9 |
| LH amplitude (IU/liter) | 4.5 ± 1.0 (3.4, 2.2–7.3) | 1.5 |
| LH (IU/liter) | 3.9 ± 0.5 (4.5, 2.5–4.9) | 2.6 |
| FSH (IU/liter) | 4.5 ± 0.6 (4.4, 3.0–6.6) | 2.5 |
| Free T (pg/ml) | 1.72 ± 0.51 (2.02, 0.37–3.23) | 11.1 |
| P (ng/ml) | 0.3 ± 0.04 (0.3, 0.2–0.4) | 0.5 |
| E2 (pg/ml) | 45.2 ± 6.3 (40.1, 32.1–66.6) | 61.7 |
| SHBG (nmol/liter)* | 51.9 ± 9.2 (48.5, 27.4–84.5) | 17.0 |
| DHEAS (μg/dl)* | 76 ± 23 (91, 13–143) | 67 |
| Fasting insulin (μIU/ml)* | 13.5 ± 3.3 (10.8, 5.7–23.3) | 58.8 |
| Tanner 4 | (n = 10) | (n = 2) |
| LH pulses per 12 h | 7.6 ± 0.5 (7, 6–10) | 9.0 ± 1.0 (9, 8–10) |
| LH amplitude (IU/liter) | 4.2 ± 0.5 (3.8, 2.6–7.3) | 1.6 ± 0.2 (1.6, 1.5–1.8) |
| LH (IU/liter) | 5.5 ± 0.7 (5.1, 2.9–10.8) | 2.7 ± 0.3 (2.7, 2.4–3.0) |
| FSH (IU/liter) | 4.1 ± 0.4 (4.0, 1.7–6.1) | 2.5 ± 0.7 (2.5, 1.8–3.1) |
| Free T (pg/ml) | 2.98 ± 0.38 (3.00, 1.52–5.24) | 6.97 ± 2.34 (6.97, 4.63–9.31) |
| P (ng/ml) | 0.3 ± 0.03 (0.3, 0.2–0.5) | 0.3 ± 0.1 (0.3, 0.2–0.4) |
| E2 (pg/ml) | 65.5 ± 10.0 (55.6, 34.6–140.2) | 75.6 ± 20.9 (75.6, 54.7–96.4) |
| SHBG (nmol/liter)* | 37.8 ± 7.5 (27.3, 10.4–82.6) | 19.3 ± 6.8 (19.3, 12.5–26.1) |
| DHEAS (μg/dl)* | 134 ± 21 (140, 54–210) | 114 ± 25 (144, 89–139) |
| Fasting insulin (μIU/ml)* | 14.5 ± 1.9 (13.8, 5.6–27.7) | 22.8 ± 15.1 (22.8, 7.7–37.9) |
| Tanner 5 | (n = 17) | (n = 9) |
| LH pulses per 12 h | 7.4 ± 0.6 (8, 3–12) | 10.6 ± 0.5 (10.6, 8–13) |
| LH amplitude (IU/liter) | 4.5 ± 0.5 (4.7, 1.9–7.5) | 2.9 ± 0.5 (2.2, 1.3–5.7) |
| LH (IU/liter) | 6.1 ± 0.9 (5.2, 2.2–16.5) | 7.7 ± 1.1 (6.8, 3.5–13.2) |
| FSH (IU/liter) | 4.5 ± 0.4 (4.0, 2.6–8.7) | 4.7 ± 0.4 (4.7, 3.3–7.5) |
| Free T (pg/ml) | 4.58 ± 0.83 (2.91, 1.80–12.35) | 9.86 ± 2.03 (8.73, 3.42–22.82) |
| P (ng/ml) | 0.4 ± 0.04 (0.4, 0.2–0.7) | 0.5 ± 0.1 (0.4, 0.2–1.0) |
| E2 (pg/ml) | 62.6 ± 6.9 (54.2, 30.1–142.9) | 63.1 ± 6.9 (60.4, 28.0–93.7) |
| SHBG (nmol/liter)* | 35.8 ± 3.8 (38.1, 13.5–62.4) | 17.6 ± 3.3 (12.8, 9.0–39.7) |
| DHEAS (μg/dl)* | 180 ± 26 (170, 41–414) | 169 ± 32 (152, 37–356) |
| Fasting insulin (μIU/ml)* | 20.9 ± 3.0 (17.3, 4.8–46.6) | 30.5 ± 4.2 (30.2, 11.5–54.8) |
Data are presented as mean ± SEM (median, minimum–maximum). Asterisks denote measurements made from a single blood sample at 0700 h. To convert from conventional to SI units: free T × 3.467 (pmol/liter); P × 3.18 (pmol/liter); E2 × 3.671 (pmol/liter); DHEAS × 0.002714 (μmol/liter); insulin × 7.175 (pmol/liter).
Table 3.
Mean hormonal values over 12 h of sampling (1900–0700 h) stratified by Tanner breast stage grouping
| Nonobese | Obese | P value | |
|---|---|---|---|
| Tanner 1–2 | (n = 8) | (n = 5) | |
| LH pulses per 12 h | 4.0 ± 0.7 (3.5, 2–7) | 1.4 ± 0.8 (1, 0–4) | 0.0241 |
| LH amplitude (IU/liter) | 1.2 ± 0.3 (1.2, 0.1–2.7) | 0.3 ± 0.2 (0.1, 0.0–0.9) | 0.0435 |
| LH (IU/liter) | 0.8 ± 0.3 (0.5, 0.1–2.8) | 0.2 ± 0.1 (0.1, 0.1–0.5) | 0.0932 |
| FSH (IU/liter) | 1.6 ± 0.3 (1.3, 0.5–3.5) | 0.8 ± 0.4 (0.3, 0.1–1.8) | >0.1 |
| Free T (pg/ml) | 0.71 ± 0.13 (0.68, 0.36–1.47) | 1.02 ± 0.21 (0.98, 0.52–1.75) | >0.1 |
| P (ng/ml) | 0.2 ± 0.03 (0.3, 0.1–0.3) | 0.2 ± 0.06 (0.2, 0.1–0.5) | >0.1 |
| E2 (pg/ml) | 34.0 ± 5.1 (35.9, 12.4–55.4) | 29.1 ± 4.3 (23.3, 21.5–39.7) | >0.1 |
| SHBG (nmol/liter)* | 58.4 ± 5.9 (51.6, 38.8–86.4) | 25.7 ± 5.3 (23.8, 10.7–43.2) | 0.0031 |
| DHEAS (μg/dl)* | 35 ± 8 (40, 7–61) | 51 ± 15 (45, 9–98) | >0.1 |
| Fasting insulin (μIU/ml)* | 8.7 ± 1.9 (6.4, 1.7–16.4) | 21.0 ± 6.6 (16.2, 6.6–44.2) | 0.0932 |
| Tanner 3–5 | (n = 32) | (n = 12) | |
| LH pulses per 12 h | 7.3 ± 0.4 (7, 3–12) | 10.2 ± 0.5 (10, 8–13) | 0.0001 |
| LH amplitude (IU/liter) | 4.4 ± 0.3 (4.1, 1.9–7.5) | 2.5 ± 0.4 (1.9, 1.3–5.7) | 0.0009 |
| LH (IU/liter) | 5.6 ± 0.5 (4.9, 2.2–16.5) | 6.4 ± 1.1 (5.9, 2.4–13.2) | >0.1 |
| FSH (IU/liter) | 4.4 ± 0.3 (4.0, 1.7–8.7) | 4.1 ± 0.4 (3.8, 1.8–7.5) | >0.1 |
| Free T (pg/ml) | 3.64 ± 0.49 (2.85, 0.37–12.35) | 9.48 ± 1.57 (9.02, 3.42–22.82) | <0.0001 |
| P (ng/ml) | 0.4 ± 0.03 (0.3, 0.2–0.7) | 0.4 ± 0.07 (0.4, 0.2–1.0) | >0.1 |
| E2 (pg/ml) | 60.7 ± 4.9 (52.6, 30.1–142.9) | 65.1 ± 5.9 (60.9, 28.0–96.4) | >0.1 |
| SHBG (nmol/liter)* | 39.0 ± 3.4 (38.4, 10.4–84.5) | 17.8 ± 2.6 (14.6, 9.0–39.7) | 0.0003 |
| DHEAS (μg/dl)* | 149 ± 17 (142, 13–414) | 151 ± 26 (138, 37–356) | >0.1 |
| Fasting insulin (μIU/ml)* | 17.5 ± 1.8 (15.8, 4.8–46.6) | 31.6 ± 4.5 (32.3, 7.7–58.8) | 0.0061 |
Data are presented as mean ± sem (median, minimum–maximum). Asterisks denote values from a single blood sample at 0700 h. To convert from conventional to SI units: free T × 3.467 (pmol/liter); P × 3.18 (pmol/liter); E2 × 3.671 (pmol/liter); DHEAS × 0.002714 (μmol/liter); insulin × 7.175 (pmol/liter).
LH pulse amplitude
Overnight LH amplitudes appeared to be higher than daytime amplitudes in nonobese girls of all Tanner stages (Fig. 1), but were reduced and with dampened nocturnal increases in obese girls. Overnight increases of LH amplitude were statistically significant in nonobese Tanner 3–5 girls (P < 0.0001, block 1 vs. blocks 2 and 3), but changes did not reach statistical significance in nonobese Tanner 1–2 girls (block 1 vs. 2, P = 0.0781; block 1 vs. 3, P = 0.0625) (Fig. 2). There was no significant overnight increase of LH amplitude in obese Tanner 1–2 girls or obese Tanner 3–5 girls. Mean LH amplitude over 12 h was 75% lower in Tanner 1–2 obese girls compared with normal (P = 0.0435) (Table 3). Similarly, LH amplitude was reduced in Tanner 3–5 obese girls at all time blocks (block 1, P = 0.0165; block 2, P = 0.0046; block 3, P = 0.0005) (Fig. 2).
Mean LH
Mean LH appeared to be lower in obese peripubertal girls, with the exception of the most mature (Tanner 5) (Fig. 1). Mean LH increased overnight in nonobese Tanner 1–2 girls (P = 0.0156 and 0.0078, block 1 vs. 2 and 3, respectively) and nonobese Tanner 3–5 girls (P = 0.0189 and 0.0098, block 1 vs. 2 and 3, respectively) (Fig. 2). In obese Tanner 1–2 girls, mean LH did not change overnight and was reduced in block 3 (P = 0.0334) compared with nonobese girls. Mean LH fell from block 1 to 2 in the obese Tanner 3–5 group (P = 0.0210). There were no differences in mean LH (obese vs. nonobese) at any time period in the Tanner 3–5 group.
Mean FSH
Mean FSH increased from block 1 to 3 in nonobese Tanner 1–2 girls (P = 0.0078) (Fig. 2). In nonobese Tanner 3–5 girls, mean FSH increased from blocks 1 to 3 (P = 0.0374). No overnight changes were observed in obese Tanner 1–2, but FSH decreased from blocks 1 to 2 in obese Tanner 3–5 girls (P = 0.0117). Although FSH appeared to be lower in obese compared with nonobese Tanner 1–2 girls, these differences were not statistically significant.
Sex steroids
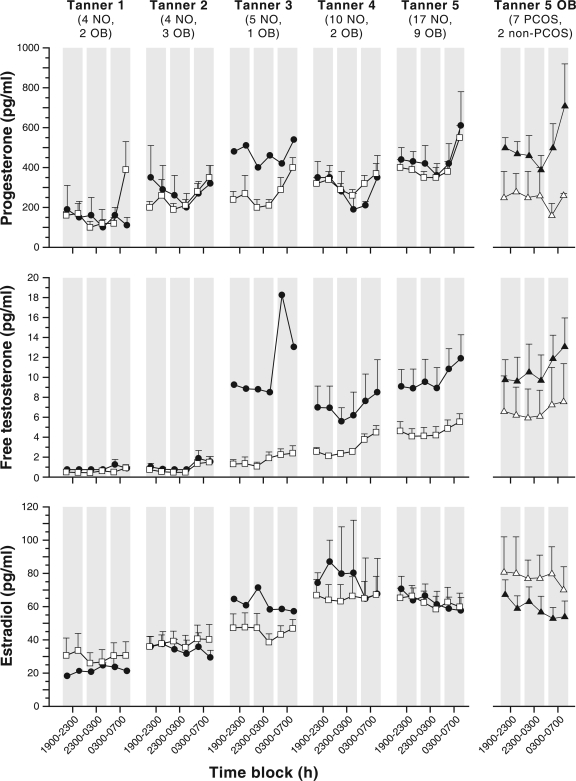
Mean overnight free T was 39% higher in obese than in nonobese Tanner 1–2 girls, but this difference was not statistically significant (Tables 2 and 3). In Tanner 3–5 girls, mean overnight free T was 2.5-fold elevated in obese girls. Mean overnight P and E2 were similar between the obese and nonobese Tanner 1–2 girls and the obese and nonobese Tanner 3–5 girls. Mean E2 concentration was greater than 20 pg/ml in all but one Tanner 1 subject.
Free T increased from block 1 to 3 in nonobese Tanner 1–2 girls (P = 0.0313) and nonobese Tanner 3–5 girls (P < 0.0001); but free T did not clearly change overnight in either group of obese girls (P = 0.0640 block 1 vs. 3 in Tanner 3–5 obese girls) (Fig. 3). P increased from blocks 1 to 3 in nonobese Tanner 1–2 girls (P = 0.0391). In nonobese Tanner 3–5 girls, P decreased from blocks 1 to 2 (P = 0.0011) but increased from blocks 1 to 3 (P = 0.0025). P decreased from blocks 1 to 2 in obese Tanner 3–5 girls (P = 0.0342). E2 concentrations decreased from block 1 to 2 in nonobese Tanner 3–5 girls (P = 0.0394), and from block 1 to 3 in obese Tanner 3–5 girls (P = 0.0068).
Figure 3.
Late evening and overnight sex steroid concentrations in obese (solid circles) and nonobese (open squares) peripubertal girls stratified by Tanner breast stage. Data are presented as mean ± sem. Four-hour mean values (using data points bounded by the shaded areas) were used for statistical comparisons. For reference, data for obese Tanner 5 girls with PCOS (solid triangles) and without PCOS (open triangles) are shown separately in the last column. To convert from conventional to SI units: P × 3.18 (pmol/liter); free T × 3.467 (pmol/liter); E2 × 3.671 (pmol/liter).
Obese Tanner 5 girls with and without PCOS
As observed in Fig. 1, LH pulse frequency in obese Tanner 5 girls appeared to be similar whether or not they met criteria for PCOS, and both groups appeared to have a higher LH frequency than nonobese Tanner 5 girls. Obese non-PCOS Tanner 5 girls appeared to have lower LH pulse amplitude compared with girls with PCOS, but both groups appeared to have lower LH pulse amplitude compared with nonobese Tanner 5 girls. Mean LH appeared lower in obese non-PCOS Tanner 5 girls compared with girls with PCOS.
Discussion
In the girls studied via overnight sampling at our institution, obesity was associated with reduced LH pulse frequency during prepuberty and early puberty but increased frequency during later puberty. Obese girls also demonstrated reduced LH pulse amplitude throughout puberty. These data add to earlier findings of relatively low morning LH values in prepubertal and early pubertal obese girls (20). Low LH amplitude in obese girls may be analogous to an inverse relationship between BMI and both mean LH and LH amplitude in PCOS (30,31,32), which in part reflects reduced gonadotrope responsiveness to GnRH (30,33) and increased LH clearance (34).
Our data also suggest that day-night changes of gonadotropin secretion during puberty are dampened in obese girls. For example, whereas nonobese Tanner 1–2 girls demonstrated significant overnight increases of LH frequency and mean LH, no substantial changes were observed in obese Tanner 1–2 girls. Similarly, whereas LH amplitude increased 1.9-fold overnight in nonobese Tanner 3–5 girls, LH amplitude did not clearly increase in obese Tanner 3–5 girls, rendering LH amplitude in blocks 2 and 3 that were less than 50% of those in nonobese Tanner 3–5 girls. Mean FSH also increased overnight in nonobese Tanner 1–2 girls, but FSH tended to be lower in obese girls with no clear overnight increase.
In nonobese girls, nighttime LH frequency remained essentially constant throughout puberty, whereas evening (a surrogate for daytime) frequency increased markedly across puberty. Thus, by later puberty, LH frequency fell overnight, as observed previously (4). In contrast to this developmental sequence, our data suggest that LH pulses change abruptly in obese girls from a relatively low frequency to a relatively high frequency during the day and night. Although LH frequency also appeared to fall overnight in obese Tanner 3–5 girls, this changed did not reach statistical significance, and LH frequency remained elevated during all time blocks.
We recognize that the inclusion of “overweight” adolescents (i.e. BMI-for-age percentile ≥85 but <95) in the nonobese group could potentially mask differences related to obesity because overweight adolescents are often considered to be at intermediate risk for obesity-associated complications in general. Among the 40 nonobese girls studied, 11 were overweight (one Tanner 2, one Tanner 3, one Tanner 4, and eight Tanner 5). For the Tanner 5 subjects, we evaluated these three groups (i.e. normal weight, overweight, and obese groups) separately. LH pulse count and LH amplitude appeared to be intermediate between normal weight and obese groups, whereas there were no clear differences of mean LH or free T (data not shown).
The mechanisms underlying the evolution of sleep-wake differences of GnRH frequency—and the potential role of sex steroids in directing these changes—are unknown. However, given that low GnRH frequencies favor FSH synthesis, diurnal slowing of pubertal GnRH frequency during puberty may be important for maintenance of FSH secretion, and hence follicular development. We have postulated that the reduction of LH pulse frequency in the morning may partly reflect negative feedback by rising P concentrations (7,20). Indeed, in the Tanner 1–2 nonobese girls studied here, LH frequency appears to fall at the same time that P increases (i.e. during the 0300–0700 h block), consistent with a negative feedback relationship. This could represent the emergence of a dominant role for sex steroid feedback in GnRH frequency regulation. In further support of this notion, girls with gonadal dysgenesis exhibited similarly high LH frequency during the evening and overnight, in contrast to low evening and high overnight LH frequency in age-matched, early pubertal controls (5).
Some of the observed differences in LH secretion may be a result of obesity per se. However, obesity does not impact LH frequency in women with or without PCOS (30,31,35); and although one study suggested an inverse relationship between BMI and LH pulse amplitude in normal women (31), other studies found no such relationship in women without PCOS (30,35). Abnormal sleep patterns in obesity could partly explain abnormal LH frequency in peripubertal obesity. Sleep was not rigorously evaluated in this study, and future studies involving formal sleep analysis will be important to examine this possibility further.
If sex steroids play a role in directing diurnal changes of LH frequency, and if peripubertal HA reduces hypothalamic sensitivity to feedback inhibition (16,36), then the presence of HA may render overnight sex steroid increases insufficient to slow GnRH pulses during the subsequent day. We suggest that an abnormal androgen milieu could in part explain the changes of LH frequency observed in the current study. Specifically, we speculate that GnRH frequency is elevated in obesity due to T-induced resistance to P negative feedback—at least once the increased GnRH activity of puberty begins. In obese girls, physical exam evidence of early puberty (i.e. breast development) may be partly related to increased estrogen production in adipose tissue (37). Thus, the lower GnRH frequencies observed in Tanner 1–2 obese girls may be related to relative immaturity of permissive factors important for the pubertal increase of hypothalamic-pituitary function [e.g. increased KiSS-GPR54 activity (38)]. However, as GnRH secretion progresses in mid to late puberty, HA-induced resistance to negative feedback may result in GnRH pulses changing abruptly from a low to a high 24-h frequency. As such, obese girls with HA may pass rapidly through the developmental stage marked by low daytime and high nighttime GnRH frequency—diurnal differences that may be critical for appropriate FSH/LH secretion, follicular development, etc.
Alternatively, it remains possible that significant HA during prepuberty and early puberty is indeed associated with elevated LH frequency. In contrast to our earlier data suggesting that free T is more than 2-fold elevated in obese Tanner 1–2 girls (20), mean free T in the current group of obese Tanner 1–2 girls was not markedly elevated. However, not all obese girls have HA (20), and the chance recruitment of obese girls without HA may explain why free T levels were not higher in this group of prepubertal and early pubertal obese girls. Mean overnight free T was unequivocally higher in obese, later pubertal girls, as previously described (19,20,21).
It is possible that, compared with obese girls without evidence of androgen excess, obese girls with hyperandrogenism were more likely to pursue participation in our studies. However, no subjects were included in or excluded from this study on the basis of androgen levels, presence or absence of hirsutism, etc.; and we have previously argued that systematic exclusion of such girls may introduce bias (21). Thus, whereas these data cannot be used to estimate the prevalence of HA or LH abnormalities in obese peripubertal girls, they may offer insight into the emergence of LH abnormalities in obese subjects.
There were no differences in 12-h P and E2 concentrations between obese and nonobese girls. P and free T showed significant overnight changes in nonobese girls. It remains unclear to what extent morning increases of P and T secretion during normal puberty reflect LH-stimulated ovarian secretion, ACTH-stimulated adrenal secretion, or both. Although our data suggested similar P and free T increases in obese girls, they were not demonstrable statistically. We did not observe early morning increases of E2 in any group, but this has been described previously (39,40). Mean fasting insulin was generally higher in obese groups as previously described (20), but this difference reached statistical significance in the Tanner 3–5 girls only. Although hyperinsulinemia may be a key driver of androgen production in obese adolescent girls, the precise pathophysiological factors involved with obesity-associated HA remain unclear.
In summary, LH pulse frequency increases overnight in prepubertal and early pubertal girls; but in the presence of obesity, LH frequency remains low with no overnight increase. In contrast, by Tanner stages 3–5, LH frequency is abnormally elevated in obese girls, possibly reflecting the effects of HA. Obesity is associated with reduced LH amplitude throughout pubertal development, analogous to findings in adult PCOS.
Acknowledgments
We gratefully acknowledge Lauren Lockhart, M.P.H.; Quirine Lamberts Okonkwo, M.D.; and Chandan Chopra for subject recruitment, study scheduling, and assistance with data management; Susan B. Cluett, CRNP, for support with subject recruitment; Christine A. Eagleson, M.D. for assistance with subject screening and General Clinical Research Center (GCRC) admission oversight; the nurses and staff of the GCRC at the University of Virginia for implementation of these sampling protocols; and the Center for Research in Reproduction Ligand Core Laboratory for performance of all assays.
Footnotes
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD28934 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and by General Clinical Research Center Grant M01 RR00847, K23 HD044742 (to C.R.M.), F32 HD-DK42895 (to K.A.P.), F32 HD055014 (to S.K.B.), and T32 HD07382 (to K.D.H. and S.C.).
Disclosure Statement: None of the authors has any potential conflicts of interest to declare.
First Published Online October 28, 2008
Abbreviations: BMI, Body mass index; CV, coefficient of variation; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; HA, hyperandrogenemia; P, progesterone; PCOS, polycystic ovary syndrome; T, testosterone.
References
- Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L 1972 Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med 287:582–586 [DOI] [PubMed] [Google Scholar]
- Oerter KE, Uriarte MM, Rose SR, Barnes KM, Cutler Jr GB 1990 Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab 71:1251–1258 [DOI] [PubMed] [Google Scholar]
- Dunkel L, Alfthan H, Stenman UH, Selstam G, Rosberg S, Albertsson-Wikland K 1992 Developmental changes in 24-hour profiles of luteinizing hormone and follicle-stimulating hormone from prepuberty to midstages of puberty in boys. J Clin Endocrinol Metab 74:890–897 [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow TL, Laughlin GA, Yen SS 1993 Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab 76:940–949 [DOI] [PubMed] [Google Scholar]
- Cemeroglu AP, Foster CM, Warner R, Kletter GB, Marshall JC, Kelch RP 1996 Comparison of the neuroendocrine control of pubertal maturation in girls and boys with spontaneous puberty and in hypogonadal girls. J Clin Endocrinol Metab 81:4352–4357 [DOI] [PubMed] [Google Scholar]
- Marshall JC 2006 Regulation of gonadotropin synthesis and secretion. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 5th ed. Philadelphia: Elsevier Saunders; 2687–2698 [Google Scholar]
- Blank SK, McCartney CR, Marshall JC 2006 The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 12:351–361 [DOI] [PubMed] [Google Scholar]
- Berga SL, Guzick DS, Winters SJ 1993 Increased luteinizing hormone and α-subunit secretion in women with hyperandrogenic anovulation. J Clin Endocrinol Metab 77:895–901 [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow T, Laughlin GA, Yen SS 1994 Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab 79:119–125 [DOI] [PubMed] [Google Scholar]
- Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ 2006 Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril 85:1049–1056 [DOI] [PubMed] [Google Scholar]
- Garcia-Rudaz MC, Ropelato MG, Escobar ME, Veldhuis JD, Barontini M 1998 Augmented frequency and mass of LH discharged per burst are accompanied by marked disorderliness of LH secretion in adolescents with polycystic ovary syndrome. Eur J Endocrinol 139:621–630 [DOI] [PubMed] [Google Scholar]
- Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley Jr WF 1988 Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab 66:165–172 [DOI] [PubMed] [Google Scholar]
- Daniels TL, Berga SL 1997 Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab 82:4179–4183 [DOI] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC 1998 Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 83:582–590 [DOI] [PubMed] [Google Scholar]
- Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC 2005 Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab 90:2810–2815 [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC 2000 Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 85:4047–4052 [DOI] [PubMed] [Google Scholar]
- Franks S 2002 Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab 16:263–272 [DOI] [PubMed] [Google Scholar]
- Witchel SF 2006 Puberty and polycystic ovary syndrome. Mol Cell Endocrinol 254–255:146–153 [DOI] [PubMed] [Google Scholar]
- Reinehr T, de Sousa G, Roth CL, Andler W 2005 Androgens before and after weight loss in obese children. J Clin Endocrinol Metab 90:5588–5595 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC 2007 Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 92:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC 2006 The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 91:1714–1722 [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL 2000 CDC growth charts: United States. Adv Data 1–27 [PubMed] [Google Scholar]
- 2006 Active healthy living: prevention of childhood obesity through increased physical activity. Pediatrics 117:1834–1842 [DOI] [PubMed] [Google Scholar]
- Barlow SE 2007 Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120(Suppl 4):S164–S192 [DOI] [PubMed] [Google Scholar]
- Zawadski JK, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, eds. Polycystic ovary syndrome. Boston: Blackwell Scientific; 377–384 [Google Scholar]
- Marshall WA, Tanner JM 1969 Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 250:E486–E493 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Gingrich MB, Hu Y, Evans WS, Marshall JC 2002 Hypothalamic regulation of cyclic ovulation: evidence that the increase in gonadotropin-releasing hormone pulse frequency during the follicular phase reflects the gradual loss of the restraining effects of progesterone. J Clin Endocrinol Metab 87:2194–2200 [DOI] [PubMed] [Google Scholar]
- Arroyo A, Laughlin GA, Morales AJ, Yen SS 1997 Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab 82:3728–3733 [DOI] [PubMed] [Google Scholar]
- Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, Hall JE 1997 Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:2248–2256 [DOI] [PubMed] [Google Scholar]
- Silfen ME, Denburg MR, Manibo AM, Lobo RA, Jaffe R, Ferin M, Levine LS, Oberfield SE 2003 Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab 88:4682–4688 [DOI] [PubMed] [Google Scholar]
- Pagan YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE 2006 Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab 91:1309–1316 [DOI] [PubMed] [Google Scholar]
- Srouji SS, Pagan YL, D'Amato F, Dabela A, Jimenez Y, Supko JG, Hall JE 2007 Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab 92:1347–1352 [DOI] [PubMed] [Google Scholar]
- Morales AJ, Laughlin GA, Butzow T, Maheshwari H, Baumann G, Yen SS 1996 Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab 81:2854–2864 [DOI] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM 2006 Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 147:1474–1479 [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS 2004 Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Kaiser UB 2005 New gatekeepers of reproduction: GPR54 and its cognate ligand, KiSS-1. Endocrinology 146:1686–1688 [DOI] [PubMed] [Google Scholar]
- Ankarberg C, Norjavaara E 1999 Diurnal rhythm of testosterone secretion before and throughout puberty in healthy girls: correlation with 17β-estradiol and dehydroepiandrosterone sulfate. J Clin Endocrinol Metab 84:975–984 [DOI] [PubMed] [Google Scholar]
- Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A 2000 Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. J Clin Endocrinol Metab 85:1074–1080 [DOI] [PubMed] [Google Scholar]