Abstract
Context: Vitamin D receptors are found in most tissues, not just those participating in the classic actions of vitamin D such as bone, gut, and kidney. These nonclassic tissues are therefore potential targets for the active metabolite of vitamin D, 1,25(OH)2D. Furthermore, many of these tissues also contain the enzyme CYP27B1 capable of producing 1,25(OH)2D from the circulating form of vitamin D. This review was intended to highlight the actions of 1,25(OH)2D in several of these tissues but starts with a review of vitamin D production, metabolism, and molecular mechanism.
Evidence Acquisition: Medline was searched for articles describing actions of 1,25(OH)2D on parathyroid hormone and insulin secretion, immune responses, keratinocytes, and cancer.
Evidence Synthesis: Vitamin D production in the skin provides an efficient source of vitamin D. Subsequent metabolism to 1,25(OH)2D within nonrenal tissues differs from that in the kidney. Although vitamin D receptor mediates the actions of 1,25(OH)2D, regulation of transcriptional activity is cell specific. 1,25(OH)2D inhibits PTH secretion but promotes insulin secretion, inhibits adaptive immunity but promotes innate immunity, and inhibits cell proliferation but stimulates their differentiation.
Conclusions: The nonclassic actions of vitamin D are cell specific and provide a number of potential new clinical applications for 1,25(OH)2D3 and its analogs. However, the use of vitamin D metabolites and analogs for these applications remains limited by the classic actions of vitamin D leading to hypercalcemia and hypercalcuria.
Vitamin D, via its active metabolite 1,25(OH)2 D, regulates numerous biologic functions in addition to bone mineral homeostasis, including hormone secretion, immune response, and cellular proliferation and differentiation.
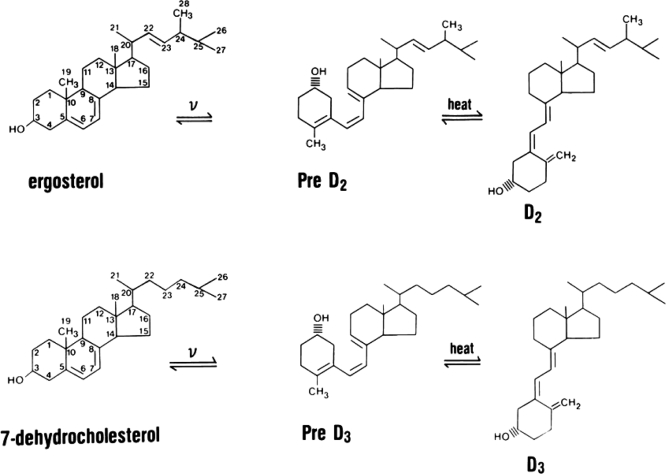
In the past few years, there has been growing appreciation for the many roles of vitamin D and its active metabolites in a large number of tissues. This has been stimulated by the appreciation that most tissues in the body have receptors for the active form of vitamin D, 1,25 dihydroxyvitamin D [1,25(OH)2D] or calcitriol. These receptors are named appropriately vitamin D receptors (VDRs), and tissues with VDR are potential target tissues. Furthermore, many of these tissues also contain the enzyme, CYP27B1, responsible for converting the major circulating metabolite of vitamin D, 25 hydroxyvitamin D (25OHD), to 1,25(OH)2D. Regulation of CYP27B1 in these nonrenal tissues generally differs from that in the kidney and may be more substrate dependent. This has led to the concept that maintenance of adequate 25OHD levels in the blood is required for vitamin D regulation of a large number of physiologic functions beyond that of the classic actions involved with bone mineral metabolism. This review is intended first to cover the basics of vitamin D production, metabolism, and molecular mechanism of action and then examine the impact of vitamin D and its metabolites on tissues that are not principally concerned with regulation of bone mineral metabolism. Two forms of vitamin D exist: vitamin D3 or cholecalciferol and vitamin D2 or ergocalciferol. The former is produced in the skin under the influence of UVB radiation (UVR); the latter is produced by UVR in a variety of plant materials and yeast (Fig. 1). Differences exist in their binding to the major transport protein in blood, vitamin D binding protein, and in their metabolism because of the differences in the chemistry of their side chains, with the result that single doses of D2 lead to lower levels of circulating 25OHD than single doses of D3 (1,2), although daily administration of D2 and D3 maintains comparable levels of 25OHD (3). At the tissue level, these differences are minor in that the biologic activity of 1,25(OH)2D2 and 1,25(OH)2D3 appear to be comparable at least with respect to binding to VDR. Therefore, references to vitamin D or D metabolites will refer to both forms unless otherwise indicated with a specific subscript.
Figure 1.
Production of vitamin D2 and vitamin D3. Ergosterol in plants and 7-dehydrocholesterol in skin are the precursors for vitamin D2 and vitamin D3, respectively. UV light B breaks the B chain of each molecule to form the pre-D isomer, which then undergoes isomerization to D. D2 and D3 differ only in the side chain in which D2 has a double bond between C22–C23 and a methyl group at C24. These differences alter somewhat its binding to DBP and metabolism.
Vitamin D3 production
Vitamin D3 (D3) is produced in the skin from 7-dehydrocholesterol through a two-step process in which the B ring is broken under UVR (e.g. sunlight), and the pre-D3 so formed isomerizes to D3 in a thermo-sensitive but noncatalytic process. Holick et al. (4,5,6) demonstrated that the formation of pre-D3 is relatively rapid, reaching a maximum within hours. Both intensity of UVR and level of pigmentation in the skin regulate the rate of pre-D3 formation but not the maximal level achieved. With continued UVR exposure, pre-D3 is converted to the biologically inactive lumisterol. Tachysterol is also formed but, like pre-D3, does not accumulate with extended UVR. The formation of lumisterol and tachysterol is reversible and can be converted back to pre-D3 as pre-D3 levels fall. Thus, prolonged exposure to sunlight will not produce toxic amounts of D3 because of the photoconversion of pre-D3 to lumisterol and tachysterol as well as the photoconversion of D3 itself to suprasterols I and II and 5,6 transvitamin D3 (4). Melanin in the epidermis, by absorbing UVR, reduces D3 production. The intensity of UVR from sunlight varies according to season and latitude, so the farther one lives from the equator, the less time of the year one can rely on solar exposure to produce D3. Clothing (7) and sunscreen (8) effectively prevent D3 production in the covered areas.
Vitamin D metabolism
To be biologically active, vitamin D must first be converted to 25OHD. There are a number of cytochrome P450 enzymes, both mitochondrial and microsomal, capable of this function (9), although CYP27A1 has received the most study. These enzymes are principally but not exclusively found in the liver and have a high capacity for substrate vitamin D. The different enzymes have different substrate specificities for the two forms of vitamin D, but this has not proven to be physiologically significant. 25OHD production is primarily substrate dependent, so serum 25OHD is a reliable indicator of vitamin D status (10).
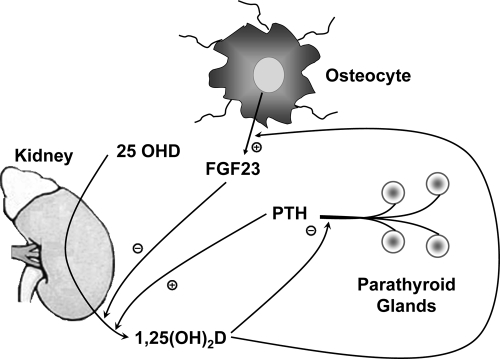
To be fully active, 25OHD must be further converted to 1,25(OH)2D via CYP27B1, a mitochondrial P450 enzyme. Although the proximal renal tubule is the major source of 1,25(OH)2D production for the body, the enzyme is also found in a number of extrarenal sites such as immune cells, epithelia of many tissues, bone, and parathyroid glands (11), in which it functions to provide 1,25(OH)2D for local consumption as an intracrine or paracrine factor. Regulation of CYP27B1 in the proximal renal tubule is controlled by PTH and fibroblast growth factor (FGF)-23, which stimulate and inhibit, respectively, its expression (Fig. 2). CYP27B1 expression is also inhibited by 1,25(OH)2D through a negative vitamin D response element in its promoter to which VDR binds indirectly (12). Additionally 1,25(OH)2D3 negatively regulates its own levels by inducing CYP24, like CYP27B1, a mitochondrial P450, that catabolizes both 1,25(OH)2D and 25OHD (13).
Figure 2.
Regulation of 1,25(OH)2D production in the kidney. PTH stimulates and FGF23 inhibits 1,25(OH)2D production in the kidney. In turn 1,25(OH)2D inhibits PTH production and secretion from the parathyroid glands and stimulates FGF23 production from bone.
Control of 1,25(OH)2D3 production (and levels) by nonrenal tissues differs. When macrophages are activated via specific toll-like receptors (TLRs), CYP27B1 is induced (14). In these cells 1,25(OH)2D3 production appears to be governed primarily by the availability of substrate (14). PTH and FGF23 do not regulate CYP27B1 in these cells due presumably to lack of their cognate receptors. Furthermore, macrophages may express a nonfunctional alternatively spliced form of CYP24 located in the cytoplasm that potentially interferes with substrate access to the mitochondrial CYP24 (15), thus reducing 25OHD and 1,25(OH)2D catabolism in these cells. The keratinocyte also contains CYP27B1, which like the macrophage enzyme can be induced by activation of specific TLRs (16). Both TNF-α and interferon (IFN)-γ stimulate 1,25(OH)2D3 production by keratinocytes (17,18), suggesting that the keratinocyte like the macrophage uses 1,25(OH)2D for important host defense mechanisms. Unlike the macrophage, the keratinocyte has a fully functional CYP24, and its induction by 1,25(OH)2D is the major means by which 1,25(OH)2D limits its own levels in the epidermis (19).
Mechanism of action
The mechanism of action of the active form of 1,25(OH)2D is similar to that of other steroid hormones and is mediated by its binding to VDR. VDR is a member of the superfamily of nuclear hormone receptors including receptors for steroid and thyroid hormones and retinoic acid. VDR functions as a heterodimer generally with the retinoid X receptor for regulation of vitamin D target genes. These heterodimeric complexes interact with specific DNA sequences [vitamin D response elements (VDREs)], generally within the promoter of target genes, resulting in either activation or repression of transcription (20,21,22,23). The control of transcription requires the additional recruitment of coregulators (24). These VDREs can be many thousand nucleotides away from the transcription start site, however (25). For activation, two major coactivator complexes have been identified: the steroid receptor activator complex (SRC) comprised of the p160 family of SRC1, SRC2, and SRC3 coactivators (26) and the vitamin D receptor interacting protein complex (DRIP) or mediator complex (22). These coactivator complexes interact with the C-terminal (activation function-2 or AF-2) domain of VDR after ligand binding and recruit cAMP response element-binding (CREB) protein-binding protein and other histone acetyl transferases (HATs) and methyltranferases to the VDR, resulting in a multisubunit complex (20,21,22,23). The HAT and methyltranferase activity of the SRC complex is thought to destabilize the interaction between DNA and the histone core, enabling transcription to occur. The DRIP complex does not have HAT activity but functions, at least in part, through recruitment of RNA polymerase II to the transcription start site. These complexes do not bind to the VDR at the same time (27). It is not clear whether these different complexes shuttle in and out of the transcription machinery, act sequentially, or act on different genes. In skin we have found that DRIP is more abundant in the proliferating keratinocyte, whereas SRC3 is more abundant in differentiated keratinocytes (28). Furthermore, different genes regulated by VDR in these cells require different coactivators (29,30), indicating that at least in the keratinocyte, these coactivator complexes serve different functions and different genes.
Corepressors block VDR-mediated transcriptional activity. Well-studied corepressors include nuclear corepressor (NCoR) and silencing mediator of retinoic acid and thyroid receptor (SMRT). These corepressors typically bind VDR in the absence of 1,25(OH)2D and are displaced when 1,25(OH)2D binding recruits the coactivators to the VDR. One recently discovered corepressor with more limited tissue distribution and function is hairless (Hr). Hr is found primarily in brain, epidermis, hair follicles, and other epithelia, although trace levels of expression have been found elsewhere (31). Lack of Hr like lack of VDR results in failure of hair follicle cycling (32). Hr binds to VDR and like other corepressors inhibits its transcriptional activity in a manner relieved by 1,25(OH)2D (33). Other transcription factors also modulate the activity of VDR. β-Catenin binds to VDR and regulates its ability to induce a number of genes (and vice versa) (34). Similarly, YY1 and CCAAT enhancer binding proteins-β and -δ modulate VDR-mediated transcription (35,36,37). These coregulators differ in their tissue distribution, providing for substantial tissue specificity in the actions of 1,25(OH)2D and VDR.
Nonclassic target tissues
The nonclassic actions of vitamin D can be categorized into three general effects: regulation of hormone secretion, regulation of immune function, and regulation of cellular proliferation and differentiation. These categories are somewhat artificial, and the effects of 1,25(OH)2D on any given tissue may involve actions in more than one of these categories. Nevertheless, the categories serve a pedagogic purpose.
Regulation of hormone secretion
The ability of 1,25(OH)2D to regulate hormone secretion plays an important role in maintaining normal bone mineral homeostasis (Fig. 2) and in the case of insulin secretion illustrates an important nonclassical action of therapeutic importance.
PTH
1,25(OH)2D inhibits the synthesis and secretion of PTH (38) and prevents the proliferation of the parathyroid gland (38,39). The parathyroid gene contains a negative VDRE through which 1,25(OH)2D exerts its suppression (38). 1,25(OH)2D also up-regulates the calcium-sensing receptor (40), which by sensitizing the parathyroid gland to calcium inhibition provides an additional means by which 1,25(OH)2D regulates PTH production and secretion. Because PTH stimulates 1,25(OH)2D production in the kidney, this inhibition of PTH production and secretion provides an important feedback loop. These actions of 1,25(OH)2D are exploited clinically through the use of 1,25(OH)2D and analogs to control secondary hyperparathyroidism in renal failure. Furthermore, the ability of the parathyroid gland to make its own 1,25(OH)2D provides an explanation for the reciprocal relationship between 25OHD and PTH levels, but not between 1,25(OH)2D and PTH levels, in the blood of subjects with vitamin D insufficiency (41).
Insulin
1,25(OH)2D stimulates insulin secretion, although the mechanism is not well defined (42,43). VDR and calbindin-D28k are found in pancreatic β-cells (44,45), and studies using calbindin-D28k null mice have suggested that calbindin-D28k, by regulating intracellular calcium, can modulate depolarization-stimulated insulin release (46). Furthermore, calbindin-D28k, by buffering calcium, can protect against cytokine mediated destruction of β-cells (47). A number of mostly case control and observational studies have suggested that vitamin D deficiency contributes to increased risk for type 2 diabetes mellitus (48).
FGF23
FGF23 is produced primarily by bone, and in particular by osteoblasts and osteocytes. 1,25(OH)2D3 stimulates this process, but the mechanism is not clear (49). Inasmuch as FGF23 inhibits 1,25(OH)2D production by the kidney, this feedback loop like that for PTH secretion maintains a balance in the levels of these important hormones. Mutations in the phosphate-regulating gene with holologies to endopeptidases on the X chromosome (PHEX) or FGF23 itself (which prevent its proteolysis) or conditions such as McCune-Albright disease and tumor-induced osteomalacia in which FGF23 is overexpressed in the involved tissue lead to hypophosphatemia and inappropriately low 1,25(OH)2D accompanied by osteomalacia. The role of (PHEX), which was originally thought to cleave FGF23, in regulating FGF23 levels is no longer clear. In contrast mutations in UDP-N-acetyl-α-D galactosamine-polypeptide N-acetylgalactosaminyl transferase (GALNT3), which glycosylates FGF23, or in FGF23, which blocks this glycosylation result in inhibited FGF23 secretion leading to hyperphosphatemia, increased 1,25(OH)2D and tumoral calcinosis (50).
Regulation of immune function
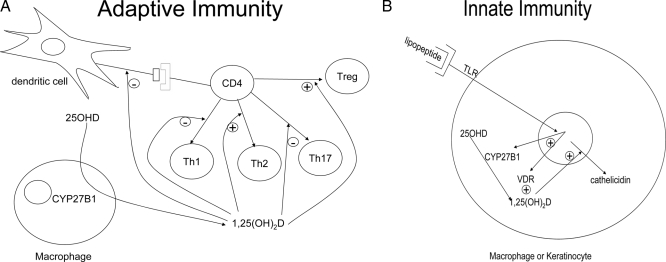
The potential role for vitamin D and its active metabolite 1,25(OH)2D in modulating the immune response was first appreciated 25 yr ago with three important discoveries: 1) the presence of VDRs in activated human inflammatory cells (51), 2) the ability of 1,25(OH)2D to inhibit T cell proliferation (52), and 3) the ability of disease activated macrophages to produce 1,25(OH)2D (i.e. express CYP27B1) (53). Vitamin D and CYP27B1 play important roles in both innate and adaptive immunity, which impact a number of clinical conditions. For example, vitamin D deficiency is a well-known accompaniment of various infectious diseases such as tuberculosis (54), and 1,25(OH)2D3 has long been recognized to potentiate the killing of mycobacteria by monocytes (55). The mechanism underlying these observations has recently been determined by the observation that the monocyte, when activated by mycobacterial lipopeptides, expresses CYP27B1, producing 1,25(OH)2D from circulating 25OHD and in turn inducing cathelicidin, an antimicrobial peptide that enhances killing of the mycobacterium. Inadequate 25OHD levels fail to support this process (14). As a second example, it has been observed that vitamin D deficiency and/or living at higher latitudes (with less sunlight) are associated with a number of autoimmune diseases including type 1 diabetes mellitus, multiple sclerosis, and Crohn’s disease (56). In a large Finnish study, providing infants with 2000 IU vitamin D for their first year of life reduced the incidence of type 1 diabetes mellitus by 80% (57). Other studies have linked vitamin D deficiency to increased risk of multiple sclerosis (58), asthma (59), and other immunologic diseases. A discussion of the mechanisms by which 1,25(OH)2D regulates adaptive and innate immunity follows (Fig. 3).
Figure 3.
Regulation of immune function by 1,25(OH)2D. 1,25(OH)2D suppresses adaptive immunity (A) by inhibiting the maturation of dendritic cells, reducing their capacity to present antigen to CD4 cells. 1,25(OH)2D further inhibits the proliferation and differentiation of CD4 cells into Th1 and Th17 cells and promotes the production of Th2 and Treg cells. On the other hand 1,25(OH)2D promotes innate immunity (B) in that when the macrophage is activated by TLRs, VDR and CYP27B1 are induced enabling the macrophage to produce 1,25(OH)2D, which then induces cathelicidin, a potent antimicrobial peptide.
Adaptive immunity
The adaptive immune response involves the ability of T and B lymphocytes to produce cytokines and immunoglobulins, respectively, to specifically combat the source of the antigen presented to them by cells such as macrophages and dendritic cells. Vitamin D exerts an inhibitory action on the adaptive immune system. In particular, 1,25(OH)2D suppresses proliferation and immunoglobulin production and retards the differentiation of B cell precursors into plasma cells (60). In addition 1,25(OH)2D inhibits T cell proliferation (52), in particular the T helper (Th)-1 cells capable of producing IFN-γ and IL-2 and activating macrophages (61). These actions prevent further antigen presentation to and recruitment of T lymphocytes (role of IFN-γ), and T lymphocyte proliferation (role of IL-2). In contrast IL-4, IL-5, and IL10 production can be increased (62), shifting the balance to a Th2 cell phenotype. CD4+/CD25+ regulatory T cells (Treg) are also increased by 1,25(OH)2D3 (63) as shown by increased FoxP3 expression and IL-10 production (64). The IL-10 so produced is one means by which Treg block Th1 development. At least in part, these actions on T cell proliferation and differentiation stem from actions of 1,25(OH)2D on dendritic cells to reduce their antigen presenting capability. The impact of 1,25(OH)2D3 on Th17 development and function is more recently discovered, and many of the effects of 1,25(OH)2D on various autoimmune diseases previously ascribed to inhibition of Th1 development and function are now being ascribed at least in part to inhibition of Th17 development and function (64). The ability of 1,25(OH)2D to suppress the adaptive immune system appears to be beneficial for a number of conditions in which the immune system is directed at self, i.e. autoimmunity. In a number of experimental models (65,66) including inflammatory arthritis, autoimmune diabetes, experimental allergic encephalitis (a model for multiple sclerosis), and inflammatory bowel disease, 1,25(OH)2D3 administration has prevented and/or treated the disease process. As indicated previously, studies in humans also show promise. However, suppression of the adaptive immune system may come at a price if such suppression leads to decreased response to infectious agents or decreased immune surveillance.
Innate immunity
Innate immune responses involve the activation of toll like receptors (TLRs) in polymorphonuclear cells, monocytes, and macrophages as well as in a number of epithelial cells including those of the epidermis, gingiva, intestine, vagina, bladder, and lungs. TLRs are transmembrane pathogen recognition receptors that interact with specific membrane patterns shed by infectious agents that trigger the innate immune response in the host (67). Activation of TLRs leads to the induction of antimicrobial peptides and reactive oxygen species, which kill the organism. Among those antimicrobial peptides is cathelicidin. The expression of this antimicrobial peptide is induced by 1,25(OH)2D in both myeloid and epithelial cells (68,69). As noted previously, both macrophages (53) and epithelial cells (70) are capable of responding to and producing 1,25(OH)2D (i.e. they both have VDR and CYP27B1). Stimulation of TLR2 by an antimicrobial peptide in macrophages (14) or stimulation of TLR2 in keratinocytes by wounding the epidermis (16) results in increased expression of CYP27B1, which in the presence of adequate substrate (25OHD) stimulates the expression of cathelicidin. Lack of substrate (25OHD), VDR, or CYP27B1 blunts the ability of these cells to respond to a challenge with respect to cathelicidin production (14,16,69). As mentioned, the innate immune system is widely distributed and operates not only in cells within the lymphopoietic system but also within epithelia of those tissues facing the outside environment in which it contributes to the protective barrier of those tissues. Therefore, it seems that it is no accident of nature that both VDR and CYP27B1 can be found in those tissues.
Regulation of proliferation and differentiation
Epidermis and hair follicle
The epidermis is unique in that under physiological conditions it is capable of not only making vitamin D but also converting it to 1,25(OH)2D in the same cell that is also fully capable of responding to the 1,25(OH)2D produced. As noted previously, 1,25(OH)2D enables the keratinocyte to mount the innate immune response and suppress the autoimmune mechanisms that contribute at least in part to psoriasis. However, 1,25(OH)2D also promotes the differentiation of keratinocytes and inhibits their proliferation (71,72). In the epidermis proliferation occurs in the basal layer, and as the keratinocytes move out of the basal layer, differentiation is initiated. As the keratinocyte moves from one layer of epidermis to the next, differentiation proceeds in a sequential fashion, ultimately resulting in the enucleated corneocyte enmeshed in a lipid-rich matrix that provides the barrier function. 1,25(OH)2D is involved in all steps of this process in that it limits proliferation in the basal layer and induces in a sequential pattern the expression of genes whose products ultimately produce the permeability barrier. The ability of 1,25(OH)2D to act sequentially on gene expression as the differentiation process unfolds is due to the differential distribution of coactivators (DRIP205 and SRC3) within the epidermis as a function of differentiation (28,73) and the differential use of these coactivators by genes involved in the early and late stages of differentiation (30,74).
Hair follicle cycling is the best example of a role for VDR independent of 1,25(OH)2D, as clearly illustrated by the development of alopecia in VDR-mutated animals (75) including humans (76) but not in CYP27B1 mutated animals (77) and humans (78). The mechanism by which VDR regulates hair follicle cycling remains unclear, but the alopecia phenotype of the VDR null animal is similar to that seen in animals with mutations in Hr (79,80) and β-catenin (81,82) that render these proteins transcriptionally inactive. As noted previously, Hr and β-catenin bind to VDR and are capable of regulating its transcriptional activity. What is not known in the hair follicle is the identity of the target genes. The VDR is found in the keratinocytes of the outer root sheath as well as in cells of the bulge in which the stem cells are also located, suggesting an important role for the VDR in regulating the proliferation and differentiation of these cells during the process of hair follicle cycling (83).
Psoriasis is a chronic, generalized, and scaly erythematous dermatosis thought to be due to a Th1- or Th17-mediated immune reaction to as-yet-unidentified antigens in the skin that may cause or at least is accompanied with increased proliferation and decreased differentiation of the keratinocytes in the epidermis. Analogs of 1,25(OH)2D, including calcipotriol, tacalcitol, and maxicalcitol as well as calcitriol itself have proved effective therapy for moderate forms of this disease (84). This form of therapy likely works by inhibiting the inflammatory component via a direct action on the T cells (85) as well as by reducing keratinocyte proliferation and enhancing their differentiation (86).
Cancer
1,25(OH)2D has been evaluated for its potential anticancer activity in animal and cell studies for approximately 25 yr (87). The list of malignant cells that express VDR is now quite extensive. The accepted basis for the promise of 1,25(OH)2D in the prevention and treatment of malignancy includes its antiproliferative, prodifferentiating effects on most cell types. In particular 1,25(OH)2D stimulates the expression of cell cycle inhibitors p21 and p27 (88) and the expression of the cell adhesion molecule E-cadherin (89) and inhibits the transcriptional activity of β-catenin (89,90,91). In keratinocytes, 1,25(OH)2D has been shown to promote the repair of DNA damage induced by UVR (92), reduce apoptosis and increase survival after UVR (93), and increase p53 (94). Epidemiological evidence supporting the importance of adequate vitamin D nutrition (including sunlight exposure) for the prevention of a number of cancers (95,96,97,98,99) is extensive. Although numerous types of cancers show reduction (100), most attention has been paid to cancers of the breast, colon, and prostate. A recent report from the Women’s Health Initiative failed to find a reduction in colon cancer in women receiving 400 IU vitamin D plus 1000 mg calcium (101), although this study has been criticized for using too low a dose of vitamin D, poor compliance, and failure to control for vitamin D and calcium supplementation in the placebo group. 25OHD levels were not recorded at the end of the study to show differences between the treated and nontreated groups. In contrast a prospective 4-yr trial with 1100 IU vitamin D and 1400–1500 mg calcium showed a 77% reduction in cancers after excluding the initial year of study (102), including a reduction in both breast and colon cancers. In this study, vitamin D supplementation raised the 25OHD levels from a mean of 28.8 to 38.4 ng/ml with no changes in the placebo or calcium only arms of the study. However, this was a relatively small study in which cancer prevention was not the primary outcome variable.
Trials of 1,25(OH)2D and its analogs for the treatment of cancer have been disappointing. In a small study involving seven subjects with prostate cancer treated with doses of 1,25(OH)2D up to 2.5 μg for 6–15 months, six of seven showed a decrease in the rise of prostate-specific antigen, a marker of tumor progression (103), and one patient showed a decline. However, hypercalciuria was common and limiting. A preliminary report of a larger study involving 250 patients with prostate cancer using 45 μg 1,25(OH)2D weekly in combination with docetaxel demonstrated a nonsignificant decline in prostate-specific antigen, although survival was significantly improved (hazard ratio 0.67) (104). The incidence of either hypercalcemia or hypercalciuria was not reported. A larger follow-up study has recently been reported (30th annual meeting of the American Society for Bone and Mineral Research, 2008) but not yet published, which failed to confirm the improved survival. Most likely until an analog of 1,25(OH)2D is developed that is both efficacious and truly nonhypercalcemic, treatment of cancer with vitamin D metabolites will remain problematic.
Footnotes
This work was supported by grants from the National Institutes of Health (PO1AR39448, RO1AR050023), Department of Veterans Affairs (Merit Review), and the American Institute for Cancer Research (07A140).
Disclosure Statement: D.B. has nothing to disclose.
First Published Online October 14, 2008
Abbreviations: D3, Vitamin D3 DRIP, vitamin D receptor interacting protein complex; FGF, fibroblast growth factor; HAT, histone acetyl transferase; Hr, hairless; IFN, interferon; 25OHD, 25 hydroxyvitamin D; 1,25(OH)2D, 1,25 dihydroxyvitamin D; SRC, steroid receptor activator complex; Th, T helper; TLR, toll-like receptor; Treg, regulatory T cells; UVR, UVB radiation; VDR, vitamin D receptor; VDRE, vitamin D response element.
References
- Armas LA, Hollis BW, Heaney RP 2004 Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89:5387–5391 [DOI] [PubMed] [Google Scholar]
- Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, Carnevale V, Scillitani A, Minisola S 2008 Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab 93:3015–3020 [DOI] [PubMed] [Google Scholar]
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD 2008 Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts Jr JT, Anderson RR, Blank IH, Parrish JA, Elias P 1980 Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 210:203–205 [DOI] [PubMed] [Google Scholar]
- Holick MF, MacLaughlin JA, Doppelt SH 1981 Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science 211:590–593 [DOI] [PubMed] [Google Scholar]
- Holick MF, Richtand NM, McNeill SC, Holick SA, Frommer JE, Henley JW, Potts Jr JT 1979 Isolation and identification of previtamin D3 from the skin of rats exposed to ultraviolet irradiation. Biochemistry 18:1003–1008 [DOI] [PubMed] [Google Scholar]
- Matsuoka LY, Wortsman J, Dannenberg MJ, Hollis BW, Lu Z, Holick MF 1992 Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol Metab 75:1099–1103 [DOI] [PubMed] [Google Scholar]
- Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF 1987 Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab 64:1165–1168 [DOI] [PubMed] [Google Scholar]
- Prosser DE, Jones G 2004 Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29:664–673 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS 2007 Extra-renal 25-hydroxyvitamin D3–1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 103:316–321 [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Fujiki R, Yoshimura K, Mezaki Y, Uematsu Y, Matsui D, Ogawa S, Unno K, Okubo M, Tokita A, Nakagawa T, Ito T, Ishimi Y, Nagasawa H, Matsumoto T, Yanagisawa J, Kato S 2003 The chromatin-remodeling complex WINAC targets a nuclear receptor to promoters and is impaired in Williams syndrome. Cell 113:905–917 [DOI] [PubMed] [Google Scholar]
- Zierold C, Darwish HM, DeLuca HF 1995 Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J Biol Chem 270:1675–1678 [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL 2006 Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M 2005 Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem 280:20604–20611 [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL 2007 Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Pillai S, Gee E, Hincenbergs M 1989 Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-γ. Endocrinology 124:655–660 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Pillai S, Gee E, Hincenbergs M 1991 Tumor necrosis factor-α regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology 129:33–38 [DOI] [PubMed] [Google Scholar]
- Xie Z, Munson SJ, Huang N, Portale AA, Miller WL, Bikle DD 2002 The mechanism of 1,25-dihydroxyvitamin D(3) autoregulation in keratinocytes. J Biol Chem 277:36987–36990 [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Liu Y, Peng X, Porta A 2003 New insights into the mechanisms of vitamin D action. J Cell Biochem 88:695–705 [DOI] [PubMed] [Google Scholar]
- DeLuca HF 2004 Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80:1689S–1696S [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP 2000 Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene 246:9–21 [DOI] [PubMed] [Google Scholar]
- Sutton AL, MacDonald PN 2003 Vitamin D: more than a bone-a-fide hormone. Mol Endocrinol 17:777–791 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW 2006 Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol 26:6469–6486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo C, Chen JD 2000 The SRC family of nuclear receptor coactivators. Genes 245:1–11 [DOI] [PubMed] [Google Scholar]
- Rachez C, Gamble M, Chang CP, Atkins GB, Lazar MA, Freedman LP 2000 The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol Cell Biol 20:2718–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Sihlbom C, Chalkley RJ, Huang L, Rachez C, Chang CP, Burlingame AL, Freedman LP, Bikle DD 2003 Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Mol Endocrinol 17:2329–2339 [DOI] [PubMed] [Google Scholar]
- Hawker NP, Pennypacker SD, Chang SM, Bikle DD 2007 Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol 127:874–880 [DOI] [PubMed] [Google Scholar]
- Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, Bikle DD, Gallo RL 2008 Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 128:816–824 [DOI] [PubMed] [Google Scholar]
- Cachon-Gonzalez MB, San-Jose I, Cano A, Vega JA, Garcia N, Freeman T, Schimmang T, Stoye JP 1999 The hairless gene of the mouse: relationship of phenotypic effects with expression profile and genotype. Dev Dyn 216:113–126 [DOI] [PubMed] [Google Scholar]
- Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam H, Aita VM, Owen J, deBlaquiere M, Frank J, Cserhalmi-Friedman PB, Leask A, McGrath JA, Peacocke M, Ahmad M, Ott J, Christiano AM 1998 Alopecia universalis associated with a mutation in the human hairless gene. Science 279:720–724 [DOI] [PubMed] [Google Scholar]
- Xie Z, Chang S, Oda Y, Bikle DD 2006 Hairless suppresses vitamin D receptor transactivation in human keratinocytes. Endocrinology 147:314–323 [DOI] [PubMed] [Google Scholar]
- Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM 2008 The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE 3:e1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan P, Peng X, Sutton AL, MacDonald PN, Croniger CM, Trautwein C, Centrella M, McCarthy TL, Christakos S 2005 Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol Cell Biol 25:472–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Aslam F, van Wijnen AJ, Roberts SG, Frenkel B, Green MR, DeLuca H, Lian JB, Stein GS, Stein JL 1997 YY1 regulates vitamin D receptor/retinoid X receptor mediated transactivation of the vitamin D responsive osteocalcin gene. Proc Natl Acad Sci USA 94:121–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval-Pandya M, Dhawan P, Barletta F, Christakos S 2001 YY1 represses vitamin D receptor-mediated 25-hydroxyvitamin D(3)24-hydroxylase transcription: relief of repression by CREB-binding protein. Mol Endocrinol 15:1035–1046 [DOI] [PubMed] [Google Scholar]
- Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM 1992 Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 89:8097–8101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KJ, Gonzalez EA 2004 Vitamin D analogs: actions and role in the treatment of secondary hyperparathyroidism. Semin Nephrol 24:456–459 [DOI] [PubMed] [Google Scholar]
- Canaff L, Hendy GN 2002 Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J Biol Chem 277:30337–30350 [DOI] [PubMed] [Google Scholar]
- Vieth R, Ladak Y, Walfish PG 2003 Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab 88:185–191 [DOI] [PubMed] [Google Scholar]
- Kadowaki S, Norman AW 1985 Demonstration that the vitamin D metabolite 1,25(OH)2-vitamin D3 and not 24R,25(OH)2-vitamin D3 is essential for normal insulin secretion in the perfused rat pancreas. Diabetes 34:315–320 [DOI] [PubMed] [Google Scholar]
- Lee S, Clark SA, Gill RK, Christakos S 1994 1,25-Dihydroxyvitamin D3 and pancreatic β-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology 134:1602–1610 [DOI] [PubMed] [Google Scholar]
- Clark SA, Stumpf WE, Sar M, DeLuca HF, Tanaka Y 1980 Target cells for 1,25 dihydroxyvitamin D3 in the pancreas. Cell Tissue Res 209:515–520 [DOI] [PubMed] [Google Scholar]
- Morrissey RL, Bucci TJ, Richard B, Empson N, Lufkin EG 1975 Calcium-binding protein: its cellular localization in jejunum, kidney and pancreas. Proc Soc Exp Biol Med 149:56–60 [DOI] [PubMed] [Google Scholar]
- Sooy K, Schermerhorn T, Noda M, Surana M, Rhoten WB, Meyer M, Fleischer N, Sharp GW, Christakos S 1999 Calbindin-D(28k) controls [Ca(2+)](i) and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and β cell lines. J Biol Chem 274:34343–34349 [DOI] [PubMed] [Google Scholar]
- Rabinovitch A, Suarez-Pinzon WL, Sooy K, Strynadka K, Christakos S 2001 Expression of calbindin-D(28k) in a pancreatic islet β-cell line protects against cytokine-induced apoptosis and necrosis. Endocrinology 142:3649–3655 [DOI] [PubMed] [Google Scholar]
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B 2007 The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK 2005 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289:G1036–G1042 [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamashita T 2007 FGF23 is a hormone-regulating phosphate metabolism—unique biological characteristics of FGF23. Bone 40:1190–1195 [DOI] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC 1983 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science 221:1181–1183 [DOI] [PubMed] [Google Scholar]
- Rigby WF, Stacy T, Fanger MW 1984 Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest 74:1451–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Sharma OP, Gacad MA, Singer FR 1983 Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest 72:1856–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustianowski A, Shaffer R, Collin S, Wilkinson RJ, Davidson RN 2005 Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J Infect 50:432–437 [DOI] [PubMed] [Google Scholar]
- Rook GA, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J, Stanford J 1986 Vitamin D3, γ interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 57:159–163 [PMC free article] [PubMed] [Google Scholar]
- Ponsonby AL, McMichael A, van der Mei I 2002 Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology 181–182:71–78 [DOI] [PubMed] [Google Scholar]
- Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM 2001 Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358:1500–1503 [DOI] [PubMed] [Google Scholar]
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A 2006 Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296:2832–2838 [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Weiss ST 2007 Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 120:1031–1035 [DOI] [PubMed] [Google Scholar]
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE 2007 Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647 [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC, Beck L, Spiegelberg HL 1995 Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr 125:1704S–1708S [DOI] [PubMed] [Google Scholar]
- Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A 2001 1α,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 167:4974–4980 [DOI] [PubMed] [Google Scholar]
- Penna G, Adorini L 2000 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164:2405–2411 [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM 2008 Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 324:23–33 [DOI] [PubMed] [Google Scholar]
- Adorini L 2005 Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol 233:115–124 [DOI] [PubMed] [Google Scholar]
- Deluca HF, Cantorna MT 2001 Vitamin D: its role and uses in immunology. FASEB J 15:2579–2585 [DOI] [PubMed] [Google Scholar]
- Medzhitov R 2007 Recognition of microorganisms and activation of the immune response. Nature 449:819–826 [DOI] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP 2005 Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077 [DOI] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH 2004 Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Nemanic MK, Whitney JO, Elias PW 1986 Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry 25:1545–1548 [DOI] [PubMed] [Google Scholar]
- Su MJ, Bikle DD, Mancianti ML, Pillai S 1994 1,25-Dihydroxyvitamin D3 potentiates the keratinocyte response to calcium. J Biol Chem 269:14723–14729 [PubMed] [Google Scholar]
- Bikle DD, Pillai S 1993 Vitamin D, calcium, and epidermal differentiation. Endocr Rev 14:3–19 [DOI] [PubMed] [Google Scholar]
- Oda Y, Ishikawa MH, Hawker NP, Yun QC, Bikle DD 2007 Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol 103:776–780 [DOI] [PubMed] [Google Scholar]
- Hawker NP, Pennypacker SD, Chang SM, Bikle DD 2007 Regulation of Human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol 127:874 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Kishimoto J, Demay MB 2001 Metabolic and cellular analysis of alopecia in vitamin D receptor knockout mice. J Clin Invest 107:961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Pike JW, Feldman D 1999 The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev 20:156–188 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM 2004 25 Hydroxyvitamin D 1α-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol 122:984–992 [DOI] [PubMed] [Google Scholar]
- Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA 1997 Cloning of human 25-hydroxyvitamin D-1α-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol 11:1961–1970 [DOI] [PubMed] [Google Scholar]
- Panteleyev AA, Botchkareva NV, Sundberg JP, Christiano AM, Paus R 1999 The role of the hairless (hr) gene in the regulation of hair follicle catagen transformation. Am J Pathol 155:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Djabali K, Chen T, Liu Y, Ioffreda M, Lyle S, Christiano AM, Holick M, Cotsarelis G 2001 Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol 117:612–617 [DOI] [PubMed] [Google Scholar]
- DasGupta R, Rhee H, Fuchs E 2002 A developmental conundrum: a stabilized form of beta-catenin lacking the transcriptional activation domain triggers features of hair cell fate in epidermal cells and epidermal cell fate in hair follicle cells. J Cell Biol 158:331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W 2001 β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545 [DOI] [PubMed] [Google Scholar]
- Cianferotti L, Cox M, Skorija K, Demay MB 2007 Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci USA 104:9428–9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce S, Epinette WW, Funicella T, Ison A, Jones EL, Loss R, Jr., McPhee ME, Whitmore C 1994 Comparative study of calcipotriene (MC 903) ointment and fluocinonide ointment in the treatment of psoriasis. J Am Acad Dermatol 31:755–759 [DOI] [PubMed] [Google Scholar]
- Bagot M, Charue D, Lescs MC, Pamphile RP, Revuz J 1994 Immunosuppressive effects of 1,25-dihydroxyvitamin D3 and its analogue calcipotriol on epidermal cells. Br J Dermatol 130:424–431 [DOI] [PubMed] [Google Scholar]
- Kragballe K, Wildfang IL 1990 Calcipotriol (MC 903), a novel vitamin D3 analogue, stimulates terminal differentiation and inhibits proliferation of cultured human keratinocytes. Arch Dermatol Res 282:164–167 [DOI] [PubMed] [Google Scholar]
- Eisman JA, Martin TJ, MacIntyre I, Moseley JM 1979 1,25-Dihydroxyvitamin-D-receptor in breast cancer cells. Lancet 2:1335–1336 [DOI] [PubMed] [Google Scholar]
- Ingraham BA, Bragdon B, Nohe A 2008 Molecular basis of the potential of vitamin D to prevent cancer. Current Med Res Opin 24:139–149 [DOI] [PubMed] [Google Scholar]
- Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A 2001 Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol 154:369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Hecht A, Pestell R, Byers SW 2003 Trans-repression of β-catenin activity by nuclear receptors. J Biol Chem 278:48137–48145 [DOI] [PubMed] [Google Scholar]
- Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW 2006 The molecular basis of vitamin D receptor and β-catenin crossregulation. Mol Cell 21:799–809 [DOI] [PubMed] [Google Scholar]
- Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, Posner GH, Ishizuka S, Halliday GM, Reeve VE, Mason RS 2005 Skin cancer prevention: a possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol 97:137–143 [DOI] [PubMed] [Google Scholar]
- De Haes P, Garmyn M, Degreef H, Vantieghem K, Bouillon R, Segaert S 2003 1,25-Dihydroxyvitamin D3 inhibits ultraviolet B-induced apoptosis, Jun kinase activation, and interleukin-6 production in primary human keratinocytes. J Cell Biochem 89:663–673 [DOI] [PubMed] [Google Scholar]
- Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, Reeve VE, Mason RS 2007 Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol 127:707–715 [DOI] [PubMed] [Google Scholar]
- Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O 1985 Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet 1:307–309 [DOI] [PubMed] [Google Scholar]
- Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR 1993 Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am J Epidemiol 137:1302–1317 [DOI] [PubMed] [Google Scholar]
- Kearney J, Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Wing A, Kampman E, Willett WC 1996 Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am J Epidemiol 143:907–917 [DOI] [PubMed] [Google Scholar]
- Garland FC, Garland CF, Gorham ED, Young JF 1990 Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med 19:614–622 [DOI] [PubMed] [Google Scholar]
- Hanchette CL, Schwartz GG 1992 Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer 70:2861–2869 [DOI] [PubMed] [Google Scholar]
- Boscoe FP, Schymura MJ 2006 Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer 6:264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE 2006 Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354:684–696 [DOI] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP 2007 Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85:1586–1591 [DOI] [PubMed] [Google Scholar]
- Gross C, Stamey T, Hancock S, Feldman D 1998 Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol). J Urol 159:2035–2039; discussion 2039–2040 [DOI] [PubMed] [Google Scholar]
- Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, Redfern CH, Fehrenbacher L, Saleh MN, Waterhouse DM, Carducci MA, Vicario D, Dreicer R, Higano CS, Ahmann FR, Chi KN, Henner WD, Arroyo A, Clow FW 2007 Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol 25:669–674 [DOI] [PubMed] [Google Scholar]