Abstract
Context: Postprandial hypoglycemia (PPH) is a frequent complication of Nissen fundoplication in children. The mechanism responsible for the PPH is poorly understood, but involves an exaggerated insulin response to a meal and subsequent hypoglycemia. We hypothesize that increased glucagon-like peptide-1 (GLP-1) secretion contributes to the exaggerated insulin surge and plays a role in the pathophysiology of this disorder.
Objective: The aim of the study was to characterize glucose, insulin, and GLP-1 response to an oral glucose load in children with symptoms of PPH after Nissen fundoplication.
Design: Ten patients with suspected PPH and a history of Nissen fundoplication and eight control subjects underwent a standard oral glucose tolerance test at The Children’s Hospital of Philadelphia. Blood glucose (BG), insulin, and intact GLP-1 levels were obtained at various time points.
Participants: Children ages 4 months to 13 years old were studied.
Main Outcome Measures: Change scores for glucose, insulin, and intact GLP-1 were recorded after an oral glucose tolerance test.
Results: All cases had hypoglycemia after the glucose load. Mean BG at nadir (± sd) was 46.7 ± 11 mg/dl for cases (vs. 85.9 ± 21.3 mg/dl; P < 0.0005). Mean change in BG from baseline to peak (± sd) was 179.3 ± 87.4 mg/dl for cases (vs. 57.8 ± 39.5 mg/dl; P = 0.003). Mean change in BG (± sd) from peak to nadir was 214.4 ± 85.9 mg/dl for cases (vs. 55.9 ± 41.1 mg/dl, P < 0.0005). Mean change in insulin (± sd) from baseline to peak was 224.3 ± 313.7 μIU/ml for cases (vs. 35.5 ± 22.2 μIU/ml; P = 0.012). Mean change in GLP-1 (± sd) from baseline to peak was 31.2 ± 24 pm (vs. 6.2 ± 9.5 pm; P = 0.014).
Conclusions: Children with PPH after Nissen fundoplication have abnormally exaggerated secretion of GLP-1, which may contribute to the exaggerated insulin surge and resultant hypoglycemia.
Children with post-prandial hypoglycemia after Nissen fundoplication have abnormally exaggerated secretion of glucagon-like peptide-1, which may contribute to the amplified insulin surge and resultant hypoglycemia.
Dumping syndrome is a frequent complication of Nissen fundoplication, a surgery performed for severe gastroesophageal reflux. It has been estimated that up to 30% of children with Nissen fundoplication develop dumping syndrome (1). In children, this syndrome is characterized by severe postprandial hypoglycemia (PPH), or late dumping syndrome, with absent or less severe gastrointestinal symptoms of early dumping syndrome. If unrecognized, hypoglycemia can lead to seizures, developmental delays, and permanent brain damage. The mechanism responsible for the PPH is not fully understood, but is thought to involve reduced postprandial gastric relaxation and accelerated gastric emptying (2), resulting in the precipitous emptying of hyperosmolar, carbohydrate-containing solutions from the stomach into the upper small bowel (3) and subsequent hyperglycemia. Although the occurrence of postprandial hyperglycemia has been blamed for the later hypoglycemia, our observations in children with PPH after Nissen fundoplication have shown that peak insulin levels after a meal are proportionally higher than the degree of hyperglycemia (4), suggesting that other factors may contribute to the hyperinsulinemia. Recently, PPH has been recognized to be a severe complication of gastric bypass surgery (5,6). An exaggerated incretin and insulin response has been described to be part of the pathophysiology in these patients (7,8), as well as pancreatic islet hyperplasia (5,6). Similarly, studies in adults with PPH after partial gastrectomy have implicated excessive release of glucagon-like peptide-1 (GLP-1) leading to exaggerated insulin release as a potential cause of the PPH observed in this population (7,9,10,11).
GLP-1 is an insulinotropic (incretin) hormone secreted by intestinal L cells that potentiate glucose-stimulated insulin release by pancreatic ß-cells (for a complete review, see Ref. 12). In addition to enhancing glucose-stimulated insulin secretion, GLP-1 has other actions that are complementary to the incretin effect, including inhibition of glucagon secretion (13,14), hepatic glucose production (15,16), gastric emptying (17,18), and appetite (19,20). Furthermore, in animal models, exogenous administration of GLP-1 receptor agonists has been shown to result in increased pancreatic β-cell mass (12). The major physiological stimulus to L cells is nutrient ingestion, with circulating levels of GLP-1 rising by 2- to 3-fold in response to a mixed meal in adult human subjects (21). The regulation of GLP-1 secretion by ingested nutrients is complex and is thought to involve indirect vagal pathways as well as direct luminal stimulation of intestinal L cells (22). The secretion of GLP-1 is related to the rate of gastric emptying (9,23). The nutrient composition of the meal influences GLP-1 secretion as well; thus, simple carbohydrates and fats stimulate GLP-1 secretion, whereas pure complex carbohydrates and protein do not appear to do so in humans when taken individually (24,25).
We hypothesize that in children with Nissen fundoplication, the rapid emptying of a meal into the intestine results in inappropriately increased GLP-1 secretion, thereby triggering an exaggerated insulin surge and subsequent hypoglycemia. If our hypothesis proves true, GLP-1 may be a target for future therapies for affected children.
Subjects and Methods
Subjects
Ten subjects, aged 4 months to 13 yr, suspected of having PPH were recruited from the outpatient and inpatient endocrinology services at The Children’s Hospital of Philadelphia. Nine of the 10 subjects had a Nissen fundoplication for severe gastroesophageal reflux; one subject had a history of gastric pull-through surgery for a tracheoesophageal fistula. We recruited eight control subjects, six of whom were children undergoing evaluation for possible hypoglycemia not due to dumping syndrome, and two subjects with severe gastroesophageal reflux were studied before Nissen fundoplication and gastrostomy tube placement with no history of hypoglycemia. Exclusion criteria for case subjects consisted of fasting hypoglycemia, liver disease and malabsorption, and bowel abnormalities. Exclusion criteria for control subjects consisted of type 1 diabetes mellitus, type 2 diabetes mellitus, insulin resistance, and obesity (body mass index > 25 kg/m2). The study was approved by The Children’s Hospital of Philadelphia institutional review board, and consent was obtained from all participants.
Methods
All subjects were admitted to the Clinical and Translational Research Center inpatient unit the night before the study. The following day, they were administered an oral glucose tolerance test (OGTT). All subjects received 1.75 g/kg (75 g maximum) of glucose (Glucola; Thermo Fisher Scientific-NERL Clinical Diagnostics, East Providence, RI) either by mouth or via gastrostomy tube. For the formula tolerance test, subjects received between 3 and 12 ounces of Pediasure (Abbott Laboratories, Columbus, OH) or their home formula orally or via gastrostomy tube. Blood glucose and insulin levels were obtained at 0, 10, 20, 30, 60, 90, 120, and 180 min. GLP-1 levels were obtained at 0, 10, 20, 30, 60, and 120 min.
Assays
Venous blood glucose was measured using a Siemens Rapid Point 400 Blood Gas analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). The analyzer has a resolution of 1 mg/dl (0.06 mmol/liter) and a within-run sd of ± 4 mg/dl (0.22 mmol/liter).
Plasma insulin was measured using the in vitro diagnostic grade insulin ELISA from ALPCO Diagnostics (Salem, NH). The assay has an analytical sensitivity of 0.798 μIU/ml (5.7 pmol/liter) and an intraassay coefficient of variation of less than 5%.
Plasma intact GLP-1 was measured using the GLP-1 ELISA kit (Millipore; Linco Research, St. Charles, MO). The kit has an analytical sensitivity of 2 pm and an intraassay coefficient of variation of less than 10%. Ten microliters of dipeptidyl peptidase IV inhibitor (Millipore) per milliliter of blood were added to samples immediately after collection to stop the degradation of GLP-1.
Statistical analysis
We performed a cross-sectional analysis of metabolic variables at baseline and in response to a glucose load in all subjects. In addition, the same metabolic parameters in response to a formula meal were analyzed in subjects with PPH. Change scores were calculated for blood glucose change from baseline to peak, blood glucose change from peak to nadir, insulin change from baseline to peak, and GLP-1 change from baseline to peak. Histograms and Kolmogorov-Smirnov one-sample tests indicated that several of the scores had skewed distributions. Results are therefore presented as median and range, as well as mean ± sd, and nonparametric tests are used. Differences between the two groups in these change scores were examined using Mann-Whitney tests. The strength of the linear relationship between these change scores was examined using Spearman correlation coefficients.
Results
Case subjects were comprised of 10 children (0 female) aged 4 months to 13 yr (47 ± 43 months) (Table 1), suspected of having PPH after Nissen fundoplication (n = 9) or gastric pull-through surgery (n = 1). The mean duration from surgery to the time of the study was 38 ± 45 months. Eight control subjects (four female) aged 15 to 69 months (39 ± 17 months) were recruited. The final diagnoses of the control subjects were: ketotic hypoglycemia (n = 5), possible defect in fatty acid oxidation (n = 1), and gastroesophageal reflux (n = 2). There was no statistically significant difference between the ages of the case subjects compared with control subjects.
Table 1.
Demographics of cases and controls including OGTT and formula tolerance test route of administration and formula information
| Age (months) | Gender | Weight (kg) | OGTT via | FTT via | Formula | Formula volume (ml) | Grams of carbohydrate/100 ml of formula | Final diagnosis | |
|---|---|---|---|---|---|---|---|---|---|
| Case no. | |||||||||
| 1 | 41 | M | 14.4 | PO | PO | Pediasure | 90 | 12.7 | PPH |
| 2 | 81 | M | 24 | PO | PO | Pediasure | 250 | 12.7 | PPH |
| 3 | 33 | M | 11.8 | GT | GT | Elecare | 200 | 7 | PPH |
| 4 | 4 | M | 6 | GT | PPH | ||||
| 5 | 34 | M | 14 | PO | PO | Neocate | 120 | 7.7 | PPH |
| 6 | 28 | M | 9 | PO | PO | Pediasure | 120 | 12.7 | PPH |
| 7 | 22 | M | 11.8 | PO/GT | PPH | ||||
| 8 | 45 | M | 14.5 | PO/NG | PO/NG | Pediasure | 350 | 12.7 | PPH |
| 9 | 24 | M | 10.6 | PO/GT | PO/GT | Peptamen Jr. | 135 | 13.7 | PPH |
| 10 | 155 | M | 36.6 | GT | GT | Nutren 2.0 | 200 | 19 | PPH |
| 11 | 43 | M | 13.7 | PO | Peptamen Jr. | 96 | 13.7 | PPH | |
| Controls | |||||||||
| 1 | 64 | M | 14 | NG | GERD | ||||
| 2 | 30 | F | 9.6 | PO | GERD | ||||
| 3 | 15 | M | 9.9 | PO | KH | ||||
| 4 | 55 | F | 17.6 | PO | KH | ||||
| 5 | 56 | M | 20 | PO | KH | ||||
| 6 | 32 | F | 10 | PO | KH | ||||
| 7 | 29 | M | 12 | PO | Possible FAO disorder | ||||
| 8 | 34 | F | 14.2 | PO | KH |
FTT, Formula tolerance test; PO, per os (by mouth); GT, gastrostomy tube; NG, nasogastric; KH, ketotic hypoglycemia; FAO, fatty-acid oxidation; M, male; F, female; GERD, gastroesophageal reflux disease.
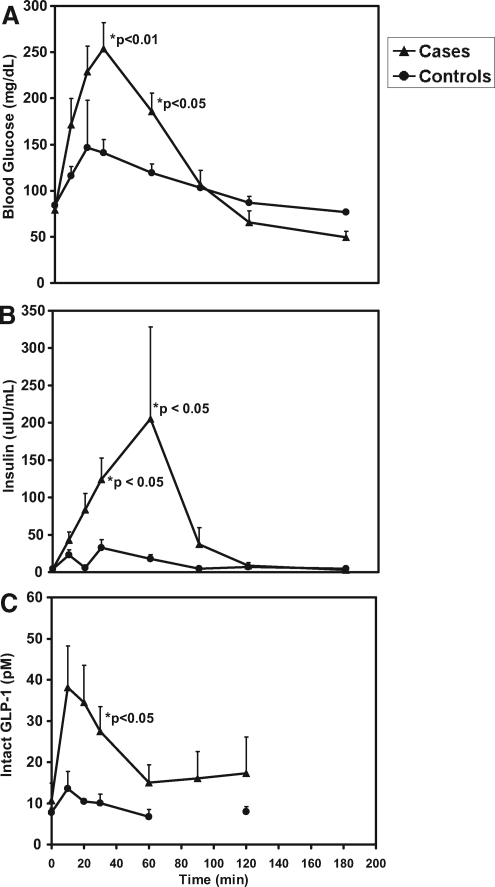
In the postabsorptive state, case and control subjects received 1.75 g/kg of glucose either by mouth or via gastrostomy tube. Baseline blood glucose levels were not different in cases and controls [79 ± 7.9 mg/dl (4.4 ± 0.44 mmol/liter) vs. 84 ± 5.8 mg/dl (4.7 ± 0.32 mmol/liter); P = 0.17]. Blood glucose rose more quickly and to a higher level in case subjects compared with controls. Mean change in blood glucose from baseline to peak (± sd) was 179.3 ± 87.4 mg/dl (10 ± 4.9 mmol/liter) in cases compared with 57.8 ± 39.5 mg/dl (3.2 ± 2.2 mmol/liter) in controls (P = 0.003). Similarly, after peaking, blood glucose dropped more significantly in cases compared with controls. Mean change in blood glucose (± sd) from peak to nadir was 214.4 ± 85.9 mg/dl (11.9 ± 4.8 mmol/liter) in cases compared with 55.9 ± 41.1 mg/dl (3.1 ± 2.3 mmol/liter) in controls (P < 0.0005). Blood glucose was significantly higher (P < 0.05) in cases compared with controls at 30 and 60 min (Fig. 1A). Hypoglycemia occurred in all cases at a median time of 120 min after the glucose load (Table 2). None of the control subjects had hypoglycemia during the study. Mean blood glucose at nadir (± sd) was 46.7 ± 11 mg/dl (2.6 ± 0.6 mmol/liter) for cases compared with 85.9 ± 21.3 mg/dl (4.8 ± 1.2 mmol/liter) for controls (P < 0.0005).
Figure 1.
A, Mean blood glucose values ± sem in cases (solid triangles) and controls (solid circles) at time points 0, 10, 20, 30, 60, 90, 120, and 180 min after OGTT. A significant difference between cases and controls is reached at 30 and 60 min (P < 0.05). B, Mean plasma insulin values ± sem in cases and controls at time points 0, 10, 20, 30, 60, 90, 120, and 180 min after OGTT. A significant difference between cases and controls is reached at 30 and 60 min (P < 0.05). C, Mean intact plasma GLP-1 values ± sem in cases and controls at time points 0, 10, 20, 30, 60, 90 (cases only), and 120 min after OGTT. Intact GLP-1 levels were not measured at 90 min in controls. A significant difference between cases and controls is reached at 30 min (P < 0.05).
Table 2.
Glucose, insulin, and GLP-1 data after OGTT in cases compared with controls
| Cases
|
Controls
|
P value | |||
|---|---|---|---|---|---|
| Median time (min) | Mean ± sd (median: range) | Median time (min) | Mean ± sd (median: range) | ||
| Glucose | |||||
| Baseline | 0 | 79 ± 7.9 mg/dl (78: 64–90) | 0 | 84 ± 5.8 mg/dl (86.5: 74–91) | 0.17 |
| Peak | 30 | 261.1 ± 82 mg/dl (258: 168–416) | 30 | 141.8 ± 41.5 mg/dl (151: 85–201) | 0.001 |
| Nadir | 120 | 46.7 ± 11 mg/dl (47.5: 23–57) | 120 | 85.9 ± 21.3 mg/dl (77.5: 59–121) | <0.0005 |
| Insulin | |||||
| Baseline | 0 | 4 ± 1.9 μ IU/ml (3: 3–8) | 0 | 4 ± 1.3 μ IU/ml (3: 3–6) | 0.92 |
| Peak | 30 | 228.3 ± 313.7 μ IU/ml (142.6: 10.5–1047) | 30 | 33.7 ± 24.7 μ IU/ml (38.4: 3–62.3) | 0.005 |
| GLP-1 | |||||
| Baseline | 0 | 10.7 ± 11.1 pm (8.2: 1.1–33.7) | 0 | 7.8 ± 2.5 pm (7.1: 5.8–12.5) | 0.63 |
| Peak | 10 | 41.9 ± 24.7 pm (43.3: 13.1–75.4) | 10 | 14 ± 9.5 pm (11.5: 6.4–33.2) | 0.014 |
Baseline plasma insulin levels were not different in the two groups [4 ± 1.9 μIU/ml (28.7 ± 13.6 pmol/liter) vs. 4 ± 1.3 μIU/ml (28.7 ± 9.3 pmol/liter), P = 0.92]. After a glucose load, plasma insulin levels increased, reaching a peak at median time of 30 min in both groups. Plasma insulin levels were significantly higher in cases compared with controls at 30 min [124.2 ± 85.7 μIU/ml (891.1 ± 614.9 pmol/liter) in cases compared with 32.9 ± 25.7 μIU/ml (236.1 ± 184.4 pmol/liter) in controls; P = 0.05] and at 60 min [205.5 ± 347.7 μIU/ml (1474.5 ± 2494.7 pmol/liter) in cases compared with 17.9 ± 13.5 μIU/ml (128.4 ± 96.9 pmol/liter) in controls; P = 0.043] (Fig. 1B). The mean change in insulin (± sd) from baseline to peak was 224.3 ± 313.7 μIU/ml (1609.4 ± 2250.8 pmol/liter) in cases compared with 35.5 ± 22.2 μIU/ml (254.7 ± 159.3 pmol/liter) in controls (P = 0.012). In one of the subjects, insulin reached a peak level of 1047.2 μIU/ml (7513.7 pmol/liter). Plasma insulin levels were appropriately suppressed after the hypoglycemia, suggesting normal regulation of insulin secretion by glucose.
We explored the pattern of GLP-1 secretion in these children with PPH, hypothesizing that increased GLP-1 secretion resulting from the rapid gastric emptying contributes to the exaggerated insulin response. Baseline intact GLP-1 levels were not different in cases vs. controls (10.7 ± 11.1 pm vs. 7.8 ± 2.5 pm; P = 0.63). Intact GLP-1 levels rise modestly after a glucose load in control children, from a baseline level of 7.8 ± 2.5 pm to a peak level of 14 ± 9.9 pm at median time of 10 min. In contrast to this modest response in normal children, children with PPH exhibit a higher and more prolonged response. Intact GLP-1 levels are higher in cases compared with controls over all time points after the glucose load, reaching a significant difference at 30 min (27.5 ± 15.8 pm in cases compared with 10 ± 5.5 pm in controls; P = 0.022) (Fig. 1C). GLP-1 peak and nadir glucose were strongly correlated (Spearman r = −0.8; P = 0.001). The mean change in GLP-1 (± sd) from baseline to peak was 31.2 ± 24 pm in cases compared with 6.2 ± 9.5 pm in controls (P = 0.014). There were no statistically significant differences identified in the responses of subjects that received glucose either by mouth or by gastrostomy tube.
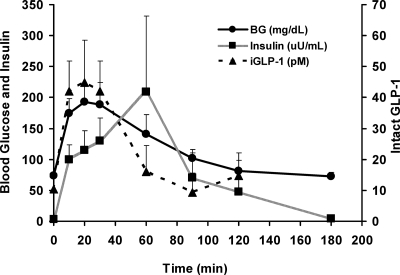
A formula tolerance test was performed in nine children (eight with Nissen fundoplication, one with gastric pull-through) with a history of PPH. Due to limitations on the allowable amount of blood to be drawn, we were unable to obtain a full set of labs on all case subjects. Control data were not obtained. Subjects received Pediasure or their home formula by mouth or via gastrostomy tube (Table 1). In response to the formula ingestion, blood glucose peaked at a median time of 20 min with a mean peak blood glucose (± sd) of 209.4 ± 87.1 mg/dl (11.6 ± 4.8 mmol/liter) (Fig. 2). The change in blood glucose from baseline to peak was 134.3 ± 91.4 mg/dl (7.5 ± 5.1 mmol/liter), and from peak to nadir was 147.9 ± 95.5 mg/dl (8.2 ± 5.3 mmol/liter). The mean peak insulin level (± sd) was 275.9 ± 334.4 μIU/ml (1979.6 ± 2399.3 pmol/liter). The change in insulin levels from baseline to peak was 272.6 ± 334.3 μIU/ml (1955.9 ± 2398.6 pmol/liter). Intact GLP-1 was measured in eight of our cases. The peak value for intact GLP-1 (± sd) was 51.4 ± 30.1 pm. The change in intact GLP-1 from baseline to peak was 41.1 ± 31.1 pm. There were no statistically significant differences identified in the responses of subjects that received formula either by mouth or gastrostomy tube.
Figure 2.
Mean blood glucose (solid circles, solid line), plasma insulin (solid square, gray line), and plasma intact GLP-1 (solid triangle, dashed line) values ± sem in cases at time points 0, 10, 20, 30, 60, 90, 120, and 180 min (glucose and insulin only at 180 min) after a formula tolerance test. This graph demonstrates the temporal relationship between glucose, insulin, and GLP-1 in children with Nissen fundoplication after a formula bolus.
The response of GLP-1 to a mixed meal in children with PPH after Nissen fundoplication is similar to their GLP-1 response after an oral glucose load.
Discussion
Our results show that children with PPH after Nissen fundoplication experience increased insulin levels and hypoglycemia compared with controls after an enteric glucose load. Additionally, they have an abnormally exaggerated secretion of GLP-1. This increased secretion may contribute to the exaggerated insulin surge and resultant hypoglycemia. The higher glucose levels after OGTT in our postsurgical patients may also contribute to higher GLP-1-mediated insulin release. Formula tolerance tests in cases also showed exaggerated secretion of both insulin and GLP-1 similar to that seen with an oral glucose load, suggesting that the rapid transport of nutrients in addition to glucose to the small intestine is capable of stimulating the L cells, resulting in abnormally elevated levels of GLP-1 and subsequently elevated insulin levels. The fact that these children received mostly liquid meals after the surgery may further exacerbate the problem because it is known that a liquid meal results in significantly more GLP-1 release than a solid meal of identical composition (26).
There is no evidence of an underlying disorder of fasting adaptation in the population studied. Fasting adaptation was evaluated as part of their initial evaluation and was found to be normal. In addition, baseline levels for all of the parameters studied were no different than those from the control subjects. Furthermore, in response to the hypoglycemia, insulin secretion was appropriately suppressed.
Most studies to date have focused on the beneficial effects of GLP-1 as an insulinotropic hormone. Little is known about the potential role of GLP-1 in the pathogenesis of hypoglycemic disorders. In patients with type 2 diabetes mellitus, acute (27,28) or chronic (29) sc administration of GLP-1 has not been associated with hypoglycemia. However, iv or sc administration of GLP-1 combined with iv glucose has been shown to induce hypoglycemia in healthy subjects (28,30). In fasted healthy subjects, sc injection of GLP-1 resulted in a fall of plasma glucose concentrations below the normal range (31). Increased levels of endogenous GLP-1 in pathological conditions have also been associated with a hypoglycemic response. In one report, a GLP-1- and somatostatin-secreting tumor resulted in postprandial hyperglycemia followed by symptomatic hypoglycemia in a 45-yr-old woman (32). Additionally, the elevated plasma levels of GLP-1 observed in partially gastrectomized adult subjects and in adult individuals after gastric bypass surgery in response to an oral glucose load or mixed meal have been proposed to be responsible for the PPH observed in this population by stimulating insulin secretion (7,8,9,10,11). To our knowledge, this is the first report in children of increased secretion of GLP-1. These findings support a potential role for GLP-1 in the pathogenesis of PPH after Nissen fundoplication in a pediatric population.
Limitations of our study include the absence of a control group for the formula tolerance test and history of fasting hypoglycemia in our control group for the OGTT. One important advantage of our study is the inclusion of children of comparable age because the metabolic response to oral glucose is likely to be influenced by the age of the subject. Because our study population consists of children, it is difficult to justify subjecting healthy control subjects to an invasive study such as an OGTT for which there would be no direct benefit to the subject. Glucose responses to the OGTT in our control population are not different from those of historical controls (33,34). There are very limited data on stimulated intact GLP-1 levels in normal adults, and these data do not exist in children. The intact GLP-1 response to a glucose load in our control population is similar to the levels reported for normal adult subjects in response to a mixed meal and to an oral glucose load (21,35).
The observation that GLP-1 secretion is significantly increased in children with PPH has important pathophysiological implications. An interesting question that should be addressed with further studies is the role of the elevated GLP-1 levels in non-β-cell targets. It has been previously reported (36) that in children with PPH after Nissen fundoplication there is inadequate glucagon response to hypoglycemia resulting in sustained hypoglycemia. It is possible that in addition to contributing to the exaggerated insulin response, GLP-1 contributes to the hypoglycemia by its effects on other targets, such as suppression of glucagon secretion or increase of peripheral glucose uptake. Furthermore, there have been reports of refusal to eat in children with dumping syndrome after Nissen fundoplication (37); given the central appetite suppressive effects of GLP-1 (19,20), its elevated levels in response to meals can explain this symptom.
Therapies that have been tried in the treatment of dumping syndrome include cornstarch, pectin, octreotide, and dietary manipulations (1,38,39,40). These therapies have had mixed results. We have had some success with the use of acarbose, an α-glucosidase inhibitor that delays the absorption of complex carbohydrates (4). However, the gastrointestinal side effects of this drug make it unfavorable for many children. Thus, many children require a regimen of continuous enteral feedings to avoid hypoglycemia but continue to be at high risk of hypoglycemic events if feedings are abruptly stopped. If the GLP-1 surge is responsible for the exaggerated insulin surge and other potential effects resulting in hypoglycemia, it is plausible that an antagonist of the GLP-1 receptor may be a potential therapy for these children.
Our data show that children with symptoms of PPH after Nissen fundoplication exhibit an exaggerated increase in secretion of GLP-1 in response to both glucose and formula boluses, which may contribute to the exaggerated insulin surge and resultant hypoglycemia. To our knowledge, this is the first study in children implicating GLP-1 in the pathogenesis of hypoglycemia. Further studies to determine the relationship between the exaggerated GLP-1 response and the subsequent hypoglycemia are needed.
Acknowledgments
The authors acknowledge the contribution of Clinical Translational and Research Center (CTRC) staff, particularly, the inpatient nurses and the biochemical laboratory technicians, as well as the nurses in The Children’s Hospital of Philadelphia Hyperinsulinism Center.
Footnotes
This work was supported by National Institutes of Health Grant 1K23 DK073663-01 (to D.D.D.L.) and by The Children’s Hospital of Philadelphia CTRC (Grant UL1-RR-024134) from the National Center for Research Resources.
Author Disclosure Summary: A.A.P., S.S., L.E.L.K., P.R.G., and D.D.D.L. have nothing to disclose.
First Published Online October 28, 2008
Abbreviations: GLP-1, Glucagon-like peptide-1; OGTT, oral glucose tolerance test; PPH, postprandial hypoglycemia.
References
- Samuk I, Afriat R, Horne T, Bistritzer T, Barr J, Vinograd I 1996 Dumping syndrome following Nissen fundoplication, diagnosis, and treatment. J Pediatr Gastroenterol Nutr 23:235–240 [DOI] [PubMed] [Google Scholar]
- Vu MK, Straathof JW, v d Schaar PJ, Arndt JW, Ringers J, Lamers CB, Masclee AA 1999 Motor and sensory function of the proximal stomach in reflux disease and after laparoscopic Nissen fundoplication. Am J Gastroenterol 94:1481–1489 [DOI] [PubMed] [Google Scholar]
- Gitzelmann R, Hirsig J 1986 Infant dumping syndrome: reversal of symptoms by feeding uncooked starch. Eur J Pediatr 145:504–506 [DOI] [PubMed] [Google Scholar]
- Ng DD, Ferry Jr RJ, Kelly A, Weinzimer SA, Stanley CA, Katz LE 2001 Acarbose treatment of postprandial hypoglycemia in children after Nissen fundoplication. J Pediatr 139:877–879 [DOI] [PubMed] [Google Scholar]
- Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV 2005 Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 353:249–254 [DOI] [PubMed] [Google Scholar]
- Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, Hanto DW, Callery M, Arky R, Nose V, Bonner-Weir S, Goldfine AB 2005 Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 48:2236–2240 [DOI] [PubMed] [Google Scholar]
- Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME 2007 Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 92:4678–4685 [DOI] [PubMed] [Google Scholar]
- Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L 2008 Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 16:298–305 [DOI] [PubMed] [Google Scholar]
- Miholic J, Orskov C, Holst JJ, Kotzerke J, Meyer HJ 1991 Emptying of the gastric substitute, glucagon-like peptide-1 (GLP-1), and reactive hypoglycemia after total gastrectomy. Dig Dis Sci 36:1361–1370 [DOI] [PubMed] [Google Scholar]
- Andreasen JJ, Orskov C, Holst JJ 1994 Secretion of glucagon-like peptide-1 and reactive hypoglycemia after partial gastrectomy. Digestion 55:221–228 [DOI] [PubMed] [Google Scholar]
- Gebhard B, Holst JJ, Biegelmayer C, Miholic J 2001 Postprandial GLP-1, norepinephrine, and reactive hypoglycemia in dumping syndrome. Dig Dis Sci 46:1915–1923 [DOI] [PubMed] [Google Scholar]
- De León DD, Crutchlow MF, Ham JY, Stoffers DA 2006 Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus. Int J Biochem Cell Biol 38:845–859 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W 1993 Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36:741–744 [DOI] [PubMed] [Google Scholar]
- Orskov C, Holst JJ, Nielsen OV 1988 Effect of truncated glucagon-like peptide-1 [proglucagon-(78–107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 123:2009–2013 [DOI] [PubMed] [Google Scholar]
- Hvidberg A, Nielsen MT, Hilsted J, Orskov C, Holst JJ 1994 Effect of glucagon-like peptide-1 (proglucagon 78-107amide) on hepatic glucose production in healthy man. Metabolism 43:104–108 [DOI] [PubMed] [Google Scholar]
- Larsson H, Holst JJ, Ahrén B 1997 Glucagon-like peptide-1 reduces hepatic glucose production indirectly through insulin and glucagon in humans. Acta Physiol Scand 160:413–422 [DOI] [PubMed] [Google Scholar]
- Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ 1993 Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38:665–673 [DOI] [PubMed] [Google Scholar]
- Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA 1996 Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 81:327–332 [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ 1998 Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzwiller JP, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J, Beglinger C 1999 Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 276:R1541—R1544 [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ 2001 Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50:609–613 [DOI] [PubMed] [Google Scholar]
- Brubaker PL, Anini Y 2003 Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can J Physiol Pharmacol 81:1005–1012 [DOI] [PubMed] [Google Scholar]
- Miholic J, Hoffmann M, Holst JJ, Lenglinger J, Mittlböck M, Bergmann H, Stacher G 2007 Gastric emptying of glucose solution and associated plasma concentrations of GLP-1, GIP, and PYY before and after fundoplication. Surg Endosc 21:309–314 [DOI] [PubMed] [Google Scholar]
- Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V 1993 Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 138:159–166 [DOI] [PubMed] [Google Scholar]
- Xiao Q, Boushey RP, Drucker DJ, Brubaker PL 1999 Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 117:99–105 [DOI] [PubMed] [Google Scholar]
- Brynes AE, Frost GS, Edwards CM, Ghatei MA, Bloom SR 1998 Plasma glucagon-like peptide-1 (7–36) amide (GLP-1) response to liquid phase, solid phase, and meals of differing lipid composition. Nutrition 14:433–436 [DOI] [PubMed] [Google Scholar]
- Knop FK, Vilsbøll T, Larsen S, Madsbad S, Holst JJ, Krarup T 2003 No hypoglycemia after subcutaneous administration of glucagon-like peptide-1 in lean type 2 diabetic patients and in patients with diabetes secondary to chronic pancreatitis. Diabetes Care 26:2581–2587 [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Krarup T, Madsbad S, Holst JJ 2001 No reactive hypoglycaemia in type 2 diabetic patients after subcutaneous administration of GLP-1 and intravenous glucose. Diabet Med 18:144–149 [DOI] [PubMed] [Google Scholar]
- Todd JF, Wilding JP, Edwards CM, Khan FA, Ghatei MA, Bloom SR 1997 Glucagon-like peptide-1 (GLP-1): a trial of treatment in non-insulin-dependent diabetes mellitus. Eur J Clin Invest 27:533–536 [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Madsbad S, Holst JJ 2001 Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 86:3853–3860 [DOI] [PubMed] [Google Scholar]
- Edwards CM, Todd JF, Ghatei MA, Bloom SR 1998 Subcutaneous glucagon-like peptide-1 (7–36) amide is insulinotropic and can cause hypoglycaemia in fasted healthy subjects. Clin Sci (Lond) 95:719–724 [DOI] [PubMed] [Google Scholar]
- Todd JF, Stanley SA, Roufosse CA, Bishop AE, Khoo B, Bloom SR, Meeran K 2003 A tumour that secretes glucagon-like peptide-1 and somatostatin in a patient with reactive hypoglycaemia and diabetes. Lancet 361:228–230 [DOI] [PubMed] [Google Scholar]
- Lestradet H, Deschamps I, Giron B 1976 Insulin and free fatty acid levels during oral glucose tolerance tests and their relation to age in 70 healthy children. Diabetes 25:505–508 [DOI] [PubMed] [Google Scholar]
- Josefsberg Z, Vilunski E, Hanukuglu A, Bialk O, Brown M, Karp M, Laron Z 1976 Glucose and insulin responses to an oral glucose load in normal children and adolescents in Israel. Isr J Med Sci 12:189–194 [PubMed] [Google Scholar]
- Meier JJ, Nauck MA, Kranz D, Holst JJ, Deacon CF, Gaeckler D, Schmidt WE, Gallwitz B 2004 Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes 53:654–662 [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Crawford JD 1987 Hypoglycemia pathogenesis in children with dumping syndrome. Pediatrics 80:937–942 [PubMed] [Google Scholar]
- Hirsig J, Baals H, Tuchschmid P, Spitz L, Stauffer UG 1984 Dumping syndrome following Nissen’s fundoplication: a cause for refusal to feed. J Pediatr Surg 19:155–157 [DOI] [PubMed] [Google Scholar]
- Borovoy J, Furuta L, Nurko S 1998 Benefit of uncooked cornstarch in the management of children with dumping syndrome fed exclusively by gastrostomy. Am J Gastroenterol 93:814–818 [DOI] [PubMed] [Google Scholar]
- Lehnert H, Beyer J, Weber P, Krause U, Schrezenmeir J 1990 Treatment of severe reactive hypoglycemia with a somatostatin analogue (SMS 201–995). Arch Intern Med 150:2401–2402 [PubMed] [Google Scholar]
- Khoshoo V, Reifen RM, Gold BD, Sherman PM, Pencharz PB 1991 Nutritional manipulation in the management of dumping syndrome. Arch Dis Child 66:1447–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]