Abstract
Context: Bicarbonate has been implicated in bone health in older subjects on acid-producing diets in short-term studies.
Objective: The objective of this study was to determine the effects of potassium bicarbonate and its components on changes in bone resorption and calcium excretion over 3 months in older men and women.
Design, Participants, and Intervention: In this double-blind, controlled trial, 171 men and women age 50 and older were randomized to receive placebo or 67.5 mmol/d of potassium bicarbonate, sodium bicarbonate, or potassium chloride for 3 months. All subjects received calcium (600 mg of calcium as triphosphate) and 525 IU of vitamin D3 daily.
Main Outcome Measures: Twenty-four-hour urinary N-telopeptide and calcium were measured at entry and after 3 months. Changes in these measures were compared across treatment groups in the 162 participants included in the analyses.
Results: Bicarbonate affected the study outcomes, whereas potassium did not; the two bicarbonate groups and the two no bicarbonate groups were therefore combined. Subjects taking bicarbonate had significant reductions in urinary N-telopeptide and calcium excretion, when compared with subjects taking no bicarbonate (both before and after adjustment for baseline laboratory value, sex, and changes in urinary sodium and potassium; P = 0.001 for both, adjusted). Potassium supplementation did not significantly affect N-telopeptide or calcium excretion.
Conclusions: Bicarbonate, but not potassium, had a favorable effect on bone resorption and calcium excretion. This suggests that increasing the alkali content of the diet may attenuate bone loss in healthy older adults.
Treatment with bicarbonate lowers bone resorption and calcium excretion in healthy older men and women.
Heredity, diet, and other lifestyle factors contribute to the problem of bone loss and fractures. Of the dietary constituents affecting bone, calcium and vitamin D have received the most attention, but there is increasing evidence that the acid/base balance of the diet is also important. Fruits and vegetables are metabolized to bicarbonate and are thus alkali-producing, whereas protein and cereal grains are metabolized to acid and thus are acid-producing or acidogenic. On average, American diets tend to be acidogenic, producing an excess of about 75 mEq of acid per day (1). With aging there is a decline in renal function (2,3), a decreased capacity to excrete hydrogen ions, and a gradually increasing metabolic acidosis. In a comprehensive review, Frassetto and Sebastian (4) identified a 6 to 7% rise in blood [H+] and a 12 to 16% decline in plasma [HCO3−] between ages 20 and 80 yr, with most of the change occurring after age 50.
An acidic environment affects bone in several ways. It inhibits osteoblastic activity (5,6,7,8,9), increases osteoclastic activity (10,11), and may also increase bone resorption by a direct, noncellular physicochemical process (12). Acidogenic (high-protein) diets induce calciuria (13,14,15,16), and administration of alkaline salts of potassium for 7 to18 d to healthy subjects on controlled high protein diets lowers biochemical markers of bone turnover and reduces calcium excretion (17,18). If sustained, these changes should have a favorable effect on calcium balance and bone mass.
There is no consensus on the individual contributions of the anion and cation to the favorable effects of potassium bicarbonate on indices of bone metabolism. Several investigators have concluded that the potassium impacts calcium balance, largely on the basis that it decreases calcium excretion (19,20). Moreover, higher potassium intakes, as seen in diets rich in fruits and vegetables, have been associated with higher femoral neck bone mineral density (BMD) and lower biochemical markers of bone turnover in adult women (21). In contrast, others have implicated the bicarbonate, which consistently lowers calcium excretion (18,22,23) and has also reduced biochemical markers of bone resorption (23,24).
This study was conducted to determine the impact of 3 months of treatment with KHCO3 and its components, potassium and HCO3, on biochemical markers of bone turnover and calcium excretion.
Subjects and Methods
Subjects
Healthy ambulatory men and women age 50 and older were recruited through direct mailings and advertisements in the community. The women were menopausal for at least 6 months. The criteria for exclusion included: vegetarianism, adrenal insufficiency, diabetes mellitus (fasting sugar >130 mg/dl), alcohol use exceeding 2 U/d, peptic ulcers or esophageal stricture, active malignancy, hyperparathyroidism, untreated thyroid disease, significant immune disorder, uncontrolled hypertension (>140/90 mm Hg), congestive heart failure, arrhythmias, myocardial infarction in the last 12 months, salt-restricted diets, gastroesophageal reflux disease requiring treatment with sodium- or alkali-containing antacids, kidney stones in the last 5 yr, creatinine (Cr) clearance less than 50 ml/min/1.73 × m2, 24-h urine calcium greater than 300 mg/d after 1 wk off of calcium supplements, total hip T-score less than −3.0, 25- hydroxyvitamin D level below 16 ng/ml, or use of oral glucocorticoids for more than 10 d in the last 3 months, use of gonadal hormones or other medications for osteoporosis in the last 2 yr, current use of diuretics, or current use of nonsteroidal antiinflammatory drugs more than three times per week.
We prescreened 519 subjects by means of a questionnaire and invited 316 for screening. Of these, 103 were found to be ineligible, 42 were potentially eligible but not enrolled, and 171 (76 men and 95 women) were enrolled. There were 148 whites, 14 blacks, and eight Asians. The protocol was approved by the Tufts Medical Center-Tufts University Health Sciences Campus Institutional Review Board, and written informed consent was obtained from each subject.
Study design and supplements
In this 84-d, double-blind, placebo-controlled trial, the subjects were randomized in blocks of four within sex and age (50–64 yr and 65 and older) strata to treatment with: KHCO3, KCl, NaHCO3, or placebo. These treatments were selected because we hypothesized that both HCO3 and potassium might have beneficial effects on our outcomes, and any benefits observed might be additive. We planned to adjust our analyses for differences in sodium excretion, because sodium is known to influence calcium excretion. At study entry, we assessed the subjects’ medical history, physical activity level, usual diet and supplement use, blood pressure, height, and weight, and analyzed blood and urine. Subjects returned on d 84 for measurement of weight and biochemical assays. They came on study d 21 for a serum potassium safety measurement and on d 21 and 49 for weights, blood pressure, and for review of adherence to supplements.
The subjects were advised to maintain their usual diets, to stop taking their own calcium and vitamin D supplements for 1 wk before screening until the end of the study, and to maintain their usual levels of physical activity and stable weight throughout the study. Each subject took a supplement tablet containing 600 mg of calcium as the triphosphate, 125 IU of vitamin D3, and 50 mg of magnesium oxide (Posture D from US Rhodia), and a multivitamin containing 400 IU of vitamin D3 with breakfast daily throughout the study (to reduce variation in these intakes and their potential effects on our outcomes).
The active treatments were given in amounts of 67.5 mmol/d, in nine gelatin capsules (containing 7.5 mmol each), and the placebo capsules contained an equal volume of microcrystalline cellulose. Subjects were asked to take one capsule three times daily during wk 1, two capsules three times daily during wk 2, and three capsules three times daily thereafter. All capsules were taken with an 8-oz glass of water immediately after meals. The capsules were made by a local compounding pharmacy (The Medical Pharmacy and Supply, Stoughton, MA).
Measurements
Height was measured with a stadiometer and weight with a digital scale. Leisure, household, and occupational activity was estimated with use of the Physical Activity Scale for the Elderly (PASE) questionnaire (25). Usual dietary intake over the last 3 months was assessed by use of the Fred Hutchinson food frequency questionnaire (26). Adherence to study supplements was assessed by review of calendars on which subjects recorded each pill that they took.
Analytic methods
Blood was drawn between 0700 and 0930 h after the subjects had fasted for 12 h. All samples from individual subjects except the safety blood on d 21 were batched for analyses. The 24-h urine measures are presented as Cr ratios, to correct for variation in the completeness of the urine collections. Serum 25-hydroxyvitamin D was measured with RIA kits from Diasorin (Stillwater, MN) with a coefficient of variation (CV) of 5.6 to 7.7%. Serum intact PTH and osteocalcin were measured by chemiluminescent immunoradiometric assays on an automated immunoassay system (IMMULITE 1000, Diagnostic Product Corp., Los Angeles, CA), with CVs of 3 to 9%. Serum calcium, potassium, and Cr and urinary potassium and sodium were measured on an automated clinical chemistry analyzer (Olympus AU400, Olympus America Inc., Melville, NY) with CVs of 3.0 to 6.0%. Twenty-four-hour urinary calcium was measured by direct-current plasma emission spectroscopy (Beckman SpectraSpan VI Direct Current Plasma Emission Spectrophotometer; Beckman Instruments, Fullerton, CA) with a CV of 3–5%. Urinary N-telopeptide (NTX) was measured by ELISA (Wampole, Princeton, NJ) with a CV of 5.6 to 7.7%. Urinary nitrogen was measured with a model FP-2000 nitrogen/protein determinator (LECO, St. Joseph, MI). This instrument employs a Dumas combustion method and detection using a thermal conductivity cell; it measures nitrogen with a precision of 15 ppm.
Net acid excretion (NAE) was measured in 24-h urine collections by a modification of the Jorgensen titration method (27), as described by Chan (28). NAE equals titratable acid plus NH4+ minus HCO3−. Briefly, titratable acid minus HCO3− was assessed after adding HCl, boiling the sample, and then titrating the sample to neutral pH. To measure the NH4+, formol was added to the sample to release the H+ from NH4+, and the sample was again titrated to neutral pH. All titrations were carried out with a TIM 900 Titration Manager (Radiometer Analytical, Loveland, CO). The precision of NAE measurements in our laboratory was determined by analyzing aliquots of a single 24-h urine collection on 15 different days. The aliquots were stored frozen at −80 C and thawed only once. The CV of the NAE measurements was 10.1%.
BMD of the total hip was measured on the screening visit with a GE Lunar model Prodigy scanner (Madison, WI). The root mean square precision of these measurements in our laboratory is 0.65% for total hip BMD (29).
Statistical analyses
The primary endpoints in this analysis were the 3-month changes in the 24-h NTX/Cr ratio (ΔNTX/Cr), urinary calcium/Cr ratio (ΔCa/Cr), and 3-month change in serum osteocalcin. Changes in other laboratory values were investigated in a similar manner. We examined changes in the endpoints by analysis of covariance and evaluated a limited set of post hoc comparisons (KHCO3 vs. NaHCO3, KCl vs. placebo, and KHCO3 vs. KCl) by the least significant differences method. We did not stratify by sex because we found no significant interactions of sex with HCO3 on the endpoints, but we did adjust for sex in all analyses. T-tests were used to compare baseline characteristics across HCO3 groups. SPSS version 15.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses, and P values <0.05 were considered to indicate statistical significance.
Status of subjects and adherence
Of the 171 enrolled, seven subjects (4%) dropped out of the study (placebo group, one with cardiac event; NaHCO3, one with high blood pressure, one with headaches, and two lost interest; KCl, one with prostate cancer; KHCO3, one lost interest). In addition, one subject in the KHCO3 group was excluded from the analyses because he was believed to have a metabolic bone disease, possibly renal tubular acidosis, on the basis of high baseline NAE, NTX, and calcium excretion values. One other subject was excluded because of high baseline NTX excretion.
Results
Selection of analysis groups
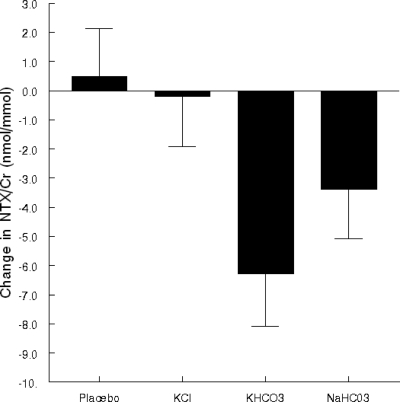
The four treatments were selected to allow us to address the question of whether the potassium, the bicarbonate, or both are important to achieve effects on calcium excretion and bone resorption. The four groups did not differ significantly in age, body weight, sex distribution, dietary intake of calcium or vitamin D, or in baseline levels of urinary NTX/Cr, serum osteocalcin, or calcium/Cr excretion (data not shown). As expected, the 3-month change in sodium/Cr excretion was higher in the NaHCO3 group than in the other groups [analysis of covariance (ANCOVA) P = 0.003, after adjustment for sex and baseline sodium/Cr excretion]. Three-month change in mean urinary NTX/Cr differed significantly among the four groups (ANCOVA P = 0.024, after adjustment for sex and baseline NTX/Cr). As shown in Fig. 1, NTX/Cr declined in the two bicarbonate groups but not in the two groups without bicarbonate. The change differed significantly between the KCl and the KHCO3 groups (P = 0.015), but not between the two bicarbonate groups (P = 0.243) or between groups not taking bicarbonate, the placebo and KCl group (P = 0.773). The patterns of change in the four groups were similar for serum osteocalcin and urine calcium/Cr. Therefore, for subsequent analyses, we combined the two HCO3 groups and the groups with no HCO3 and focused on the effect of HCO3 treatment on the endpoints, adjusting for ΔK/Cr, ΔNa/Cr, sex, and baseline value of the endpoint.
Figure 1.
Mean 3-month change in urinary NTX/Cr by treatment group, adjusted for sex and baseline NTX/Cr.
Main results
The clinical characteristics of the 162 subjects by HCO3 group are shown in Table 1. There were no significant group differences in any of these baseline characteristics. During the study, weight did not change significantly in either group (0.18 ± 1.96 kg, P = 0.422 in the HCO3 group; and −0.04 ± 1.50, P = 0.803 kg in the no HCO3 group). Mean adherence in the 153 subjects with adequate adherence data was 94.6 ± 12.5% in the HCO3 group and 86.5 ± 22.8% in the no HCO3 group; among the 145 subjects who were taking any pills at the end of the study, mean adherence was 96.3 ± 6.2% and 91.4 ± 14.8%, respectively.
Table 1.
Baseline characteristics (mean ± sd) of the 162 study subjects by treatment group
| No HCO3 | HCO3 | P | |
|---|---|---|---|
| n | 84 | 78 | |
| Age (yr) | 63.3 ± 7.7 | 62.4 ± 7.6 | 0.453 |
| Female (%) | 58.3 | 55.1 | 0.681 |
| Height (cm) | 167.0 ± 8.8 | 168.6 ± 9.9 | 0.293 |
| Weight (kg) | 72.2 ± 13.6 | 74.9 ± 137 | 0.207 |
| Dietary calcium intake (mg/d) | 772 ± 386 (83) | 880 ± 431 | 0.095 |
| Dietary vitamin D (IU/d) | 206 ± 144 (83) | 247 ± 135 | 0.066 |
| Physical activity score | 168 ± 84 (81) | 156 ± 82 (75) | 0.366 |
Numbers in parentheses indicate sample size.
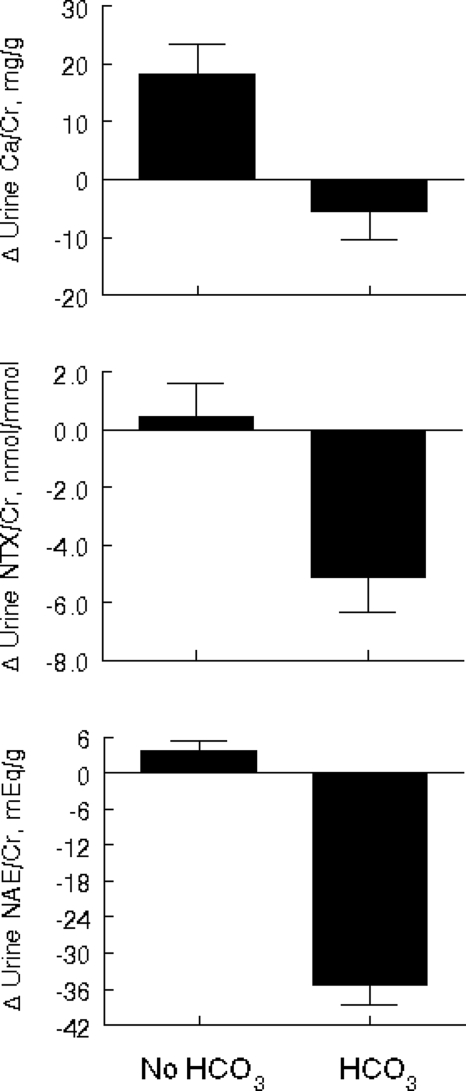
The baseline biochemical measurements and changes over the 3-month study in the HCO3 and no HCO3 groups (after adjustment for sex, baseline value, and changes in sodium/Cr and K/Cr excretion, except when they were the dependent variables) are shown in Table 2. There were no statistically significant group differences in any of these measures at baseline except NAE/Cr, which was higher in the HCO3 group. As expected, ΔNAE/Cr differed significantly in the two groups (P < 0.001). Subjects supplemented with HCO3 for 3 months had significantly greater adjusted mean changes in calcium/Cr excretion (P = 0.002) and urinary NTX/Cr (P = 0.002) than subjects in the no HCO3 group. These changes are illustrated in Fig. 2.
Table 2.
Mean ± sem baseline and 3-month changes in laboratory values by bicarbonate treatment status
| No HCO3 (n = 84) | HCO3 (n = 78) | P | |
|---|---|---|---|
| Serum | |||
| Calcium (mg/dl) | |||
| Baseline | 9.38 ± 0.04 | 9.27 ± 0.04 | 0.063 |
| Change | −0.02 ± 0.04 | 0.07 ± 0.04 | 0.112 |
| Phosphorus (mg/dl) | |||
| Baseline | 3.39 ± 0.05 | 3.43 ± 0.06 | 0.653 |
| Change | 0.10 ± 0.04 | 0.01 ± 0.04 | 0.129 |
| PTH (pg/ml) | |||
| Baseline | 53.5 ± 1.93 | 57.9 ± 2.3 | 0.141 |
| Change | −1.5 ± 1.6 | −2.0 ± 1.9 | 0.807 |
| Potassium (mEq/liter) | |||
| Baseline | 4.49 ± 0.04 | 4.39 ± 0.04 | 0.080 |
| Change | 0.07 ± 0.04a | 0.001 ± 0.037 | 0.200 |
| Osteocalcin (ng/ml) | |||
| Baseline | 7.36 ± 0.41 | 7.44 ± 0.49 | 0.906 |
| Change | 0.36 ± 0.21 | −0.17 ± 0.22 | 0.077 |
| Urine | |||
| Calcium/Cr (mg/g) | |||
| Baseline | 114.04 ± 7.05 | 125.30 ± 7.36 | 0.271 |
| Change | 18.34 ± 5.00 | −5.38 ± 5.16 | 0.001 |
| Potassium/Cr (mEq/g) | |||
| Baseline | 57.94 ± 2.38 | 58.82 ± 2.61 | 0.802 |
| Change | 12.49 ± 3.20 | 14.95 ± 3.32 | 0.597 |
| Sodium/Cr (mEq/g) | |||
| Baseline | 111.49 ± 4.79 | 105.18 ± 4.34 | 0.333 |
| Change | 5.98 ± 4.31 | 18.15 ± 4.48 | 0.053 |
| NTX/Cr (nmol/mmol) | |||
| Baseline | 38.07 ± 1.84 | 38.19 ± 1.92 | 0.964 |
| Change | 0.44 ± 1.19 | −5.11 ± 1.23 | 0.001 |
| NAE/Cr (mEq/g) | |||
| Baseline | 24.6 ± 1.7 | 31.0 ± 2.5 | 0.034 |
| Change | 3.6 ± 1.8 | −35.2 ± 3.3 | <0.001 |
Changes are adjusted for sex, baseline laboratory value, and (except when it is the dependent variable) for the change in urinary sodium to Cr and the change in urinary potassium to Cr.
One subject with missing value.
Figure 2.
Mean 3-month changes in urinary Ca/Cr, NTX/Cr, and NAE/Cr by HCO3 group, adjusted for sex, baseline value, and 3-month changes in sodium/Cr and K/Cr.
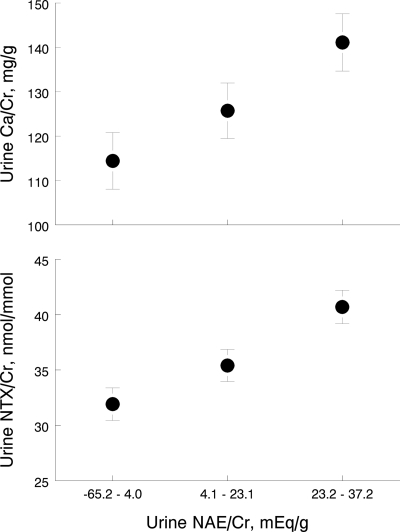
In ANCOVA, NAE/Cr on d 84 was significantly associated with calcium excretion on d 84 (β = 0.61, P < 0.001, after adjustment for sex, baseline calcium/Cr excretion, and for sodium/Cr and K/Cr excretion on d 84). Similarly, NAE/Cr on d 84 was significantly associated with NTX/Cr on d 84 (β = 0.18, P < 0.001, after adjustment for sex, baseline urinary NTX/Cr, and sodium/Cr and K/Cr excretion on d 84). To illustrate these associations, Fig. 3 displays the adjusted mean 24-h urine calcium/Cr and NTX/Cr values by tertile of NAE/Cr on d 84 in the 162 subjects.
Figure 3.
Mean urinary NTX/Cr and Ca/Cr by NAE/Cr tertile at the end of the study, adjusted for sex, baseline value, and for sodium/Cr and K/Cr at the end of the study.
Serum potassium concentration was determined in each subject after 3 wk in the study as a safety measure. Three subjects treated with potassium had serum potassium levels over 5 mg/dl, the upper end of the reference range, and their values were 5.1, 5.1, and 5.2 mg/dl; five subjects not treated with potassium had values over 5.0 mg/dl, and their values were 5.1, 5.2, 5.2, 5.3, and 5.4 mg/dl. Blood pressure did not exceed 140/90 mm Hg at entry in any subject. One subject in the NaHCO3 group had blood pressure exceed 145/95 mm Hg during the trial; she was unavailable for a repeat blood pressure check and so was asked to discontinue the study pills. The supplements were generally well tolerated, but 12 discontinued treatment because of gastrointestinal symptoms (placebo, one subject; KHCO3, one; NaHCO3, three; and KCl, seven), and four discontinued pills for reasons unrelated to the pills.
Discussion
Supplementation with 67.5 mmol/d of bicarbonate for 3 months had the favorable effects of decreasing calcium excretion and lowering the biochemical marker of bone resorption, NTX. Subjects in the HCO3 group had a relative decrease in calcium excretion compared with subjects in the no HCO3 group. All subjects took a calcium supplement daily throughout the study, which probably accounts for the increase in calcium excretion seen in the no HCO3 group and may have reduced the treatment-related decrease in the HCO3 group. In this study, we chose to use a neutral calcium supplement, calcium triphosphate; use of an alkali-producing supplement such as calcium carbonate or calcium citrate would have neutralized some of the acid load in these subjects. Our finding is consistent with the short-term studies that have reported that alkaline salts of potassium administered over a period of up to 3 wk lower 24-h calcium excretion (17,18), and it extends these observations to demonstrate that the effect is present after 3 months. A recent report indicated that treatment of postmenopausal women with 33.5 mmol/d of potassium citrate, when compared with a similar dose of potassium chloride, reduced excretion of calcium in 2-h fasting collections at 6, 9, and 12 months but not at 3 months (30). The effect of alkaline salts on calcium absorption remains to be defined.
Treatment with HCO3 lowered bone resorption, as indicated by a 13.4% decrease in urinary NTX/Cr, compared with no change in the no HCO3 group. Reducing the rate of bone resorption in older adults to levels observed in healthy young adults is desirable because lower turnover rates have been associated with reduced rates of bone loss (31,32) and lower fracture rates (33). We confirm findings of previous shorter-term (7- to 18-d) studies (17,18) and show that the bicarbonate effect is apparent after 3 months. The recent 1-yr intervention study cited above also found that 33.5 mmol/d of potassium citrate (compared with potassium chloride) decreased excretion of the bone resorption markers, pyridinoline and deoxypyridinoline, after 3 months but not after 1 yr; despite this, however, the intervention lowered rates of bone loss from the spine and hip (30). In contrast, Macdonald et al. (34) reported a significant effect of treatment with 55 mmol/d of KHCO3 (vs. true placebo) on one biochemical marker of bone resorption at 4 to 6 wk (2-h fasting urinary free deoxypyridinoline crosslinks/Cr), but no effect on BMD at the spine or hip over a 2-yr period. Treatment with HCO3 had no significant effect on serum osteocalcin in our study, in agreement with some (30,34) but not with others who have noted modest increases in serum osteocalcin after 14 and 18 d of HCO3 treatment (17,35). The reason for the divergent findings is not apparent at this time.
Treatment with HCO3 had no significant effect on serum PTH levels in this study. This finding is in agreement with other metabolic studies in normal subjects in which administration of oral acid and alkaline loads has not been found to alter PTH secretion over 1- to 2-wk periods (18,24,36,37).
The NEA measurements in this study were valuable because they gave a quantitative estimate of the combined influences of net acid- or base-producing content of the subjects’ self-selected diets and of their intervention (including compliance with the pills) on these outcomes. This integrated estimate allowed us to identify significant linear relationships between change in NAE and changes in NTX and calcium excretion, confirming that it is the reduction in acid load that was the active component of the treatment.
Like many others in the United States, our study population generally consumed acid-producing diets, as indicated by their positive mean NAE at entry into the study. Treatment with 67.5 mmol/d of HCO3 reduced the mean NAE to around 0, illustrating that, on average, this dose was adequate to neutralize the net acid load of their usual diets. Because of variation in self-selected diets and in treatments, however, there was wide variation in NAE among the subjects at the end of this study. In the group as a whole at the end of the study, those with the lowest levels of NAE had the lowest levels of calcium and NTX excretion. The linear relationship between NAE and excretion of calcium and NTX on the final visit raises the possibility that a higher dose of HCO3 may be more beneficial for bone. Further research is needed to clearly define the longer-term effects of potassium bicarbonate on rates of bone turnover and bone loss. It will also be of interest to determine whether a net neutral or alkali-producing diet, achieved through the combination of high fruit and vegetable and reduced cereal grain intake or by coadministration of alkali, will influence the effect of dietary protein on the skeleton. Achieving alkali-producing diets would require drastic changes in food choices and be challenging in older people who tend to have long-established dietary patterns. Should it be shown to be beneficial, an alternative approach may be to administer bicarbonate in supplement form or to lower the acid-producing capacity of selected foods through alkali fortification.
Supplementation with potassium did not significantly alter calcium excretion or markers of bone turnover in this study. This is in contrast to earlier reports of Lemann et al. (19) and Jones et al. (20) who found that increasing potassium intake decreased urinary calcium excretion. The apparently conflicting observation that higher potassium intake is associated with higher BMD in healthy perimenopausal women (21) may result from the fact that potassium-rich diets tend to be alkali-producing, in that they are rich in fruits and vegetables. Treatment with potassium did enhance sodium excretion, as has been documented widely.
In conclusion, we have found that reducing the acidogenicity of the diet into the alkali-producing range with bicarbonate lowers calcium excretion and the bone resorption rate in healthy older men and women consuming rather typical acid-producing American diets. Treatment with 67.5 mmol/d of potassium bicarbonate was safe and well tolerated in this population. Increasing intake of alkali merits further consideration as a safe and low-cost approach to improving skeletal health in older men and women.
Acknowledgments
We express our appreciation to Dr. Catherine Gordon of Children’s Hospital in Boston for serving as the Safety Monitor of this study and to the staff of the Metabolic Research Unit, the Nutrition Evaluation Laboratory, and to the study participants for their valuable contributions to this study.
Footnotes
Reprints will not be available.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 21, 2008
Abbreviations: ANCOVA, Analysis of covariance; BMD, bone mineral density; Cr, creatinine; CV, coefficient of variation; NAE, net acid excretion; NTX, N-telopeptide.
References
- Lemann Jr J 1999 Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. Nephron 81(Suppl 1):18–25 [DOI] [PubMed] [Google Scholar]
- Davies DF, Shock NW 1950 Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29:496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW 1976 The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol 31:155–163 [DOI] [PubMed] [Google Scholar]
- Frassetto L, Sebastian A 1996 Age and systemic acid-base equilibrium: analysis of published data. J Gerontol A Biol Sci Med Sci 51:B91–B99 [DOI] [PubMed] [Google Scholar]
- Bhargava U, Bar-Lev M, Bellows CG, Aubin JE 1988 Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone 9:155–163 [DOI] [PubMed] [Google Scholar]
- Ecarot-Charrier B, Glorieux FH, van der RM, Pereira G 1983 Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol 96:639–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S 1983 In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol 96:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague SM, Krieger NS, Bushinsky DA 1993 Aluminum inhibits bone nodule formation and calcification in vitro. Am J Physiol 264:F882–F890 [DOI] [PubMed] [Google Scholar]
- Sprague SM, Krieger NS, Bushinsky DA 1994 Greater inhibition of in vitro bone mineralization with metabolic than respiratory acidosis. Kidney Int 46:1199–1206 [DOI] [PubMed] [Google Scholar]
- Arnett TR, Dempster DW 1986 Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology 119:119–124 [DOI] [PubMed] [Google Scholar]
- Arnett TR, Spowage M 1996 Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 18:277–279 [DOI] [PubMed] [Google Scholar]
- Bushinsky DA 1996 Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Physiol 271:F216–F222 [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, Mitnick ME, Gundberg CM, Caseria DM, Ellison AF, Carpenter TO, Insogna KL 1999 Changes in bone turnover in young women consuming different levels of dietary protein. J Clin Endocrinol Metab 84:1052–1055 [DOI] [PubMed] [Google Scholar]
- Linkswiler HM, Joyce CL, Anand CR 1974 Calcium retention of young adult males as affected by level of protein and of calcium intake. Trans NY Acad Sci 36:333–340 [DOI] [PubMed] [Google Scholar]
- Pannemans DL, Schaafsma G, Westerterp KR 1997 Calcium excretion, apparent calcium absorption and calcium balance in young and elderly subjects: influence of protein intake. Brit J Nutr 77:721–729 [DOI] [PubMed] [Google Scholar]
- Hegsted M, Linkswiler HM 1981 Long-term effects of level of protein intake on calcium metabolism in young adult women. J Nutr 111:244–251 [DOI] [PubMed] [Google Scholar]
- Sebastian A, Morris Jr RC 1994 Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 331:279 [DOI] [PubMed] [Google Scholar]
- Maurer M, Riesen W, Muser J, Hulter HN, Krapf R 2003 Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol 284:F32–F40 [DOI] [PubMed] [Google Scholar]
- Lemann Jr J, Gray RW, Pleuss JA 1989 Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int 35:688–695 [DOI] [PubMed] [Google Scholar]
- Jones JW, Sebastian A, Hulter HN, Schambelan M, Sutton JM, Biglieri EG 1982 Systemic and renal acid-base effects of chronic dietary potassium depletion in humans. Kidney Int 21:402–410 [DOI] [PubMed] [Google Scholar]
- Macdonald HM, New SA, Fraser WD, Campbell MK, Reid DM 2005 Low dietary potassium intakes and high dietary estimates of net endogenous acid production are associated with low bone mineral density in premenopausal women and increased markers of bone resorption in postmenopausal women. Am J Clin Nutr 81:923–933 [DOI] [PubMed] [Google Scholar]
- Lutz J 1984 Calcium balance and acid-base status of women as affected by increased protein intake and by sodium bicarbonate ingestion. Am J Clin Nutr 39:281–288 [DOI] [PubMed] [Google Scholar]
- Buclin T, Cosma M, Appenzeller M, Jacquet AF, Decosterd LA, Biollaz J, Burckhardt P 2001 Diet acids and alkalis influence calcium retention in bone. Osteoporos Int 12:493–499 [DOI] [PubMed] [Google Scholar]
- Sellmeyer DE, Schloetter M, Sebastian A 2002 Potassium citrate prevents increased urine calcium excretion and bone resorption induced by a high sodium chloride diet. J Clin Endocrinol Metab 87:2008–2012 [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA 1993 The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162 [DOI] [PubMed] [Google Scholar]
- Block G, Woods M, Potosky A, Clifford C 1990 Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43:1327–1335 [DOI] [PubMed] [Google Scholar]
- Jorgensen K 1957 Titrimetric determination of the net excretion of acid/base in urine. Scand J Clin Lab Invest 9:28–291 [DOI] [PubMed] [Google Scholar]
- Chan JC 1972 The rapid determination of urinary titratable acid and ammonium and evaluation of freezing as a method of preservation. Clin Biochem 5:94–98 [DOI] [PubMed] [Google Scholar]
- White J, Harris SS, Dallal GE, Dawson-Hughes B 2003 Precision of single vs bilateral hip bone mineral density scans. J Clin Densitom 6:159–162 [DOI] [PubMed] [Google Scholar]
- Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R 2006 Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol 17:3213–3222 [DOI] [PubMed] [Google Scholar]
- Elders PJ, Netelenbos JC, Lips P, van Ginkel FC, Khoe E, Leeuwenkamp OR, Hackeng WH, van der Stelt PF 1991 Calcium supplementation reduces vertebral bone loss in perimenopausal women: a controlled trial in 248 women between 46 and 55 years of age. J Clin Endocrinol Metab 73:533–540 [DOI] [PubMed] [Google Scholar]
- Garnero M, Delmas P 2001 Biochemical markers of bone turnover in osteoporosis. In: Marcus R, Feldman D, Kelsey J, eds. Osteoporosis. San Diego: Academic Press 459–477 [Google Scholar]
- Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD 1996 Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11:1531–1538 [DOI] [PubMed] [Google Scholar]
- Macdonald HM, Black AJ, Aucott L, Duthie G, Duthie S, Sandison R, Hardcastle AC, Lanham New SA, Fraser WD, Reid DM 2008 Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: a randomized controlled trial. Am J Clin Nutr 88:465–474 [DOI] [PubMed] [Google Scholar]
- Sakhaee K, Maalouf NM, Abrams SA, Pak CY 2005 Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab 90:3528–3533 [DOI] [PubMed] [Google Scholar]
- Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris Jr RC 2002 Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr 76:1308–1316 [DOI] [PubMed] [Google Scholar]
- Lemann Jr J, Gray RW, Maierhofer WJ, Cheung HS 1986 The importance of renal net acid excretion as a determinant of fasting urinary calcium excretion. Kidney Int 29:743–746 [DOI] [PubMed] [Google Scholar]