Abstract
Context: Dipeptidyl peptidase 4 (DPP-4) inhibitors are proposed to lower blood glucose in type 2 diabetes mellitus (T2DM) by prolonging the activity of the circulating incretins, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). Consistent with this mechanism of action, DPP-4 inhibitors improve glucose tolerance after meals by increasing insulin and reducing glucagon levels in the plasma. However, DPP-4 inhibitors also reduce fasting blood glucose, an unexpected effect because circulating levels of active GIP and GLP-1 are low in the postabsorptive state.
Objective: The objective of the study was to examine the effects of DPP-4 inhibition on fasting islet function.
Design: We conducted a randomized, double-blind, placebo-controlled trial.
Setting: The study was performed in General Clinical Research Centers at two University Hospitals.
Subjects: Forty-one subjects with T2DM were treated with metformin or diet, having good glycemic control with glycosylated hemoglobin values of 6.2–7.5%.
Intervention: Subjects were treated with vildagliptin (50 mg twice daily) or placebo for 3 months, followed by a 2-wk washout.
Major Outcome Measure: We measured insulin secretion in response to iv glucose and arginine before and after treatment and after drug washout.
Results: There were small and comparable reductions in glycosylated hemoglobin in both groups over 3 months. Vildagliptin increased fasting GLP-1 levels in subjects taking metformin, but not those managed with diet, and raised active GIP levels slightly. DPP-4 inhibitor treatment improved the acute insulin and C-peptide responses to glucose (50 and 100% respectively; P < 0.05) and increased the slope of the C-peptide response to glucose (33%; P = 0.023).
Conclusion: Vildagliptin improves islet function in T2DM under fasting conditions. This suggests that DPP-4 inhibition has metabolic benefits in addition to enhancing meal-induced GLP-1 and GIP activity.
Three months of vildagliptin treatment in subjects with well-controlled type 2 diabetes improves fasting β-cell function, with only minor effects on plasma glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1.
Dipeptidyl peptidase 4 (DPP-4) inhibitors are a new class of drugs now available for the treatment of type 2 diabetes mellitus (T2DM) (1,2). DPP-4 is a widely expressed serine protease with particularly high levels of activity in the liver, kidney, and intestine (3,4). Although there are numerous endogenous peptides that may be metabolized by DPP-4, the two best-established targets of this enzyme are the gastrointestinal hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). GIP and GLP-1, collectively referred to as incretins, are secreted during meal absorption, stimulate insulin secretion, and are essential for normal glucose tolerance (5,6,7). DPP-4 rapidly removes the two N-terminal amino acids from GIP and GLP-1 after their release from intestinal endocrine cells, abolishing their insulinotropic activity (8,9). DPP-4 inhibitors were developed to attenuate this process and increase the concentration of active incretins as a means of improving glucose metabolism (10). Although many DPP-4 inhibitors are being evaluated in clinical trials, vildagliptin and sitagliptin are agents that have been approved for the treatment hyperglycemia in patients with T2DM.
The mechanism(s) by which DPP-4 inhibitors reduce blood glucose is currently the subject of debate. Based on their effect in protecting the bioactive forms of GLP-1 and GIP, it has been generally accepted that vildagliptin and sitagliptin would enhance the actions of the incretins and be most effective after meals. In fact, both vildagliptin and sitagliptin stimulate postprandial insulin secretion relative to plasma glucose and reduce plasma glucagon levels, one of the hallmark effects of GLP-1 (11,12). However, DPP-4 inhibitors also lower fasting glucose levels, to an extent greater than what would be expected from agents working exclusively in the postprandial period (13,14,15,16). Recently, Balas et al. (17) have shown that 100 mg of vildagliptin given to subjects with T2DM before the evening meal increased incretin hormone levels, improved islet function, and suppressed endogenous glucose production overnight, suggesting that the effects of DPP-4 inhibitors may not be limited to the immediate postprandial period. To understand better the actions of DPP-4 inhibitors to regulate basal islet cell function, we studied subjects with T2DM that was well controlled by metformin or diet, treated with vildagliptin or placebo, using iv stimuli in the fasting state.
Subjects and Methods
Subjects
Individuals with established T2DM treated with diet alone or metformin monotherapy for at least 6 months were recruited for participation in this study. All patients were weight stable (<2.5 kg change) in the 6 months before the study, were free of serious concurrent medical conditions, and had good to moderate glycemic control as reflected by glycosylated hemoglobin (HbA1c) values of 6.5–7.5%. Before any study procedures, subjects provided written informed consent. The study was approved by the University of Cincinnati and University of Vermont institutional review boards.
Metabolic testing
Subjects had a screening visit to verify inclusion criteria and consent to the study. They next presented to the Clinical Research Centers (CRC) at the Cincinnati VA Hospital (n = 26) or the University of Vermont (n = 15) in the morning after an overnight fast. After placement of iv catheters, fasting blood samples were obtained, and subjects received an iv bolus of glucose (0.3 g/kg) over 60 sec to initiate an insulin-supplemented frequently sampled iv glucose tolerance test (IVGTT) (9). Twenty minutes after the glucose bolus, the subjects received an infusion of human regular insulin (0.06 mU/kg) over 5 min, and blood was sampled at specified intervals for an additional 220 min. At the conclusion of the 4-h IVGTT, subjects received a progressively increasing infusion of a 20% dextrose solution to increase blood glucose levels to a target of 25 mm over 60 min; blood was sampled at 5- to 10-min intervals, and the glucose infusion was adjusted to raise plasma glucose levels by approximately 0.3 mm/min. At the conclusion of the 60-min glucose ramp, the glucose infusion was continued to maintain blood glucose concentrations of approximately 25 mm, an iv bolus of 5 g arginine was given, and blood was sampled at 2-min intervals for 10 min. All blood samples were collected on ice in tubes with heparin, aprotinin, and diprotin A (18). Plasma was separated by centrifugation and stored for the measurement of glucose, insulin, C-peptide, glucagon, and intact GIP and GLP-1.
Randomization
After initial metabolic testing, subjects were randomized to receive vildagliptin (50 mg by mouth twice daily) or a matching placebo. Subjects were contacted by phone weekly to collect information on blood glucose and adverse effects. They visited the CRC at monthly intervals for collection of blood to monitor drug safety and returned for metabolic testing after 12 wk of treatment; subjects received their assigned medication, vildagliptin or placebo, immediately before testing. Subjects then discontinued their medication and returned for a third set of metabolic tests 2–3 wk later.
Plasma measurements and calculation of parameters of islet hormone secretion
Glucose was measured using a glucose oxidase method. Plasma insulin, glucagon, and C-peptide were measured by RIA using previously reported methods (19). Plasma DPP-4 activity was measured using an enzymatic method as described previously (20). Concentrations of GIP and GLP-1 were measured using specific assays for intact peptides (18). The GIP assay used an antiserum that was specific for the N terminus of GIP, having less than 0.1% cross-reactivity with GIP[3–42], the product of DPP-4. Intra- and interassay coefficients of variation (CVs) were 6 and 10%, respectively. The GLP-1 assay used an antiserum specific for the N terminus of GLP-1[7–36 amide] with less than 0.1% to GLP-1[9–36 amide], the metabolite created by DPP-4. The detection limit of the GLP-1 assay was 2 pm with intra- and interassay CVs of 6 and 16%, respectively.
Fasting values for glucose and hormones were taken as the mean of the three measurements taken before the IVGTT. The acute β-cell response to glucose was computed as the mean increment in plasma insulin [acute insulin response to glucose (AIRg)] or C-peptide [acute C-peptide response to glucose (AC-PRg)] above fasting in the 10 min after the iv glucose bolus. Indices of insulin sensitivity (SI) and glucose effectiveness (SG) were derived using the minimal model of glucose kinetics (21). The disposition index (DI), a measure of insulin secretion corrected for the degree of SI, was calculated as the product of AIRg and SI (21). β-Cell sensitivity to glucose was taken as the slope of plasma insulin or C-peptide vs. plasma glucose during the glucose ramp. The potentiation of glucose-stimulated β-cell secretion by arginine at 25 mm glucose was computed as the average insulin and C-peptide responses to glucose in the 10 min after the arginine bolus (22); this measure (AIRmax and AC-PRmax) has been used as an index of maximal insulin secretory capacity because insulin secretion is not increased further with higher levels of blood glucose. Parameters of α-cell secretion (acute glucagon response to glucose, α-cell sensitivity to glucose and acute glucagon response max) were computed similarly using plasma glucagon levels.
Statistical analysis
Demographic and clinical parameters are presented as mean ± sd and were compared between subjects in the vildagliptin and placebo groups using t-tests. Biochemical and experimental measures are presented as mean ± sem. Fasting levels of glucose and hormones were compared between the two treatment groups using t-tests. Islet hormone secretion and glucose tolerance at baseline and 12 wk were compared among the subjects using a two-way ANOVA for repeated measures, with time and treatment as the parameters. A similar comparison to baseline was made after drug washout.
Results
Twenty-one subjects were randomized to receive vildagliptin and 20 to placebo. One subject from each group withdrew before randomization. One of these subjects (placebo) underwent placement of an implantable cardiac defibrillator for treatment of a preexisting cardiomyopathy after baseline testing. The second subject did not complete baseline testing because of a latex allergy. Data from these two subjects are not included in the analysis. The demographic and clinical characteristics of the subjects in the vildagliptin and placebo arms are presented in Table 1. The groups were comparable in age, duration of diabetes, initial HbA1c, and the percentage taking metformin vs. diet and exercise. There was a tendency for a greater percentage of males, and subjects identifying themselves as Caucasian in the vildagliptin group. The placebo group tended to be heavier than the subjects randomized to vildagliptin (P = 0.06).
Table 1.
Demographic and clinical characteristics of the subjects
| Age (yr) | BMI (kg/m2) | Duration (yr) | HbA1c | Met/Diet | M/F | C/AA | |
|---|---|---|---|---|---|---|---|
| Vildagliptin | 55 ± 7 | 30.6 ± 5.4 | 3.6 ± 2.3 | 6.7 ± 0.4 | 13/7 | 14/6 | 16/4 |
| Placebo | 55 ± 8 | 34.1 ± 5.6 | 3.5 ± 3.1 | 6.8 ± 0.4 | 14/5 | 9/10 | 12/7 |
Duration refers to duration of known diabetes. Met/Diet, Treatment with metformin or diet; M/F, male/female; C/AA, Caucasian/African-American; BMI, body mass index.
Over the 3 months of active treatment, both the vildagliptin and the placebo groups demonstrated significant improvements in HbA1c (6.7 ± 0.4 to 6.3 ± 0.4% and 6.5 ± 0.4 to 6.3 ± 0.4%, respectively; P < 0.001), but the magnitude of change was not significantly different between the two groups (P = 0.11 for the interaction of time and treatment). Vildagliptin was well tolerated by the subjects, and no drug-related adverse events were observed during the course of the study.
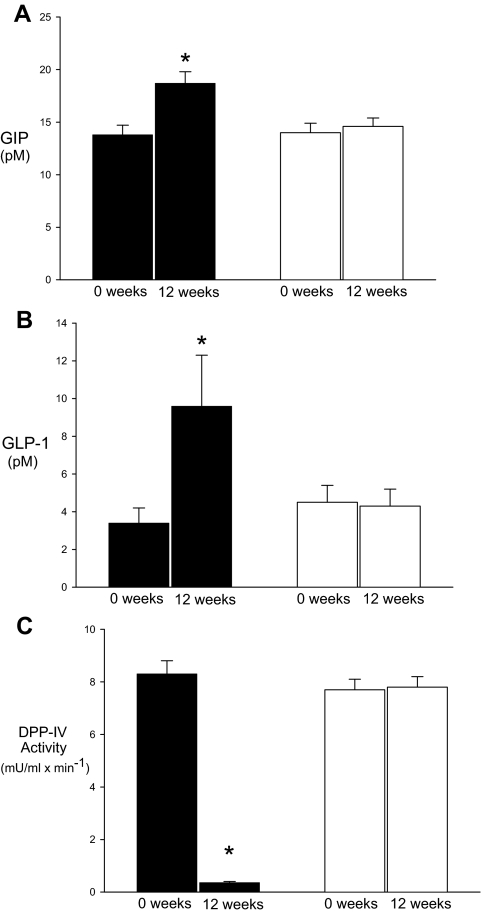
Figure 1 shows plasma DPP-4 activity and GLP-1 and GIP concentrations during the baseline and 12-wk studies in the placebo and vildagliptin groups. As expected, plasma DPP-4 activity at baseline was significantly reduced after 12 wk of vildagliptin (8.7 ± 0.58 and 0.35 ± 0.07 mU/ml × min−1; P < 0.01). Despite the fact that subjects had been fasting for 10–12 h before the study, plasma levels of intact GLP-1 were higher with vildagliptin treatment (baseline, 3.5 ± 0.2 pm; 12 wk, 8.3 ± 1.5 pm; P < 0.05). Consistent with previous findings demonstrating an effect of metformin to raise plasma concentrations of intact GLP-1 (23,24), only subjects taking vildagliptin with metformin had elevated levels of GLP-1 (baseline, 2.1 ± 0.1 pm; 12 wk, 11.2 ± 3.9 pm; P < 0.05). Subjects taking vildagliptin alone did not have significant changes in fasting GLP-1 (baseline, 5.7 ± 2.4 pm; 12 wk, 6.9 ± 3.3 pm; P = 0.44). Fasting levels of intact GIP also increased with vildagliptin between the baseline and 12-wk studies (13.7 ± 1.0 and 18.6 ± 1.1 pm; P < 0.01), but there were no apparent differences between subjects taking metformin and those treated by diet alone. In the placebo-treated subjects, DPP-4 activity between the baseline and 3-month studies did not differ (7.7 ± 0.20 and 7.8 ± 0.26 mU/ml × min). Likewise, plasma levels of intact GLP-1 (4.5 ± 0.5 and 4.4 ± 0.4 pm) and intact GIP (13.9 ± 0.9 and 14.6 ± 0.8 pm) were similar between the baseline and 12-wk studies.
Figure 1.
Fasting levels of intact GIP (A) and GLP-1(B) concentrations, and DPP-4 activity (C) in diabetic subjects treated with vildagliptin (black bars) or placebo (white bars). Measurements are shown from pretreatment (0 wk) and posttreatment (12 wk) studies. *, Posttreatment values that are significantly different from pretreatment results (P < 0.05).
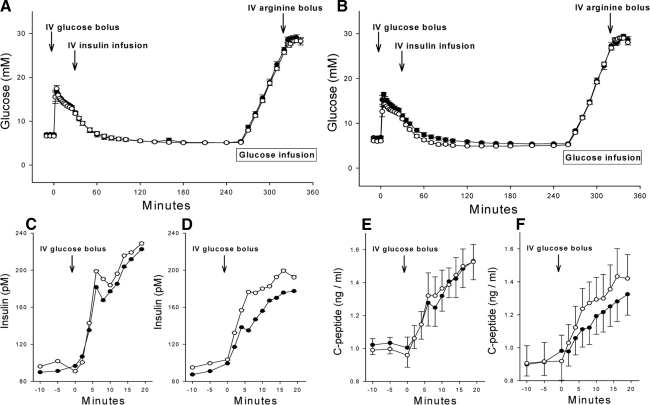
There were no differences in fasting glucose, insulin, C-peptide, or glucagon between the groups after the 3-month treatment period (Table 2). The glucose values during the IVGTT, glucose ramp, and arginine stimulation components of the protocol are shown in Fig. 2 for the vildagliptin and placebo groups before randomization and after 3 months of treatment. The mean glucose levels did not differ during these tests between the treatment groups or before and after randomization; thus, the glycemic stimuli during the islet function tests were equivalent (Fig. 2, A and B). Importantly for the assessment of α- and β-cell sensitivity to glucose, the glucose ramps were comparable among the studies. In the vildagliptin-treated subjects, the slopes of plasma glucose vs. time during the ramp were 0.37 ± 0.01 and 0.36 ± 0.01 mm/min, respectively, for the baseline and 12-wk evaluations. The mean difference in slope between the two studies was 0.003 ± 0.01 mm/min with a CV of 9.0 ± 1.5% for the slopes in the two tests of each subject. The slope of the glucose ramp in the placebo group at baseline and 12 wk was similar, 0.36 ± 0.01 and 0.35 ± 0.01 mm/min, with a mean difference of 0.015 ± 0.01 and a mean CV of 7.5 ± 1.4% between the two studies.
Table 2.
Fasting glucose and hormone concentrations and parameters derived from the IVGTT
| Placebo
|
Vildagliptin
|
P value | |||
|---|---|---|---|---|---|
| Baseline | 12 wk | Baseline | 12 wk | ||
| Fasting | |||||
| Glucose (mm) | 7.0 ± 0.3 | 6.6 ± 0.3 | 6.6 ± 0.3 | 6.0 ± 0.2 | 0.233 |
| Insulin (pm) | 97.1 9.8 | 97.5 ± 10.4 | 102.8 ± 19.8 | 97.9 ± 22.4 | 0.916 |
| C-peptide (ng/ml) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.675 |
| Glucagon (ng/liter) | 20.5 ± 1.6 | 19.2 ± 1.8 | 22.0 ± 1.9 | 21.3 ± 1.8 | 0.484 |
| IVGTT | |||||
| AIRg (pm · min) | 65.6 ± 26.3 | 72.3 ± 32.3 | 35.9 ± 11.4 | 72.1 ± 16.8 | 0.033a |
| AC-PRg (ng/ml · min) | 0.19 ± 0.06 | 0.24 ± 0.09 | 0.12 ± 0.04 | 0.28 ± 0.05 | 0.044a |
| SI (pm · min−1/10−5) | 2.0 ± 0.3 | 1.8 ± 0.3 | 2.0 ± 0.3 | 2.4 ± 0.3 | 0.298 |
| SG (10−2/min) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 0.875 |
| DI | 154 ± 78 | 151 ± 83 | 73 ± 27 | 130 ± 28 | 0.016a |
P values are for change over the course of the study based on significant interaction of time and treatment in two-way ANOVA.
For values <0.05.
Figure 2.
Plasma glucose concentrations in pretreatment (closed circles) and posttreatment (open circles) studies of diabetic subjects receiving placebo (A) or vildagliptin (B). The protocol included a frequently sampled IVGTT from 0–240 min, a graded glucose infusion from 260 to 320 min, and an arginine bolus from 320 to 335 min. The acute insulin (C and D) and C-peptide (E and F) responses to an iv glucose bolus are shown for subjects receiving placebo (C and E) and vildagliptin (D and F).
The parameters of glucose tolerance derived from minimal model analysis of the IVGTT are shown in Table 2. Three months of vildagliptin treatment increased AIRg by 50% (P = 0.033 respectively for the interaction of time and treatment) and AC-PRg by 100% (Fig. 2, C–F; P = 0.044), but did not have a significant effect on SI, SG, and the glucose disappearance constant (kg). The DI, insulin secretion expressed relative to SI, was increased approximately 80% after treatment with vildagliptin (P = 0.016), consistent with a direct effect on β-cell function independent of peripheral insulin demand.
During the glucose ramp, the slope of C-peptide vs. glucose increased significantly in the subjects treated with vildagliptin compared with the placebo, 0.047 ± 0.005 vs. 0.035 ± 0.005 ng/ml × mm−1 (P = 0.023 for interaction of time and treatment); the slope of insulin vs. glucose was also increased, 9.3 ± 1.6 vs. 7.7 ± 1.6 pm/mm, trending toward statistical significance (P = 0.09). In the placebo-treated subjects, the pre- and posttreatment slopes of C-peptide vs. glucose, 0.057 ± 0.006 and 0.054 ± 0.009 ng/ml × mm−1, and insulin vs. glucose, 12.6 ± 3.1 and 12.5 ± 2.2 pm/mm, did not change over the period of observation.
After 12 wk of vildagliptin, there was an approximately 20% increase in AIRmax (846 ± 124 vs. 1010 ± 148 pm × min; P = 0.075 for the interaction of time and treatment) and an approximately 10% increase in AC-PRmax (3.1 ± 1.3 vs. 3.4 ± 1.5 ng/ml × min; P = 0.049). These findings demonstrate a modest effect of chronic DPP-4 inhibition to increase the maximal capacity for insulin release in diabetic subjects. Values of AIRmax and AC-Pmax before and after treatment did not differ in the placebo-treated subjects (1084 ± 638 vs. 1073 ± 589 pm × min, and 3.7 ± 1.6 vs. 3.6 ± 1.6 ng/ml × min, respectively).
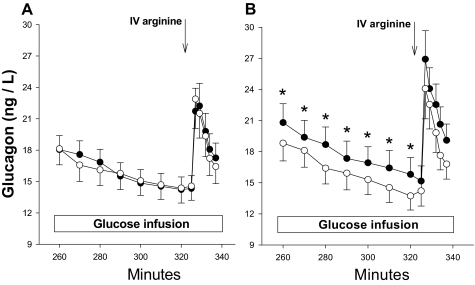
Treatment with vildagliptin did not affect the suppression of glucagon after the iv bolus of glucose, but glucagon values at the conclusion of the 4-h IVGTT were significantly lower than placebo-treated subjects and remained lower throughout the graded glucose infusion (P < 0.001 for an interaction of time and treatment; Fig. 3). The greater suppression of glucagon in the vildagliptin-treated group at 12 wk was maintained during the 60-min glucose ramp although the slope of glucagon vs. glucose was not different in the baseline and 12-wk evaluations. This enhancement of glucagon suppression during graded glucose infusion was distinct from the effect on β-cell secretion, where the change in slope was consistent with an increase in sensitivity to glucose. However, the resetting of the level at which glucagon is maintained at a comparable level of hyperglycemia suggests that chronic DPP-4 inhibition also affects α-cell function.
Figure 3.
Plasma glucagon concentrations from the pretreatment (closed circles) and posttreatment (open circles) studies in diabetic subjects treated with placebo (A) or vildagliptin (B). *, Posttreatment values that are significantly different from pretreatment results (P < 0.05).
To determine whether the effects of vildagliptin on islet cell function were related to plasma levels of GLP-1, measures of β-cell function were compared in the vildagliptin group between subjects treated with metformin, who had higher 12-wk GLP-1 levels, and those on diet therapy, who had no change in GLP-1. Although the number of subjects in each group was relatively small, the effect of treatment was similar (Table 3).
Table 3.
Comparison of changes in β -cell function in 7 diet- and 12 metformin-treated patients taking vildagliptin
| Week 0 | Week 12 | Change | |
|---|---|---|---|
| AIRg (pm × min) | |||
| Metformin | 41 ± 17 | 82 ± 27 | 41 ± 25 |
| Diet | 16 ± 7 | 55 ± 10 | 39 ± 8 |
| DI | |||
| Metformin | 99 ± 41 | 126 ± 41 | 27 ± 24 |
| Diet | 34 ± 18 | 149 ± 37 | 115 ± 28 |
| Slope C-peptide vs. glucose (ng/ml × mm−1) | |||
| Metformin | 0.040 ± 0.007 | 0.051 ± 0.007 | 0.011 ± 0.003 |
| Diet | 0.025 ± 0.003 | 0.040 ± 0.003 | 0.017 ± 0.003 |
| Fasting glucagon (ng/liter) | |||
| Metformin | 21.7 ± 2 | 20.6 ± 1.8 | −1.0 ± 1.1 |
| Diet | 22.4 ± 4 | 22.7 ± 4 | 0.26 ± 1.2 |
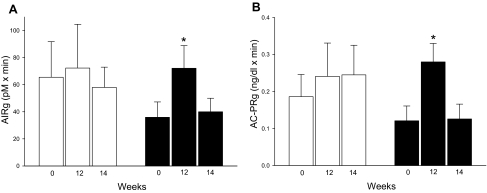
After 2 wk of drug washout, the parameters measured during the IVGTT, the glucose ramp, or the arginine infusion did not differ from the baseline measures in the vildagliptin and placebo subjects (Fig. 4).
Figure 4.
The acute insulin (A) and C-peptide (B) responses to glucose in diabetic subjects treated with placebo (white bars) or vildagliptin (black bars). For each measure, the results are shown for pretreatment (0 wk), posttreatment (12 wk), and after drug washout (14 wk). *, Posttreatment values that are significantly different from pretreatment results (P < 0.05).
Discussion
In this study, we demonstrate that treatment with vildagliptin significantly improves islet function in fasting subjects with well-controlled T2DM. These findings are compatible with the clinical effect of DPP-4 inhibitors to reduce fasting as well as postprandial glucose (13,14,15,16). Although there was an apparent interaction of metformin and vildagliptin to elevate fasting concentrations of intact GLP-1, and for vildagliptin treatment to raise intact GIP, circulating concentrations of the incretins were lower than what has been reported in humans after meals. Based on these findings, it appears that DPP-4 inhibition can promote β-cell function in the presence of low levels of plasma incretins, and this effect seems likely to contribute to the therapeutic response to vildagliptin.
Previous studies of the effects of vildagliptin on β-cell function in diabetic subjects have used the response to meal or glucose ingestion as the means to assess insulin secretion (17,20,25,26,27,28,29). This approach is logical because the effects of DPP-4 inhibition would be expected to be greater after meals when secretion of the incretins is highest. However, despite 2- to 3-fold elevations in intact GLP-1 and GIP and significant reductions in the meal-induced glycemic excursion, postprandial insulin levels in vildagliptin-treated subjects have not been substantially different than those of untreated controls (20,28,29). Nonetheless, it has been argued that similar insulin levels in the presence of improved postprandial glycemia represent improved β-cell function. This contention has been borne out by the use of mathematical modeling to show improvements in several parameters of β-cell function (25,26,27). In the present study, we took an alternative approach, using methods to assess insulin release in the fasting state under controlled levels of blood glucose. Our results demonstrate that treatment with vildagliptin has beneficial effects on several common measures of β-cell secretion in fasting patients with T2DM.
We recruited subjects with well-controlled diabetes for this study to minimize any variability in plasma glucose on study outcomes. All the subjects in the vildagliptin group had some reduction of their HbA1c over the 3 months of treatment, but so did most of the placebo-treated subjects, such that there was no difference in HbA1c between the groups. Although this result differs from effectiveness trials with vildagliptin (13,15,19,27), our study population was distinct in that they had HbA1c levels that were at least 1% lower than previously studied populations, and treatment effects tend to be lower in patients with better glycemic control. For the purposes of this study, having similar fasting glucose and HbA1c levels was important and beneficial because it removed the potentially confounding effect of variable glycemic control as a factor contributing to changes in islet cell function. In other words, the improvements in insulin secretion seen with vildagliptin could not be attributed to a reduction of glucose toxicity.
Despite their good metabolic control and HbA1c values within a relatively narrow range, the diabetic subjects in this study had impaired β-cell function at baseline. Approximately one third of the subjects had a complete absence of first phase insulin release (an AIRg <10 pm × min), and the remainder had responses that were well below what has been reported for nondiabetic subjects (30). Although vildagliptin did not affect fasting levels of insulin or C-peptide, these are relatively insensitive markers of β-cell function (31). However, treatment caused a significant improvement of the acute insulin response to glucose, the sensitivity of insulin release to glucose, and maximal arginine-stimulated β-cell secretion, measures of insulin secretion that are among the classic lesions of T2DM (32,33). Given the matched glucose levels during the iv tests of insulin secretion, these results demonstrate that vildagliptin treatment improves the β-cell response in subjects with T2DM.
Suppression of fasting and meal-stimulated glucagon has been a consistent and robust effect noted in a number of previous reports of vildagliptin treatment (20,28,29). In the present study, effects on glucagon levels were present but subtle. This is likely because the hyperglycemia induced during the IVGTT and glucose ramp tend to dampen α-cell secretion and may minimize further inhibition secondary to DPP-4 inhibition. Indeed, in previous studies infusions of GLP-1 did not lower plasma glucagon beyond the suppressive effects of hyperglycemia (9).
Studies in cultured β-cells and rodent models have demonstrated effects of GLP-1 and GIP signaling on β-cell replication, growth, and apoptosis, with an overall effect of increasing islet mass (6). The potential for drugs that work through the incretin pathways to increase the numbers of β-cells, or at least decrease the rate of their loss, in humans with T2DM has been the source of recent commentary (7,34). We hypothesized that if chronic increased levels of circulating incretins had beneficial effects on β-cell function and/or mass, the effects of vildagliptin would be apparent even after washout of the drug. However, islet hormone secretion after a 2-wk washout of the 3-month treatment did not differ from baseline. The lack of a persistent benefit with vildagliptin has been reported in studies for periods of up to 1 yr that have also demonstrated that any drug effects are dependent on active usage (26,35,36). Interestingly, more recent work suggests that there may be some persistent effects of vildagliptin on β-cell function after 2 yr of treatment (28).
Our findings that DPP-4 inhibitor treatment improved islet function in fasted subjects with T2DM is consistent with the results reported by Utzschneider et al. (36) in subjects with impaired glucose tolerance and by Balas et al. (17) in subjects with T2DM given single doses of vildagliptin. Plasma concentrations of intact GIP were elevated 30–40% in our vildagliptin-treated subjects compared with baseline. This change is very modest compared with the 8- to 10-fold rise in GIP seen after meals, with typical changes ranging from approximately 10 pm fasting to 100 pm postprandially (18). Plasma levels of intact GLP-1 were twice as high in the metformin-treated patients as the group mean at baseline, but the absolute plasma levels did not approach concentrations seen after meals, typically 15–25 pm (20,29). Moreover, changes in the levels of GLP-1 varied among the subjects, in that only seven of the 13 subjects receiving vildagliptin with metformin had 12-wk fasting GLP-1 levels greater than 4 pm, the average pretrial basal value. We cannot exclude the possibility that the changes in insulin secretion seen in the subjects treated with DPP-4 inhibitor were due to small increases in plasma GIP and GLP-1. However, it is also possible that the effects of drug treatment were independent of plasma incretins. Speaking to this latter possibility is the lack of a difference in β-cell responses among vildagliptin-treated subjects with and without increases in plasma GLP-1 from baseline to 12 wk. This inference raises questions as to the mechanism by which DPP-4 inhibitors mediate their effects on the β-cell. One possibility is that protection from DPP-4 metabolism enhances GLP-1 activation of afferent nerves in the intestinal mucosa or portal vein (9,37), actions that are not reflected by systemic changes in plasma GLP-1 levels. Another possibility is that vildagliptin protects islet peptides such as pituitary adenylate cyclase activating polypeptide (PACAP) or glucagon, also metabolized by DPP-4 (38), but which stimulate insulin secretion by paracrine action in the islet.
There are several important limitations to this study. Because subjects were studied only after 3 months of treatment, and not after the first dose of vildagliptin, we cannot distinguish the effects of acute from chronic treatment. However, Balas et al. (17) previously demonstrated that a single dose of vildagliptin improved insulin secretion after a meal in subjects with poorly controlled T2DM. Therefore it seems most likely that the improvements we noted with iv stimulated β-cell function were due to the dose of vildagliptin taken immediately before the study. Although it is also plausible that the effects of vildagliptin were caused by some cumulative action of the drug over 3 months, the rapid washout of these effects speaks against this. Our data also do not address the relative impact of vildagliptin-mediated islet hormone secretion on blood glucose levels because the glycemic levels were matched by design. Finally, because this study was limited to subjects with well-controlled diabetes, it is not possible to conclude that the effects of DPP-4 inhibition on fasting islet function seen in this group can be generalized to patients with more severe impairments of glucose control, although vildagliptin treatment in clinical trials has been similarly efficacious in patients with both poor and moderately controlled levels of glycemia (13,19).
In summary, this study demonstrates enhancement of insulin secretion in subjects with T2DM treated with vildagliptin. Vildagliptin caused small elevations in fasting plasma GLP-1 in subjects taking metformin but not those treated with diet alone. However, the β-cell effects of vildagliptin cannot be conclusively attributed to acute elevations of plasma GLP-1 because they were observed in almost all of the treated subjects regardless of GLP-1 concentration. These findings are consistent with the effect of vildagliptin to lower fasting, as well as postprandial, glucose levels in patients with T2DM. Moreover, they suggest that there are additional mechanisms of action for DPP-4 inhibitors beyond enhancing postprandial incretin effects.
Acknowledgments
We acknowledge the nursing staff at the Cincinnati Veterans Affairs Medical Center, Colleen Rogge, Fay Hailes, Brenda Westrup, Cathy Bailey, and Beverly Smith, for their care of the research subjects and expert assistance with the studies. We thank Drs. Carolyn Deacon and Jens Holst for their expert analysis of plasma GIP and GLP-1. We also acknowledge Brian Conklin and Johnson & Johnson for providing glucometers and test strips.
Footnotes
Conflict of Interest: D.A.D. is a consultant for Merck and Novartis and has received research grants from Amylin and Lilly. R.E.P. is a consultant for Novartis, Takeda, Novo Nordisk, GlaxoSmithKline (GSK), Roche, and Boehringer-Ingelheim, and has received research grants or participated in clinical trials sponsored by Novartis, Merck, Takeda, Novo Nordisk, GSK, Sanofi-Aventis, and Lilly. B.E.D. was a consultant for Novartis. The other authors have no conflicts to disclose.
This work was prospectively entered in the Registry of Clinical Trials as no. NCT00351585.
Support for this work included a research grant from Novartis Pharmaceuticals, Public Health Service (PHS) Grant DK57900 (to D.A.D.), and PHS grants to the General Clinical Research Centers at the University of Vermont College of Medicine (M01RR00109) and the Cincinnati Children’s Hospital and Cincinnati VA Medical Center (M01RR0808), and the Geriatric Research Education and Clinical Center at the Baltimore VA Medical Center.
First Published Online October 28, 2008
Abbreviations: AC-PRg, Acute C-peptide response to glucose; AIRg, acute insulin response to glucose; CV, coefficient of variation; DI, disposition index; DPP-4, dipeptidyl peptidase; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; HbA1c, glycosylated hemoglobin; IVGTT, iv glucose tolerance test; SG, glucose effectiveness; SI, insulin sensitivity; T2DM, type 2 diabetes mellitus.
References
- Ahren B, Schmitz O 2004 GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res 36:867–876 [DOI] [PubMed] [Google Scholar]
- Pratley RE, Salsali A 2007 Inhibition of DPP-4: a new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin 23:919–931 [DOI] [PubMed] [Google Scholar]
- Deacon CF 2004 Circulation and degradation of GIP and GLP-1. Horm Metab Res 36:761–765 [DOI] [PubMed] [Google Scholar]
- Mentlein R 1999 Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides. Regul Pept 85:9–24 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ 2007 Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- Drucker DJ 2006 The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- Salehi M, Aulinger BA, D'Alessio DA 2008 Targeting β-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins. Endocr Rev 29:367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, McIntosh CH, Pederson RA 1995 Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136:3585–3596 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Paty BW, Fuller BD, Prigeon RL, D'Alessio DA 2003 Effects of GLP-1-(7–36)NH2, GLP-1-(7–37), and GLP-1- (9–36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab 88:1772–1779 [DOI] [PubMed] [Google Scholar]
- Ahren B 2007 Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care 30:1344–1350 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA 1996 Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care 19:580–586 [DOI] [PubMed] [Google Scholar]
- Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Goke B 2006 Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahren B, Gomis R, Standl E, Mills D, Schweizer A 2004 Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care 27:2874–2880 [DOI] [PubMed] [Google Scholar]
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE 2006 Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 29:2632–2637 [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Schweizer A, Mills D, Dejager S 2007 Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract 76:132–138 [DOI] [PubMed] [Google Scholar]
- Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D 2006 Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res 38:423–428 [DOI] [PubMed] [Google Scholar]
- Balas B, Baig MR, Watson C, Dunning BE, Ligueros-Saylan M, Wang Y, He YL, Darland C, Holst JJ, Deacon CF, Cusi K, Mari A, Foley JE, DeFronzo RA 2007 The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab 92:1249–1255 [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ 2001 Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50:609–613 [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A 2007 Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Care 30:217–223 [DOI] [PubMed] [Google Scholar]
- Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A 2004 Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 89:2078–2084 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider YZ, Bowden CR, Cobelli C 1979 Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667—E677 [DOI] [PubMed] [Google Scholar]
- Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr 1984 Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannucci E, Tesi F, Bardini G, Ognibene A, Petracca MG, Ciani S, Pezzatini A, Brogi M, Dicembrini I, Cremasco F, Messeri G, Rotella CM 2004 Effects of metformin on glucagon-like peptide-1 levels in obese patients with and without type 2 diabetes. Diabetes Nutr Metab 17:336–342 [PubMed] [Google Scholar]
- Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Messeri G, Rotella CM 2001 Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care 24:489–494 [DOI] [PubMed] [Google Scholar]
- Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, Deacon CF, Holst JJ, Foley JE 2005 Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed β-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab 90:4888–4894 [DOI] [PubMed] [Google Scholar]
- Mari A, Scherbaum WA, Nilsson PM, Lalanne G, Schweizer A, Dunning BE, Jauffret S, Foley JE 2008 Characterization of the influence of vildagliptin on model-assessed cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab 93:103–109 [DOI] [PubMed] [Google Scholar]
- Pratley RE, Schweizer A, Rosenstock J, Foley JE, Banerji MA, Pi-Sunyer FX, Mills D, Dejager S 2008 Robust improvements in fasting and prandial measures of β-cell function with vildagliptin in drug-naive patients: analysis of pooled vildagliptin monotherapy database. Diabetes Obes Metab 10:931–938 [DOI] [PubMed] [Google Scholar]
- Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Wang Y, Dunning BE, Foley JE 18 March 2008 Evidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab 10.1111/j.1463–1326.2008.00875.x [DOI] [PubMed] [Google Scholar]
- Azuma K, Radikova Z, Mancino J, Toledo FG, Thomas E, Kangani C, Dalla Man C, Cobelli C, Holst JJ, Deacon CF, He Y, Ligueros-Saylan M, Serra D, Foley JE, Kelley DE 2008 Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab 93:459–464 [DOI] [PubMed] [Google Scholar]
- Quddusi S, Vahl TP, Hanson K, Prigeon RL, D'Alessio DA 2003 Differential effects of acute and extended infusions of glucagon-like peptide-1 on first- and second-phase insulin secretion in diabetic and nondiabetic humans. Diabetes Care 26:791–798 [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Mari A 2004 β-Cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia 47:943–956 [DOI] [PubMed] [Google Scholar]
- Kahn SE 2001 Clinical review 135: the importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86:4047–4058 [DOI] [PubMed] [Google Scholar]
- Weyer C, Bogardus C, Mott DM, Pratley RE 1999 The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ 2006 Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu Rev Med 57:265–281 [DOI] [PubMed] [Google Scholar]
- Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Jauffret S, Foley JE 2008 Efficacy and tolerability of vildagliptin in drug-naive patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab 10:675–682 [DOI] [PubMed] [Google Scholar]
- Utzschneider KM, Tong J, Montgomery B, Udayasankar J, Gerchman F, Marcovina SM, Watson CE, Ligueros-Saylan MA, Foley JE, Holst JJ, Deacon CF, Kahn SE 2008 The dipeptidyl peptidase-4 inhibitor vildagliptin improves β-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care 31:108–113 [DOI] [PubMed] [Google Scholar]
- Burcelin R, Da Costa A, Drucker D, Thorens B 2001 Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50:1720–1728 [DOI] [PubMed] [Google Scholar]
- Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, Sinha Roy R 2003 The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38). J Biol Chem 278:22418–22423 [DOI] [PubMed] [Google Scholar]