Abstract
Context: The prevalence of type 2 diabetes (T2D), particularly among young adults, has been rising steadily during the past 2 decades. T2D, especially in its early-onset subtype, is under genetic control. TRIB3 inhibits insulin-stimulated Akt phosphorylation and subsequent insulin action. A TRIB3 gain-of-function polymorphism, Q84R (rs2295490), impairs insulin signaling.
Objective: The objective of the study was to verify the association of TRIB3 Q84R with: 1) T2D, either subtyped or not according to age at diagnosis (early-onset, <45 yr, or ≥ 45 yr); 2) insulin secretion and sensitivity in nondiabetic individuals; or 3) in vitro insulin secretion from isolated human islets.
Design: Four different case-control samples comprising a total of 5469 whites were examined. Insulinogenic and insulin sensitivity indexes and their interplay (disposition index) were assessed in 645 nondiabetic individuals at oral glucose tolerance test, glucose (16.7 mmol/liter)-induced in vitro insulin secretion was assessed in islets isolated from 54 nondiabetic donors.
Results: In the whole sample, the R84 variant was nominally associated with T2D (odds ratio 1.17, 95% confidence interval 1.00–1.36, P = 0.04). When stratifying according to age of diabetes onset, R84 carriers had an increased risk of early-onset T2D (odds ratio 1.32, 95% confidence interval 1.10–1.58, P = 0.002). Among 645 nondiabetic subjects, R84 carriers had higher glucose levels (P = 0.005) and lower insulinogenic (P = 0.03) and disposition index (P = 0.02) during the oral glucose tolerance test. R84 islets were more likely to display relatively low glucose-stimulated insulin release (P = 0.04).
Conclusions: The TRIB3 R84 variant is associated with early-onset T2D in whites. Alteration in the insulin secretion/insulin sensitivity interplay appears to underlie this association.
Tribbles homolog 3 (TRIB3) Q84R polymorphism is associated with type 2 diabetes as a likely consequence of altered interplay between insulin secretion and action.
Type 2 diabetes (T2D) is a typical complex disease that recognizes both genetic and environmental factors (1,2). Its prevalence, especially among young adults, is dramatically increasing to the point of assuming the characteristics of an epidemic and therefore posing severe problems to health care systems (3,4,5). T2D, especially in its early-onset subtype, is under genetic control (6,7,8).
Insulin resistance and defective insulin secretion are major determinants of T2D (9). During the past few years, several genes coding for inhibitors of insulin signaling and action have been reported to modulate the risk of insulin resistance and related metabolic abnormalities. One of these inhibitors is TRIB3, a mammalian tribbles homolog, which binds to Akt (10,11,12) and inhibits insulin-stimulated Akt phosphorylation and subsequent activation (10,11,12). Whereas TRIB3 knockout mice do not manifest major defects in glucoregulation (13), mice overexpressing TRIB3 have increased liver glucose output and become hyperglycemic (10). We recently described a TRIB3 missense polymorphism, Q84R (i.e. glutamine to arginine at position 84, rs2295490) that is associated with in vivo insulin resistance and related clinical outcomes (11,14). In addition, cultured human hepatoma cells transfected with TRIB3 R84 cDNA showed a reduced insulin-stimulated Ser473 Akt phosphorylation in respect to cells transfected with Q84 TRIB3, thus indicating that the R84 is a functional variant with a stronger inhibitory activity on insulin signaling compared with the wild-type Q84 variant (11). Similar results also have been recently reported in human umbilical vein endothelial cells naturally carrying the R84 variant (12). Of particularly importance in the specific context of T2D are preliminary results suggesting that the R84 variant may also play a deleterious role in pancreatic β-cells by inducing apoptosis and inhibiting cell proliferation and insulin secretion (15). Taken together, these data strongly point to the TRIB3 R84 variant as a plausible candidate in the modulation of risk in T2D.
Our aim was to investigate the role of TRIB3 R84 variant on the risk of T2D. Unfortunately, the Q84R polymorphism was not among the single-nucleotide polymorphisms (SNPs) examined by the large genome-wide association studies of T2D that were recently published and are publicly available (16). In addition, none of the SNPs in the Affymetrix and Illumina arrays used by these previous studies was in sufficiently strong linkage disequilibrium with Q84R (i.e. r2 > 0.4) to function as a good proxy of this variant. To detect small genetic effects as the ones thus far reported for T2D (i.e. allelic odds ratios < 1.5) (2), we established the GENIUS (the Genetics of type 2 diabetes in Italy and United States) consortium combining the resources available at four different research centers (for statistical power see Subjects and Methods). Because several studies suggest that patients with early-onset T2D have a stronger genetic load compared with T2D with an older onset (6,7,8), we tested for association with T2D also according to its age at diagnosis. In line with previous epidemiological studies, an age at diagnosis younger than 45 yr was used as a cutoff to define early-onset T2D (4,5). Of note, 45 yr is also the age the American Diabetes Association currently recommends for T2D screening in adults (17).
Subjects and Methods
Study subjects
The GENIUS consortium involves four centers from Italy and the United States (San Giovanni Rotondo and Catanzaro, Italy, Boston, MA, and Dallas, TX), which established a coordinated research program to identify genes and molecular mechanisms underlying the pathophysiology of T2D and related abnormalities. The four principal investigators signed a formal collaborative agreement establishing a decision-making steering committee and procedures for coordinating and sharing participation in analyses and publications. The steering committee meets at least once a year.
The study was approved by local institutional review boards and was conducted according to the Helsinki Declaration. Written informed consent was obtained from each participant.
Case-control for T2D
Study subjects were all whites (as per self-report) and were recruited at: the Scientific Institute Casa Sollievo della Sofferenza in San Giovanni Rotondo (SGR), Italy, at the University Magna Graecia of Catanzaro, Italy, at the Joslin Diabetes Center, Boston (MA), and at the University of Texas Southwestern, Dallas (TX). Controls were 2451 unrelated nondiabetic subjects (fasting plasma glucose <6.9 mmol/liter, not taking medications known to interfere with glucose and lipid metabolism). Cases were 3018 unrelated patients with T2D defined according to the 2003 American Diabetes Association criteria. Among diabetic cases, 1041 had early-onset T2D (i.e. diagnosed before age 45 yr), and 1977 had T2D diagnosed at 45 yr or older. Clinical characteristics of cases and controls contributed by each center are shown in Table 1. The age at diagnosis of T2D was based on self-reported information.
Table 1.
Clinical features in nondiabetic control subjects and patients with type 2 diabetes
| Boston | Catanzaro | Dallas | SGR | Whole sample | |
|---|---|---|---|---|---|
| Controls | |||||
| Number | 356 | 1218 | 272 | 605 | 2451 |
| Gender (M/F) | 185/171 | 518/700 | 113/159 | 225/380 | 1041/1410 |
| Age (yr) | 57.0 ± 17.7 | 48.7 ± 14.4 | 38.4 ± 15.7 | 36.5 ± 11.8 | 45.8 ± 16.1 |
| BMI (kg/m2) | 26.4 ± 4.6 (n = 346) | 29.7 ± 6.4 (n = 1185) | 26.0 ± 6.1 (n = 258) | 25.5 ± 4.6 (n = 603) | 27.7 ± 6.0 (n = 2392) |
| All patients with T2D | |||||
| Number | 1222 | 500 | 76 | 1220 | 3018 |
| Gender (M/F) | 726/496 | 244/256 | 53/23 | 635/585 | 1658/1360 |
| Age (yr) | 61.1 ± 8.4 | 62.6 ± 10.1 | 56.6 ± 9.7 | 61.7 ± 9.4 | 61.5 ± 9.2 |
| BMI (kg/m2) | 32.4 ± 6.3 (n = 1191) | 30.4 ± 5.6 (n = 498) | 34.3 ± 8.9 (n = 71) | 30.9 ± 5.5 (n = 1182) | 31.5 ± 6.0 (n = 2942) |
| Patients with T2D diagnosed at age < 45 yr | |||||
| Number | 492 | 138 | 19 | 392 | 1041 |
| Gender (M/F) | 272/220 | 70/68 | 14/5 | 212/180 | 568/473 |
| Age (yr) | 55.6 ± 8.2 | 56.1 ± 9.9 | 49.0 ± 12.2 | 55.8 ± 9.4 | 55.6 ± 9.0 |
| BMI (kg/m2) | 33.8 ± 7.1 (n = 463) | 30.4 ± 5.5 (n = 136) | 35.8 ± 10.1 (n = 19) | 31.0 ± 5.6 (n = 380) | 32.2 ± 6.6 (n = 998) |
| Patients with T2D diagnosed at age ≥ 45 yr | |||||
| Number | 730 | 362 | 57 | 828 | 1977 |
| Gender (M/F) | 454/276 | 174/188 | 39/18 | 423/405 | 1090/887 |
| Age (yr) | 64.8 ± 6.2 | 65.1 ± 9.1 | 59.1 ± 7.3 | 64.6 ± 7.9 | 64.6 ± 7.6 |
| BMI (kg/m2) | 31.5 ± 5.6 (n = 728) | 30.4 ± 5.7 (n = 362) | 33.7 ± 8.4 (n = 52) | 30.8 ± 5.4 (n = 802) | 31.1 ± 5.7 (n = 1944) |
Number of individuals whose BMI was recorded is shown in parentheses. M/F, Males/females.
Study of insulin secretion and sensitivity
A total of 645 individuals (243 males/402 females) who were part of the controls recruited in Catanzaro, Italy, underwent an oral glucose tolerance test (OGTT; 75 g) with glucose and insulin levels measured before and after glucose load.
Insulinogenic index (IGI) was calculated according to the formula: insulin level (milliunits per liter) at 30 min − insulin levels (milliunits per liter) at 0 min/glucose level (millimoles per liter) at 30 min − glucose level (millimoles per liter) at 0 min (18). The Matsuda index [insulin sensitivity index (ISI)] was calculated as follows: 10,000/square root of [fasting glucose (millimoles per liter) × fasting insulin (milliunits per liter)] × [mean glucose × mean insulin during OGTT] (19). Of note, potential violations of the hyperbolic shape (IGI × ISI = k, where k is a constant value) were investigated through a goodness-of-fit test for the following nonlinear model: IGI = k/ISI. Data obtained (r2 = 0.36, P < 0.0001) clearly indicate that the hyperbolic shape was confirmed.
The disposition index was calculated as follows: insulinogenic index × Matsuda index (19).
Islet studies
Isolated human islets were prepared by collagenase digestion and density gradient purification (20) from 54 nondiabetic multiorgan donors. Islet secretory function was evaluated as insulin release under the presence of 3.3 or 16.7 mmol/liter glucose as previously described (20). Stimulation index was calculated by dividing insulin release at 16.7 mmol/liter glucose over insulin release at 3.3 mmol/liter glucose (20).
Genotyping
DNA was extracted from whole blood by standard methods. Genotyping was performed by restriction fragment length polymorphism as previously described (11). Failure rate of genotyping in each sample was less than 2%.
Genotyping quality was checked by directly sequencing 10% of randomly selected samples. The agreement rate of resequenced samples was greater than 99%. The proportion of the Q84R genotypes does not significantly deviate from Hardy-Weinberg equilibrium in both controls and cases of each individual sample as well as in the pooled analysis (n = 15 comparisons, P > 0.001).
Statistical analysis
Values for continuous variables are expressed as mean ± sd. Comparisons between groups were conducted by unpaired student’s t or Mann-Whitney (as appropriate) tests and among groups by one-way ANOVA or, after adjustment for covariates, by analysis of covariance. Hardy-Weinberg equilibrium and the association between genotype and categorical variables were examined by χ2 tests. Multivariate logistic regression analysis was used to model the effect of the polymorphism on dichotomous outcomes and to estimate odd ratios and 95% confidence interval. Because no evidence of significant heterogeneity was detected among the four samples with respect to the effect of TRIB3 R84 variant on risk of T2D (P for gene × sample interaction = 0.74 in the whole sample, P = 0.15 for early-T2D, and P = 0.44 for T2D diagnosed at older age), data from the four different centers were analyzed together after adjusting for place of recruitment. P < 0.05 was considered significant. In this combined analysis, we had 80% power (α = 0.05) to detect an association with an odd ratio as small as 1.25 and 1.20 under a dominant model with early-onset T2D and with T2D diagnosed at 45 yr or older, respectively. All analyses were performed by Statistical package (version 14; SPSS, Chicago IL).
Results
Association with T2D
The proportion of individuals carrying the RR genotype was very low (≤3% in all samples). Thus, to increase statistical power, as a first analytical attempt, data from QR and RR individuals were pooled and analyzed together, according to a dominant genetic model. In the whole sample, carriers of the R84 variant were more prevalent among patients with T2D than nondiabetic subjects [odds ratio (OR) 1.17, 95% confidence interval (CI) 1.00–1.36, P = 0.04)] (Table 2). A similar trend, although not reaching statistical significance, was observed with the additive (OR 1.11, 95% CI 0.97–1.27, P = 0.12) but not with the recessive (OR 0.90, 95% CI 0.59–1.37, P = 0.61) genetic model. According to the dominant model, after stratifying for age of diabetes onset, TRIB3 R84 carriers had a 32% increased risk of early-onset T2D (OR 1.32, 95% CI 1.10–1.58, P = 0.002) (Table 2). This association was not affected by adjustment for either body mass index (BMI; OR 1.34, 95% CI 1.10–1.62) or multiple comparisons (data not shown). By contrast, no significant association was observed with T2D diagnosed at older age (Table 2). We then analyzed the role of TRIB3 R84 variant on the age of diabetes onset among diabetic patients. Although no association was observed with age at onset considered as a continuous variable (P = 0.7, data not shown), diabetic individuals carrying the R84 were significantly more likely to have developed diabetes before rather than after age 45 (OR 1.19, 95% CI 1.01–1.40, P = 0.038).
Table 2.
TRIB3 Q84R genotype and the risk of type 2 diabetes
| Sample | Genotype
|
Dominant genetic model
|
||||
|---|---|---|---|---|---|---|
| QQ (%) | QR (%) | RR (%) | QR+RR (%) | OR (95% CI) | P | |
| Boston | ||||||
| Controls (n = 356) | 262 (73.6) | 90 (25.3) | 4 (1.1) | 94 (26.4) | ||
| All T2D (n = 1222) | 844 (69.1) | 341(27.9) | 37(3.0) | 378 (30.9) | 1.26 (0.96–1.64) | 0.09 |
| T2D (<45 yr) (n = 492) | 332 (67.5) | 145 (29.5) | 15 (3.0) | 160 (32.5) | 1.35 (1.00–1.82) | 0.05 |
| T2D (≥45 yr) (n = 730) | 512 (70.1) | 196 (26.8) | 22 (3.1) | 218 (29.9) | 1.17 (0.88–1.56) | 0.28 |
| Catanzaro | ||||||
| Controls (n = 1218) | 895 (73.5) | 284 (23.3) | 39 (3.2) | 323 (26.5) | ||
| All T2D (n = 500) | 351 (70.2) | 139 (27.8) | 10 (2.0) | 149 (29.8) | 1.17 (0.91–1.52) | 0.22 |
| T2D (<45 yr) (n = 138) | 85 (61.6) | 50 (36.2) | 3 (2.2) | 53 (38.4) | 1.71 (1.18–2.46) | 0.004 |
| T2D (≥45 yr) (n = 362) | 266 (73.5) | 89 (24.6) | 7 (1.9) | 96 (26.5) | 0.99 (0.76–1.29) | 0.95 |
| Dallas | ||||||
| Controls (n = 272) | 199 (73.2) | 68 (25) | 5 (1.8) | 73 (26.8) | ||
| All T2D (n = 76) | 52 (68.4) | 24 (31.6) | 0 | 24 (31.6) | 1.78 (0.61–2.29) | 0.63 |
| T2D (<45 yr) (n = 19) | 16 (84.2) | 3 (15.8) | 0 | 3 (15.8) | 0.72 (0.19–2.66) | 0.62 |
| T2D (≥45 yr) (n = 57) | 36 (63.2) | 21 (36.8) | 0 | 21 (36.8) | 1.74 (0.94–3.23) | 0.08 |
| SGR | ||||||
| Controls (n = 605) | 430 (71.0) | 157 (26.0) | 18 (3.0) | 175 (29.0) | ||
| All T2D (n = 1220) | 856 (70.2) | 333 (27.3) | 31 (2.5) | 364 (29.8) | 1.11 (0.77–1.60) | 0.56 |
| T2D (<45 yr) (n = 392) | 268 (68.4) | 113 (28.8) | 11 (2.8) | 124 (31.6) | 1.16 (0.88–1.54) | 0.29 |
| T2D (≥45 yr) (n = 828) | 588 (71.0) | 220 (26.6) | 20 (2.4) | 240 (29.0) | 1.02 (0.81–1.29) | 0.85 |
| Whole sample | ||||||
| Controls (n = 2451) | 1786 (72.9) | 599 (24.4) | 66 (2.7) | 665 (27.1) | ||
| All T2D (n = 3018) | 2103 (69.7) | 837 (27.7) | 78 (2.6) | 915 (30.3) | 1.17 (1.00–1.36) | 0.04a |
| T2D (<45 yr) (n = 1041) | 701 (67.3) | 311 (29.9) | 29 (2.8) | 340 (32.7) | 1.32 (1.10–1.58) | 0.002a |
| T2D (≥45 yr) (n = 1977) | 1402 (70.9) | 526 (26.6) | 49 (2.5) | 575 (29.1) | 1.07 (0.93–1.24) | 0.34a |
Data were analyzed after adjusting for age and gender in ″all T2D″ and for gender, after stratifying for age at diagnosis. P values in bold are significant.
In the whole sample, data were analyzed after also adjusting for place of recruitment.
Association with intermediate traits related to glucose homeostasis
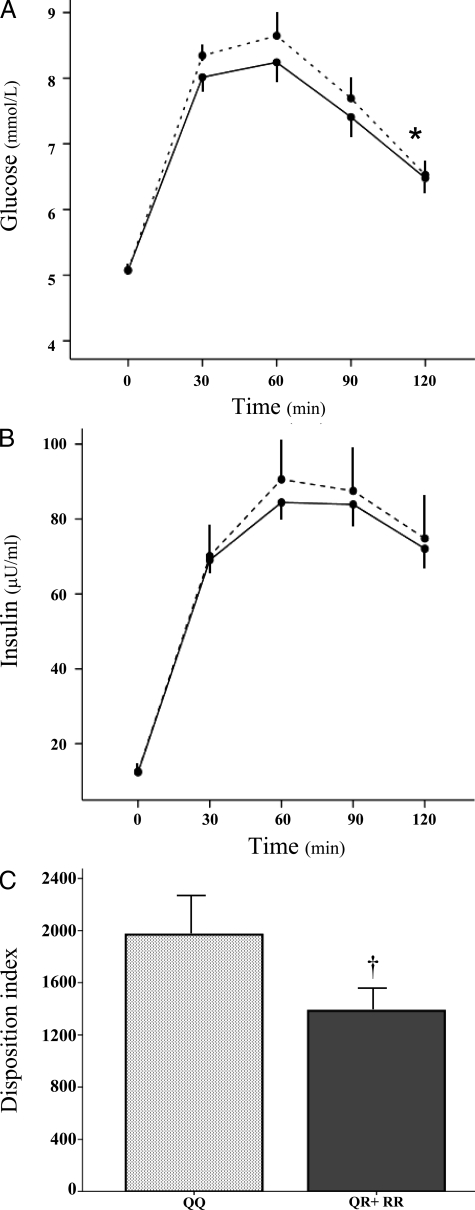
To obtain mechanistic insights into the association between TRIB3 R84 variant and early-onset T2D, we measured early insulin secretion (by the insulinogenic index), insulin sensitivity (by the Matsuda index), and the interplay between these two major determinants of glucose homeostasis (by the disposition index) during OGTT in 645 of the 1218 control individuals from Catanzaro. Because of the small number of RR individuals (n = 18) and the a priori hypothesis derived from the dominant effect observed in the case-control study for early-onset T2D, R84 homozygotes and heterozygotes were considered together as R84 carriers. Clinical features of study subjects according to their R84 carrier status are shown in Table 3. At each time point of the OGTT, mean glucose levels were higher in R84 carriers than QQ individuals (P = 0.005, by two-way ANOVA) (Fig. 1A). A similar trend was also observed for mean insulin levels (Fig. 1B), although it did not reach statistical significance (P = 0.28). The insulinogenic index was lower in the R84 carriers than the QQ group (20.2 ± 18.3 vs. 24.5 ± 28.7, P = 0.03), whereas the Matsuda index was similar in the two genotype groups (82.1 ± 50.1 vs. 84.7 ± 51.4, P = 0.56). Most importantly, the disposition index, which takes into account both insulin secretion and insulin sensitivity, was significantly reduced in R84 carriers compared with QQ individuals (P = 0.02) (Fig. 1C).
Table 3.
Features of the 645 nondiabetic subjects who underwent OGTT, according to TRIB3 Q84R genotype
| Genotype
|
||
|---|---|---|
| QR+RR | ||
| Number of subjects (M/F) | 478 (174/304) | 167 (69/98) |
| Age (yr) | 44.5 ± 14.5 | 45.0 ± 13.5 |
| BMI (kg/m2) | 30.3 ± 7.4 | 30.4 ± 6.4 |
| Fasting glucose (mmol/liter) | 5.1 ± 0.7 | 5.1 ± 0.6 |
| Subjects with IGR (%) | 36.8 | 34.7 |
Data are means ± sd. M/F, Males/females; IGR, impaired glucose regulation (i.e. impaired fasting glucose and/or impaired glucose tolerance).
Figure 1.
Glucose, insulin, and disposition index values at OGTT. Plasma glucose (A) and serum insulin (B) levels during OGTT in QQ (solid lines) and QR+RR (dotted lines) individuals are shown. Disposition index values (calculated as insulinogenic index × insulin sensitivity index) are shown in QQ (light gray bar) and QR+RR (dark gray bar) individuals (C). *, P = 0.005 by two-way ANOVA test; †, P = 0.02 by Student’s t test.
Human islets study
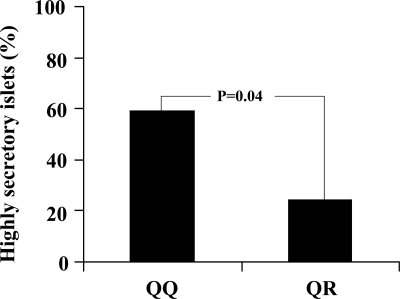
To further evaluate the effects of R84 variant on insulin secretion, we studied isolated human islets from 54 nondiabetic multiorgan donors (age: 56.8 ± 17.9 yr; sex: 29 males/25 females; BMI: 24.5 ± 3.5 kg/m2), prepared as previously described (20). Basal (3.3 mmol/liter glucose) and glucose-stimulated insulin release (16.7 mmol/liter glucose) were 0.038 ± 0.01 and 0.11 ± 0.07 μU/islet · min in 42 QQ and 0.036 ± 0.02 and 0.092 ± 0.100 SU/islet · min in 12 QR islets, respectively. The stimulation index (glucose stimulated over basal insulin release) was 2.81 ± 1.36 in QQ islets compared with 2.22 ± 0.99 in QR islets (P = 0.13). The proportion of islets displaying a relatively high insulin secretion (i.e. a stimulation index above the median value of the entire sample: 2.3) was significantly lower among those carrying the QR genotype (P = 0.04) (Fig. 2).
Figure 2.
Insulin secretion in isolated human islets according to TRIB3 Q84R genotype. Proportion of highly secretory islets from 42 QQ and 12 QR nondiabetic donors. Islets were defined as highly secretory as indicated by stimulation index (calculated by dividing insulin release at 16.7 mmol/liter glucose over insulin release at 3.3 mmol/liter glucose) above 2.3, the median value of the whole sample.
Discussion
TRIB3 binds to Akt (10) and inhibits (10,11,12) insulin-stimulated Akt phosphorylation and activation, a key step in insulin signaling and action. We recently described a TRIB3 missense polymorphism, Q84R, that is associated with in vivo insulin resistance and related traits (11,14). In cultured cells, the R84 variant is a stronger inhibitor of insulin-stimulated Ser473 Akt phosphorylation compared with the wild-type Q84 variant (11,12,15). Thus, this TRIB3 missense polymorphism is a plausible candidate of T2D. We now present evidence that carriers of the R84 variant have a 17% increased risk of T2D. After stratifying for age of disease onset, carriers of the R84 variant have a 32% increased risk of early-onset T2D, whereas no evidence of association with T2D diagnosed at older age is observed. This finding reinforces the hypothesis of a stronger genetic component of the early-onset form of T2D (6,7,8). Of note, a significant association with early-onset T2D was observed in two different samples from the consortium, indicating that this finding is a replicable one. As for any epidemiological study based on self-reported data, the ascertainment of true age of diabetes onset in a cross-sectional study is not straightforward. Although, in this scenario, using a cutoff may help improve measurement precision in that one can, at least, be sure about the ascertainment of young cases, caution has to be used about the association with early-onset T2D, observed at post hoc analysis, until further studies replicate this finding. A preliminary study in 1461 individuals from Poland and a subset of the Boston sample recently reported that R84 homozygotes have an increased risk of T2D (OR 3.4 and 95% CI 1.2–6.8) (15). Unfortunately, the Q84R polymorphism was not among the SNPs examined by the recently published large genome-wide association studies of T2D (16). In addition, none of the SNPs used by these previous studies can function as a good proxy of the Q84R polymorphism.
Population stratification is an unlikely explanation of our findings. First, the association was observed among Italian subjects from Southern Italy, a population that shows limited substructure in a principal component analysis of Genome Wide Association Studies data (21). Second, the frequency of the R allele in the Italian subjects of our study (0.152) was virtually identical with that in the North European subjects of the HapMap CEPH sample of Utah residents with European ancestry panel (0.147) and in a panel of 150 subjects from the United Kingdom that we expressly typed to study ethnic variation of this allele (0.142). Thus, this polymorphism does not show the north-south gradient that accounts for most of the substructure observed among U.S. subjects of European descent (22).
It is well recognized that a reduction in both insulin sensitivity and secretion is needed for T2D to develop (9). To obtain mechanistic insights about the role of the TRIB3 R84 variant in the risk modulation of T2D, early insulin secretion, insulin sensitivity, and the disposition index were measured in nondiabetic individuals undergoing OGTT. Glucose and insulin profiles after oral glucose load (Fig. 1, A and B) clearly suggest that R84 carriers are characterized by both relative insulin deficiency (not enough insulin is secreted, especially in the first 30 min, to maintain normoglycemia) and a certain degree of insulin resistance (glucose level are higher in R84 carriers than noncarriers despite slightly higher insulin levels during the entire period of OGTT). Indeed, R84 carriers showed a reduced early phase insulin secretion and, most importantly, disposition index (Fig. 1C), which takes into account both early insulin secretion and insulin sensitivity and has been recently reported to be the best predictor of future T2D (18,23). The insulin sensitivity Matsuda index was not reduced in R84 carriers, probably because insulin resistance inferred from increased endogenous insulin levels (as done with this index) may be underestimated in individuals with a reduced insulin secretory capacity such as R84 carriers seem to be. This interpretation is supported by the fact that R84 carriers were found to be insulin resistant compared with QQ individuals when studied by euglycemic, hyperinsulinemic clamp, a method that does not rely on endogenous insulin production for measuring insulin sensitivity (11). Overall, our data from the OGTT in nondiabetic individuals suggest that abnormalities in the interplay between insulin secretion and insulin sensitivity are likely to play a major role in the increased risk of T2D displayed by R84 carriers. A direct deleterious role of the R84 variant in altering insulin secretion is also suggested by in vitro data showing that human isolated islets from TRIB3 R84 carrying donors tend to have reduced glucose-stimulated insulin release compared with islets from QQ donors.
In functional studies on transfected cells, an inhibitory role of R84 variant on insulin-stimulated Akt phosphorylation has been observed in vitro both in cells from peripheral insulin target tissues (11,12) and insulin secreting β-cells (15), making plausible a direct role of this variant on both in vivo insulin sensitivity (11) and β-cell function and survival (15). A similar deleterious role on both in vitro (20) and in vivo insulin secretion (24) has been reported for the G972R polymorphism of IRS-1, which has been clearly associated with insulin resistance (25,26,27), although its role on the risk of T2D has been questioned by large studies (28,29). Similarly, evidence of a role on in vivo insulin secretion has been reported for the K121Q polymorphism of ENPP1 (30), which has been associated with insulin resistance (31) and T2D (32) Taken together, present data on TRIB3 Q84R and previous reports on IRS1 G972R (24,25,26) and ENPP1 K121 Q (30,31,32) are consistent with a pathogenic role of altered insulin signaling in inducing both peripheral insulin resistance and impaired insulin secretion (33).
In conclusion, our data suggest that the TRIB3 R84 variant is associated with a 32% increased risk of early-onset T2D among whites. Considering that this association was observed in two independent samples from the GENIUS Consortium, that the R84 variant has been associated with T2D in a previous study (15), that no evidence of population stratification due to the north-south gradient described to account for most of substructure observed among subjects of European descent was observed, that functional in vitro data on isolated human islets tend to support the association we describe, and that previous in vivo and in vitro studies are consistent with a deleterious role of this variant on both peripheral insulin target tissues (11,12) and β-cell function (15), the possibility of a false-positive finding seems unlikely. On the other hand, most of our results are based on post hoc analyses; in addition, the caveat of the multiple comparisons we performed also has to be taken into consideration.
Therefore, given the problematic history of association studies, we believe it would be prudent to replicate these findings in additional large studies and reach overall genome-wide significance before TRIB3 can be conclusively considered as a T2D gene.
Footnotes
This work was partly supported by the Italian Ministry of Health (Ricerca Corrente 2006 and 2008, to S.P.); European Community’s FP6 EUGENE 2 (Grant LSHM-CT-2004-512013, to G.S); National Institutes of Health Grants HL073168, DK055523, and DK036836 (to A.D. and the Genetics Core of the Diabetes and Endocrinology Research Center at the Joslin Diabetes Center) and DK072158 (to N.A.); and The European Union (Integrated Project EuroDia LSHM-CT-2006-518153, to P.M.).
Author Disclosure Summary: S.P., D.S., M.C., Y.-Y.Z., E.M., S.D.G., F.P., R.L., C.P., F.A., P.M., B.D., N.A., A.D., G.S., and V.T. have nothing to declare.
First Published Online November 4, 2008
Abbreviations: BMI, body mass index; CI, confidence interval; GENIUS, Genetics of type 2 diabetes in Italy and United States; IGI, insulinogenic index; ISI, insulin sensitivity index; OGTT, oral glucose tolerance test; OR, odds ratio; SGR, San Giovanni Rotondo; SNP, single-nucleotide polymorphism; T2D, type 2 diabetes.
References
- Hansen L, Pedersen O 2005 Genetics of type 2 diabetes mellitus: status and perspectives. Diabetes Obes Metab 7:122–135 [DOI] [PubMed] [Google Scholar]
- Frayling TM 2007 Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 8:657–662 [DOI] [PubMed] [Google Scholar]
- Bloomgarden ZT 2004 Type 2 diabetes in the young: the evolving epidemic. Diabetes Care 27:998–1010 [DOI] [PubMed] [Google Scholar]
- Hillier TA, Pedula KL 2001 Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care 24:1522–1527 [DOI] [PubMed] [Google Scholar]
- Hillier TA, Pedula KL 2003 Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care 26:2999–3005 [DOI] [PubMed] [Google Scholar]
- Weijnen CF, Rich SS, Meigs JB, Krolewski AS, Warram JH 2002 Risk of diabetes in siblings of index cases with type 2 diabetes: implications for genetic studies. Diabet Med 19:41–50 [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Kammerer CM, Reinhart LJ, Stern MP 1994 NIDDM in Mexican-American families. Heterogeneity by age of onset. Diabetes Care 17:567–573 [DOI] [PubMed] [Google Scholar]
- Frayling TM, Wiltshire S, Hitman GA, Walker M, Levy JC, Sampson M, Groves CJ, Menzel S, McCarthy MI, Hattersley AT 2003 Young-onset type 2 diabetes families are the major contributors to genetic loci in the Diabetes UK Warren 2 genome scan and identify putative novel loci on chromosomes 8q21, 21q22, and 22q11. Diabetes 52:1857–1863 [DOI] [PubMed] [Google Scholar]
- Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR 1992 Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 17:925–929 [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, Montminy M 2003 TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 6:1574–1577 [DOI] [PubMed] [Google Scholar]
- Prudente S, Hribal ML, Flex E, Turchi F, Morini E, De Cosmo S, Bacci S, Tassi V, Cardellini M, Lauro R, Sesti G, Dallapiccola B, Trischitta V 2005 The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes 54:2807–2811 [DOI] [PubMed] [Google Scholar]
- Andreozzi F, Formoso G, Prudente S, Hribal ML, Pandolfi A, Bellacchio E, Di Silvestre S, Trischitta V, Consoli A, Sesti G 2008 TRIB3 R84 variant is associated with impaired insulin mediated nitric oxide production in human endothelial cells. Arterioscler Thromb Vasc Biol 28:1355–1360 [DOI] [PubMed] [Google Scholar]
- Okamoto H, Latres E, Liu R, Thabet K, Murphy A, Valenzeula D, Yancopoulos GD, Stitt TN, Glass DJ, Sleeman MW 2007 Genetic deletion of Trb3, the mammalian Drosophila tribbles homolog, displays normal hepatic insulin signaling and glucose homeostasis. Diabetes 56:1350–1356 [DOI] [PubMed] [Google Scholar]
- De Cosmo S, Prudente S, Andreozzi F, Morini E, Rauseo A, Scarpelli D, Zhang YY, Xu R, Perticone F, Dallapiccola B, Sesti G, Doria A, Trischitta V 2007 Glutamine to arginine substitution at amino acid 84 of mammalian tribbles homolog TRIB3 and CKD in whites with type 2 diabetes. Am J Kidney Dis 50:688–689 [DOI] [PubMed] [Google Scholar]
- Wee Liew C, Bochenski J, Hu J, Krowleski AS, Kulkarni RN Effects of TRB3 on β-cell insulin exocytosis. Proc 68th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 2008, 186-OR (Abstract) [Google Scholar]
- Zeggini E, Scott LJ, Saxena R, Voight BF, for the Diabetes Genetics Replication and Meta-analysis DIAGRAM Consortium 2008 Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Associations 2004 Screening for diabetes. Diabetes Care 27(Suppl 1):S11–S14 [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Williams K, De Fronzo RA, Stern M 2007 What is the best predictor of future type 2 diabetes? Diabetes Care 30:1544–1548 [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 9:1462–1470 [DOI] [PubMed] [Google Scholar]
- Marchetti P, Lupi R, Federici M, Marselli L, Masini M, Boggi U, Del Guerra S, Patane G, Piro S, Anello M, Bergamini E, Purrello F, Lauro R, Mosca F, Sesti G, Del Prato S 2002 Insulin secretory function is impaired in isolated human islets carrying the Gly(972)->Arg IRS-1 polymorphism. Diabetes 51:1419–1424 [DOI] [PubMed] [Google Scholar]
- Tian C, Plenge RM, Ransom M, Lee A, Villoslada P, Selmi C, Klareskog L, Pulver AE, Qi L, Gregersen PK, Seldin MF 2008 Analysis and application of European genetic substructure using 300 K SNP information. PLoS Genet 1:e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Butler J, Patterson N, Capelli C, Pascali VL, Scarnicci F, Ruiz-Linares A, Groop L, Saetta AA, Korkolopoulou P, Seligsohn U, Waliszewska A, Schirmer C, Ardlie K, Ramos A, Nemesh J, Arbeitman L, Goldstein DB, Reich D, Hirschhorn JN 2008 Discerning the ancestry of European Americans in genetic association studies. PLoS Genet 1:e236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissén M, Isomaa B, Forsen B, Homström N, Saloranta C, Taskinen MR, Groop L, Tuomi T; Botnia Study Group 2005 Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 54:166–174 [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Fritsche A, Volk A, Stefan N, Madaus A, Maerker E, Teigeler A, Koch M, Machicao F, Haring H 2001 The Gly972Arg polymorphism in the insulin receptor substrate-1 gene contributes to the variation in insulin secretion in normal glucose-tolerant humans. Diabetes 50:882–885 [DOI] [PubMed] [Google Scholar]
- Clausen JO, Hansen T, Bjorbaek C, Echwald SM, Urhammer SA, Rasmussen S, Andersen CB, Hansen L, Almind K, Pedersen O 1995 Insulin resistance: interactions between obesity and a common variant of insulin receptor substrate-1. Lancet 346:397–402 [DOI] [PubMed] [Google Scholar]
- Marini MA, Frontoni S, Mineo D, Bracaglia D, Cardellini M, De Nicolais P, Baroni A, D'Alfonso R, Perna M, Lauro D, Federici M, Gambardella S, Lauro R, Sesti G 2003 The Arg972 variant in insulin receptor substrate-1 is associated with an atherogenic profile in offspring of type 2 diabetic patients. J Clin Endocrinol Metab 88:3368–3371 [DOI] [PubMed] [Google Scholar]
- Almind K, Inoue G, Pedersen O, Kahn CR 1996 A common amino acid polymorphism in insulin receptor substrate-1 causes impaired insulin signaling. Evidence from transfection studies. J Clin Invest 11:2569–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggini E, Parkinson J, Halford S, Owen KR, Frayling TM, Walker M, Hitman GA, Levy JC, Sampson MJ, Feskens EJ, Hattersley AT, McCarthy MI 2004 Association studies of insulin receptor substrate 1 gene (IRS1) variants in type 2 diabetes samples enriched for family history and early age of onset. Diabetes 53:3319–3322 [DOI] [PubMed] [Google Scholar]
- Florez JC, Sjögren M, Burtt N, Orho-Melander M, Schayer S, Sun M, Almgren P, Lindblad U, Tuomi T, Gaudet D, Hudson TJ, Daly MJ, Ardlie KG, Hirschhorn JN, Altshuler D, Groop L 2004 Association testing in 9,000 people fails to confirm the association of the insulin receptor substrate-1 G972R polymorphism with type 2 diabetes. Diabetes 53:3313–3318 [DOI] [PubMed] [Google Scholar]
- Baratta R, Rossetti P, Prudente S, Barbetti F, Sudano D, Nigro A, Farina MG, Pellegrini F, Trischitta V, Frittitta L 2008 Role of the ENPP1 K121Q polymorphism on glucose homeostasis. Diabetes 57:3360–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci S, De Cosmo S, Prudente S, Trischitta V 2007 ENPP1 gene, insulin resistance and related clinical outcomes. Curr Opin Clin Nutr Metab Care 4:403–409 [DOI] [PubMed] [Google Scholar]
- McAteer JB, Prudente S, Bacci S, Lyon HN, Hirschhorn JN, Trischitta V, Florez JC; ENPP1 Consortium 2008 The ENPP1 K121Q polymorphism is associated with type 2 diabetes in European populations: evidence from an updated meta-analysis in 42,042 subjects. Diabetes 4:1125–1130 [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR 2006 From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol 68:123–158 [DOI] [PubMed] [Google Scholar]