Summary
We assessed the effect of herpes simplex virus type 2 (HSV-2) acquisition on the plasma HIV RNA and CD4 cell levels among individuals with primary HIV infection using a retrospective cohort analysis. We studied 119 adult, antiretroviral-naive, recently HIV-infected men with a negative HSV-2–specific enzyme immunoassay (EIA) result at enrollment. HSV-2 acquisition was determined by seroconversion on HSV-2 EIA, confirmed by Western blot analysis. Ten men acquired HSV-2 infection a median of 1.3 years after HIV infection (HSV-2 incidence rate of 7.4 per 100 person-years of follow-up). The median time of follow-up after acquiring HSV-2 infection was 303 days. All men except 1 were asymptomatic during HSV-2 acquisition, and only 1 HSV-2 seroconverter, who was asymptomatic, had a transient increase in blood HIV load (0.5 log10 copies/mL over 11 days). The HSV-2 incidence rate was high in our cohort of recently HIV-infected individuals; however, HSV-2 acquisition did not significantly change the plasma HIV dynamics and CD4 cell levels.
Keywords: HIV RNA, incident herpes simplex virus-2, viral dynamics
Herpes simplex virus type 2 (HSV-2) shedding in the genital tract has been correlated with increased plasma HIV RNA levels in people coinfected with HSV-2 and HIV.1,2 This elevation of plasma HIV RNA levels may persist for up to 6 weeks, independent of symptoms.2 The highest frequency of HSV-2 genital shedding, symptomatic and asymptomatic, occurs during the first year after HSV-2 acquisition.3,4 Acute HSV-2 infection induces a strong local inflammatory reaction that recruits macrophages and activated natural killer cells to the genital tract.5 These cells release cytokines (eg, interferon-α, tumor necrosis factor-γ, RANTES) that may inhibit HSV-2 replication but also recruit lymphocytes that are permissive for HIV replication.5,6 This could explain the observations that higher titers of HIV in genital mucosa of HSV-2–infected men were associated with an increase in plasma HIV RNA levels.7 Based on these findings, we hypothesized that the more frequent episodes of HSV-2 genital shedding during the first year after HSV-2 infection would produce a sustained increase in plasma HIV RNA levels. We therefore conducted a longitudinal study to investigate the effect of HSV-2 acquisition on plasma HIV RNA and CD4 cell count levels.
POPULATION AND METHODS
Subjects for this study were those who were enrolled into the University of California, San Diego (UCSD) site of the Acute Infection and Early Disease Research Program (AIEDRP) network between June 1996 and June 2005, as reported elsewhere.8 In a previous study, we investigated changes in plasma HIV RNA dynamics and CD4 cell counts between the AIEDRP participants who were HSV-2–seropositive or –seronegative at the time of enrollment. The present study consisted only of AIEDRP participants who tested negative, using an HSV-2–specific enzyme immunoassay (EIA), at enrollment. We then identified those who acquired HSV-2 infection during follow-up and assessed whether HSV-2 acquisition subsequently changed their pattern of HIV blood plasma dynamics and CD4 cell counts.
We defined recent acquisition of HSV-2 infection by HSV-2 EIA seroconversion confirmed by an HSV-2 Western blot test (University of Washington, Seattle, WA) or HerpeSelect Western blot IgG test (Focus Technologies, Cypress, CA). First, we tested the last available serum samples from each eligible participant with a specific HSV-2 EIA. Individuals with a positive HSV-2 EIA result and a negative or indeterminate HSV-2 Western blot result were considered HSV-2 seroconverters only if at least 2 Western blot IgG test results in consecutive time point samples were positive. Second, once individuals with new HSV-2 seroconversion were identified, we proceeded with serial retrospective HSV-2 EIA and HSV-2 Western blot IgG testing on stored blood samples until we identified a negative HSV-2 EIA and Western blot IgG time point sample. The outcomes of the study were the calculation of (1) the rate of HSV-2 seroconversion in the cohort and (2) the change of plasma HIV RNA levels after HSV-2 seroconversion.
The estimated date of HSV-2 infection was assumed to be the midpoint between the last positive HSV-2 EIA/Western blot IgG result and the first negative HSV-2 EIA/Western blot IgG result in the retrospective testing of collected samples. Participants who acquired HSV-2 infection were included in the analysis until they initiated antiretroviral therapy or were lost to follow-up. We generated plots of HIV viral dynamics in each HSV-2 seroconverter with intervals around the time of the estimated date of HSV-2 acquisition. The first point of the interval represents the date 2 weeks before the last negative HSV-2 EIA result so as to account for the potential window of HSV-2 EIA seroconversion.9 The second point represents the date of the first positive HSV-2 EIA result. Categoric variables were analyzed using the Fisher exact test. Bivariate analyses were performed before and after HSV-2 seroconversion using Wilcoxon signed-rank tests. Based on a paired t test, assuming that the SD in viral loads in the absence of any HSV-2 effect is 0.5 log10 copies/mL, we estimated that with a minimum of 9 HSV-2 seroconverters, we would attain 75% power to detect a mean difference in plasma HIV RNA levels of 0.5 log10 copies/mL, assuming a 2-sided α-value of 0.05; this increases to 88% power for 0.6 log10 copies/mL and to 95% power for a 0.7-log10 copies/mL difference. Hence, even using simple statistical tests, a 2-sided P value, and a subset of the data (only the viral loads before and after HSV-2 infection), our study would be adequately powered to detect clinically relevant differences in viral load of the same order of magnitude as natural variability in viral load at “setpoint.” To control for the dynamic nature of plasma HIV RNA levels, we performed longitudinal analysis of HIV RNA levels using a generalized additive model (GAM), which used patient-specific intercept terms and nonparametric smooths (modeled using thin-plate regression splines) to capture possibly nonlinear patterns of viral dynamics. The model generates “effective degrees of freedom” (EDF) for each patient (estimated using generalized cross-validation), which indicates how much the curve deviates from a straight line (EDF = 1), with high values of EDF indicating extremely “wiggly” lines. This model included a covariate indicating the first time point at which the individual was HSV-2–seropositive, allowing us to test whether HSV-2 serostatus was associated with a “jump” in viral load, using the viral load data from each individual as his own control. We used the statistical software R, version 2.4.1 (R Foundation for Statistical Computing, Vienna, Austria);10 all reported P values are 2-sided.
RESULTS
As of June 2005, 172 subjects in the UCSD AIEDRP cohort had a negative HSV-2 EIA result at baseline. Twelve started antiretrovirals within 1 month of study enrollment, and 41 subjects were excluded from the analysis because they did not have follow-up serum samples. Those excluded were less likely to be Native American individuals and had a lower median CD4 cell count at enrollment than those included in the final cohort, but there were no significant differences in terms of plasma HIV RNA levels and stage (acute or early) of primary HIV at enrollment between those who were included and those who were excluded (Table 1). Our final study cohort consisted of 119 men (see Table 1). More than 90% reported sex with men as their primary HIV risk factor. Ten subjects seroconverted for HSV-2 infection during the period of follow-up. Five were seropositive for HSV-1 infection at the time of AIEDRP enrollment. Only 1 individual acquired HSV-1 and HSV-2 infection during the follow-up period. The median age at HSV-2 seroconversion was 36 years (range: 32 to 45 years). We found no significant difference in the risk of HSV-2 acquisition attributable to race or ethnicity (P = 0.46, Fisher exact test). Among the HSV-2 seroconverters, the median CD4 count and plasma HIV RNA level at enrollment were 545 cells/mm3 (range: 314 to 1193 cells/mm3) and 4.30 log10 copies/mL (range: 2.89 to 6.86 log10 copies/mL), respectively. All men except 1 were asymptomatic for HSV-2 infection. The single possibly symptomatic participant reported mild dysuria within 4 weeks of the estimated date of HSV-2 infection, although the clinical assessment at that time did not attribute the symptoms to HSV-2 infection.
TABLE 1.
Baseline Demographic Characteristics, CD4 Cell Count, and Blood HIV Load of Excluded Subjects and Studied Participants
| Variable | HIV+/HSV-2−, Excluded n = 53 |
HIV+/HSV-2−, Cohort n = 119 |
HIV+/ HSV-2 Nonseroconveters n = 109 |
HIV+/HSV-2 Seroconverters n = 10 |
|
|---|---|---|---|---|---|
| Age, median (range), y | 36 (23 to 54) | 35 (21 to 56) | 34 (21 to 56) | 36 (31 to 45) | |
| Race, no. (%) subjects | |||||
| White | 34 (83)* | 91 (76) | 83 (76) | 8 (80) | |
| Native American | 0 (0) | 15 (13) | 14 (13) | 1 (10) | |
| Black | 2 (5) | 9 (7) | 9 (8) | 0 (0) | |
| Asian | 3 (7) | 3 (3) | 2 (2) | 1 (10) | |
| Unknown | 2 (5) | 1 (1) | 1 (1) | 0 (0) | |
| Ethnicity, no. (%) subjects | |||||
| Not Hispanic | 16 (39) | 44 (37) | 39 (36) | 5 (50) | |
| Hispanic | 8 (20) | 24 (20) | 22 (20) | 2 (20) | |
| Unknown | 17 (41) | 51 (43) | 48 (44) | 3 (30) | |
| HIV stage, no. (%) subjects | |||||
| Acute | 14 (34) | 30 (25) | 28 (26) | 2 (20) | |
| Early | 27 (66) | 89 (75) | 81 (74) | 8 (80) | |
| CD4 T-cell count, median (range), cells/mm3 | 431 (123 to 932)† | 520 (107 to 1380) | 510 (107 to 1380) | 545 (314 to 1193) | |
| HIV load, median (range), log10 copies/mL | 5.20 (2.88 to 7.28) | 5.04 (1.70 to 7.19) | 5.04 (1.70 to 7.19) | 4.30 (2.89 to 6.86) | |
P ≤ 0.015 in comparison to the HIV-1+/HSV-2− cohort studied using the Fisher exact test.
P ≤ 0.018 in comparison to the HIV-1+/HSV-2− cohort studied using the Wilcoxon 2-sample test.
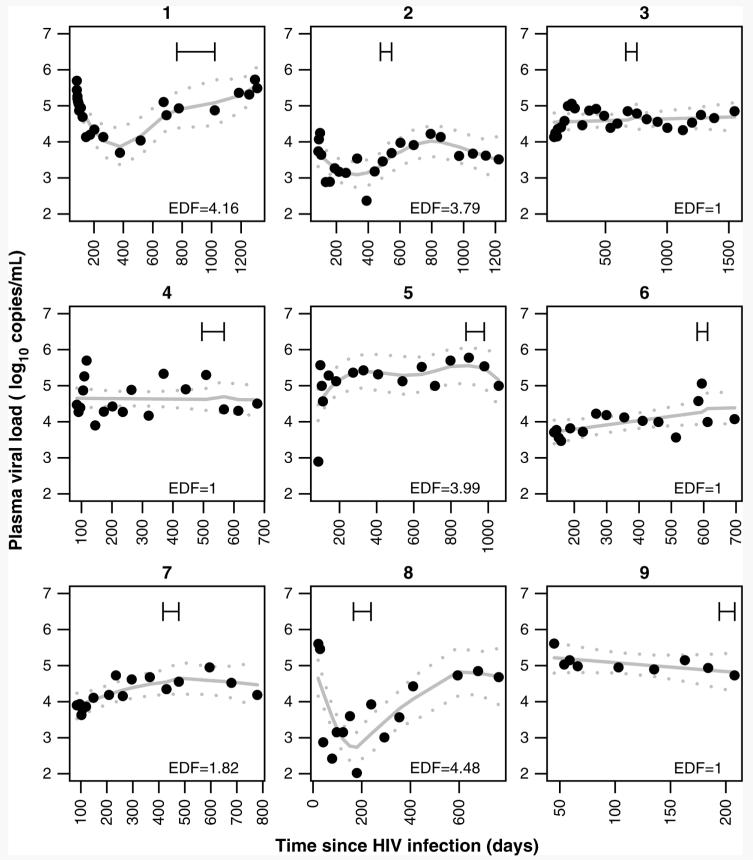
The time at risk of the 10 HSV-2 seroconverters was 136.28 person-years. The overall HSV-2 incidence rate was 7.3 cases per 100 years of follow-up (95% confidence interval [CI]: 3.5 to 12.6). The median time of the estimated date of HSV-2 acquisition occurred 471 days after the estimated date of HIV-1 infection (range: 111 to 846 days). Five of 10 HSV-2 seroconverters started antiretroviral therapy a median of 279 days (range: 80 to 777 days) after the estimated date of HSV-2 infection. None of the 10 HSV-2 seroconverters received therapeutic or prophylactic HSV-2 therapy. One subject acquired HSV-2 infection around the time when antiretroviral therapy was initiated and was excluded from the analysis of HIV dynamics because the effect of HSV-2 infection on plasma HIV RNA levels could not be ascertained in the setting of antiretroviral therapy. The median period of follow-up was 779 days after the estimated date of HIV infection. The median plasma HIV RNA level increased 0.17 log10 copies/mL (range of plasma HIV RNA level change: −1.58 to 0.49 log10 copies/mL) between the sample time points before and after (range: 12 to 49 days) the estimated date of HSV-2 acquisition, which was not a statistically significant change (P = 0.57, Wilcoxon signed-rank test). The median CD4 count after HSV-2 acquisition was 44 cells/mm3 lower (range of CD4 count difference: −182 to 220 cells/mm3) than before HSV-2 infection; this was also not a statistically significant change (P = 0.36, Wilcoxon signed-rank test). As shown in Figure 1, only 2 patients showed transient changes in their plasma HIV RNA levels around the estimated interval of HSV-2 acquisition. One showed a decline in his plasma HIV RNA levels (1.6 log10 copies/mL over 14 days; see Figure 1, panel 8), and 1 had an increase in his plasma HIV RNA levels (0.5 log10 copies/mL over 11 days; see Figure 1, panel 6) after HSV-2 acquisition. The plasma HIV RNA level for this person (see Fig. 1, panel 6) was already increasing based on the nearest plasma HIV RNA level obtained before the window for HSV-2 acquisition, and he was asymptomatic during this time. To test for overall changes in the plasma HIV RNA levels in any of the HSV-2 seroconverters, we fitted a generalized additive model to the HIV RNA levels from each individual, allowing subject-specific curves and a jump in HIV RNA levels at the first time point at which the subject was HSV-2–seropositive. This model generates estimates of the EDF, which indicate how much the curves deviate from a straight line (EDF = 1), with higher values of EDF corresponding to more “dynamic” patterns. Overall, the model fitted well (adjusted R2 = 68%), capturing nonlinear dynamics in patients 1, 2, 5, 7, and 8. The mean change in plasma HIV RNA level associated with HSV-2 serostatus was small (+0.08 log10 copies/mL), however, and not statistically significant from 0 (P = 0.64). Similar results were obtained if we considered the time point just before HSV-2 seropositivity (ie, in case the actual time of HSV-2 acquisition was earlier than estimated based on intervals of HSV-2 testing [+0.14 log10 copies/mL; P = 0.37]). Hence, after controlling for the dynamic nature of plasma HIV RNA levels during acute and early HIV infection, the change in plasma HIV RNA level associated with HSV-2 seroconversion was even less dramatic than when we considered only 2 plasma HIV RNA measurements per patient before and after HSV-2 seroconversion.
FIGURE 1.
Longitudinal plots describing HIV viral dynamics of 9 individuals who acquired HSV-2 infection. The intervals represent the calculated interval of time during which individuals could have acquired HSV-2 infection. The first point of the CI represents the date 2 weeks before the last negative HSV-2 EIA result so as to account for the potential seroconversion window time of HSV-2 EIA. The second point represents the date of the first positive HSV-2 EIA result. Note that the subject in panel 6 is the man who had a transient finding. The solid lines represent the fit of a generalized additive model, in which a nonparametric smooth was fitted to each individual, including a covariate indicating the first time point at which the individual was HSV-2–seropositive. Dotted lines represent 95% CIs. EDF for each curve are indicated, with higher values of EDF corresponding to more nonlinear curves.
DISCUSSION
To our knowledge, this study represents the largest cohort (N = 10) with the longest follow-up of HIV-infected men who acquired HSV-2 infection. We found a 4-fold higher incidence rate for HSV-2 than previously reported among newly HIV diagnosed subjects, and participants in our cohort acquired HSV-2 infection in less time after their estimated date of HIV acquisition than in an ethnically diverse cohort of HIV-infected persons (median: 1.3 vs. 4 years).11
The hypothesis that HSV-2 replication induces increased plasma HIV RNA levels12 was investigated in individuals with recently acquired HSV-2 infection, because the most frequent episodes of HSV-2 genital shedding are expected in this group.4 We conducted individual-level analyses to ascertain the clinical significance of recently acquired HSV-2 infection per participant and found that there was no sustained effect on plasma HIV RNA levels in any individual after almost 1 year of follow-up from the estimated date of HSV-2 acquisition (see Fig. 1). Although 10 seroincident HSV-2 cases is a relatively small number, our study had 75% power to detect a 0.5-log10 difference in plasma HIV RNA levels.
The strengths of our study are the strict serologic methods used to identify individuals with new HSV-2 infection and the use of a large and well-characterized primary infection cohort in which multiple blood samples and HIV load measurements (N = 163) were available over a long period. A major limitation of our study is that we did not measure HSV-2 genital shedding or reactivation; therefore, increases of plasma HIV RNA levels associated with HSV-2 genital shedding may have been missed because of an inability to evaluate associations of HIV levels during concurrent HSV-2 reactivation, when increased HIV levels would be more likely to occur. If this phenomenon was only transient after HSV-2 acquisition, the HSV-2 effect on plasma HIV RNA levels likely would not be clinically significant, as has been proposed among a female cohort.13 Additionally, individuals who enrolled in the AIEDRP cohort but who were excluded from these analyses had lower CD4 cell counts than those included in the final cohort (medians: CD4 = 420 vs. CD4 = 520 cells/mm3, respectively); it is unknown if this difference may have a role in susceptibility to acquire HSV-2 infection.14 As with other geographically limited studies, our results must be tempered by the fact that they are based on a local cohort of men in San Diego and our results cannot be necessarily generalized to other groups.
In summary, HSV-2 acquisition in this cohort of North American men with recent HIV infection did not influence their plasma HIV RNA levels after 1 year of HSV-2 acquisition. We believe that clinical trials assessing the effect of HSV-2 suppressant therapy on plasma HIV RNA levels need to last at least 9 to 12 months to establish its long-term efficacy at the individual level, because the effect of HSV-2 on plasma HIV RNA levels in men seems to be transient or intermittent.
ACKNOWLEDGMENTS
The authors are indebted to all the study participants. They thank Dr. Christopher Mathews for his comments in the redaction of this manuscript; Nancy Keating, Deya Collier, Prentice Higley, Rita Aguilar, and Sharon Wilcox for their assistance in the virology laboratory; and Joanne Santangelo, Paula Potter, Tari Gilbert, DeeDee Pacheco, Michael Giancola, Jack Degnan and Jill Kunkel for their clinical assistance.
Supported by the 2005 Bristol-Myers Squibb Fellow Virology Research Award Program and grants AI27670 and AI43638; University of California, San Diego Center for AIDS Research grants AI 36214, AI29164, AI47745, AI57167, AI55276, and MH62512 from the National Institutes of Health; and the San Diego Veterans Affairs Healthcare System.
Footnotes
The authors have no conflicts of interest.
REFERENCES
- 1.Mole L, Ripich S, Margolis D, et al. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 2.Schacker T, Zeh J, Hu H, et al. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 3.Sacks SL, Griffiths PD, Corey L, et al. HSV shedding. Antiviral Res. 2004;63(Suppl 1):S19–S26. doi: 10.1016/j.antiviral.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 5.Duerst RJ, Morrison LA. Innate immunity to herpes simplex virus type 2. Viral Immunol. 2003;16:475–490. doi: 10.1089/088282403771926300. [DOI] [PubMed] [Google Scholar]
- 6.Harandi AM, Svennerholm B, Holmgren J, et al. Protective vaccination against genital herpes simplex virus type 2 (HSV-2) infection in mice is associated with a rapid induction of local IFN-gamma-dependent RANTES production following a vaginal viral challenge. Am J Reprod Immunol. 2001;46:420–424. doi: 10.1034/j.1600-0897.2001.d01-34.x. [DOI] [PubMed] [Google Scholar]
- 7.Schacker T, Ryncarz AJ, Goddard J, et al. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998;280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Cachay ER, Frost SD, Richman DD, et al. Herpes simplex virus type 2 infection does not influence viral dynamics during early HIV-1 infection. J Infect Dis. 2007;195:1242–1244. doi: 10.1086/513568. [DOI] [PubMed] [Google Scholar]
- 9.Ashley-Morrow R, Krantz E, Wald A. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex Transm Dis. 2003;30:310–314. doi: 10.1097/00007435-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 10.R Development Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2007. Available at: http://www.R-project.org. Accessed March 15, 2007. [Google Scholar]
- 11.Ramaswamy M, Sabin C, McDonald C, et al. Herpes simplex virus type 2 (HSV-2) seroprevalence at the time of HIV-1 diagnosis and seroincidence after HIV-1 diagnosis in an ethnically diverse cohort of HIV-1-infected persons. Sex Transm Dis. 2006;33:96–101. doi: 10.1097/01.olq.0000187211.61052.c7. [DOI] [PubMed] [Google Scholar]
- 12.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 13.Serwadda D, Gray RH, Sewankambo NK, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 14.McFarland W, Gwanzura L, Bassett MT, et al. Prevalence and incidence of herpes simplex virus type 2 infection among male Zimbabwean factory workers. J Infect Dis. 1999;180:1459–1465. doi: 10.1086/315076. [DOI] [PubMed] [Google Scholar]