Abstract
Muscle atonia is a central feature of adult REM sleep which has recently been demonstrated to be a component of sleep in rats as young as 2 days of age (P2). The neural generation of atonia, which depends on mesopontine and medullary structures, is not fully understood in adults and has never been described in infants. In the present experiments we used electrical stimulation in decerebrated pups to identify an inhibitory area within the medial medulla of P7-10 rats. Muscle tone inhibition was consistently found on or near the midline within the ventromedial medulla, dorsal to the inferior olive, in an area that includes the nucleus gigantocellularis, nucleus paramedianis, and raphe obscurus. Chemical infusions in the same region revealed inhibitory responses to quisqualic acid but not to carbachol or corticotropin-releasing factor. Next, extracellular recordings within the medullary inhibitory area revealed neurons with atonia-on profiles; tone-on neurons were also found, typically at more lateral sites. Finally, in non-decerebrated pups, chemical lesions within the inhibitory area resulted in significant reductions in atonia durations, as well as decoupling of atonia from a second component of infant sleep, myoclonic twitching; specifically, twitches occasionally occurred during periods of high muscle tone, a condition reminiscent of “REM without atonia” as described in adults.
In summary, we document the existence of an area within the ventromedial medulla of infant rats that (i) causes atonia when stimulated; (ii) contains units that exhibit atonia-related discharge profiles during sleep-wake cycling; and (iii) when lesioned, results in the partial loss of atonia and decoupling of the components of sleep. All together, these findings demonstrate that muscle atonia is actively regulated very early in ontogeny.
Keywords: REM sleep, active sleep, myoclonic twitching, medulla, glutamate, development
The active inhibition of muscle tone is a central feature of REM sleep in adult mammals (Chase and Morales, 1990, Chase and Morales, 2000). Stimulation studies have revealed two primary inhibitory areas within the brainstem: one in the rostral pons and one in the ventromedial medulla (Magoun, 1944, Siegel, 2000). In adult rats and cats, the pontine inhibitory area includes the nuclei reticularis pontis oralis and caudalis (Hajnik et al., 2000); the medullary inhibitory area includes the nucleus gigantocellularis (Gi), nucleus paramedianis (PM), and nucleus magnocellularis (Lai and Siegel, 1988). Atonia during REM sleep is believed to result from glutamatergic and cholinergic excitation of medullary inhibitory nuclei by the pontine inhibitory area, which in turn produces glycinergic inhibition of spinal and hypoglossal motoneurons (Lai and Siegel, 1988, Chase and Morales, 1990, Chase and Morales, 2000, Hajnik et al., 2000). Although the medullary area comprises the final common path for the production of atonia (Siegel and Lai, 1994; for review, see Gottesmann, 1999), inactivation of the pontine inhibitory area inhibits the atonia that would normally result from medullary stimulation, suggesting bidirectional communication between the two regions (Siegel and Lai, 1994, Kohyama et al., 1998, Siegel, 2000). When either of these two regions is lesioned, animals exhibit “REM without atonia,” characterized by the expression of various REM sleep components against a background of high muscle tone (Morrison, 1988, Siegel, 2000).
Recent work in our laboratory supports the notion that the state defined by nuchal atonia and myoclonic twitching satisfies most standard criteria of sleep (Campbell and Tobler, 1984, Hendricks et al., 2000). Specifically, in rats as young as P2, myoclonic twitching is only expressed against a background of nuchal atonia (Karlsson and Blumberg, 2002). In addition, sensory threshold increases during sleep under normal conditions (Seelke and Blumberg, 2004) as well as during sleep deprivation (Blumberg et al., in press-b). Moreover, sleep-wake cycles are regulated by brainstem and hypothalamic structures (Karlsson et al., 2004) and, during sleep, bursts of hippocampal theta occur (Karlsson and Blumberg, 2003).
The developmental relations between infant sleep and adult sleep continue to be debated (Frank and Heller, 2003, Blumberg et al., in press-a). Particularly relevant to the present study is the contention that brainstem and more rostral structures only begin to participate in the regulation of sleep when the neocortical EEG begins exhibiting state-dependent differentiation at P12 (Frank and Heller, 2003). If this contention is correct, then the “central executive mechanisms” that mediate REM-sleep atonia in adults should not be active before P12.
Because the neural substrates of REM sleep atonia are not fully understood in adults and have never been described in infants, it is possible that infant atonia results from the passive withdrawal of excitation from spinal motoneurons. Alternatively, atonia during infant sleep could result from active inhibition, as in adults, but arise from distinct neurophysiological mechanisms. If infant and adult atonia are produced by similar mechanisms, however, then the infant brainstem should contain inhibitory neurons within the ventromedial medulla that produce atonia when stimulated (Hajnik et al., 2000) and exhibit atonia-related discharge properties (Sakai, 1988, Siegel et al., 1991); moreover, lesions of this area should produce a state reminiscent of “REM without atonia” (Schenkel and Siegel, 1989). Here, we find support for each of these predictions, thereby providing the earliest demonstration in an altricial mammal of a common atonia-producing mechanism in infants and adults.
MATERIALS AND METHODS
All experiments were performed in accordance with National Institutes of Health guidelines for the care of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Subjects
Sixty-two P7-10 Sprague-Dawley Norway rats (Rattus norvegicus) of both sexes from 33 litters were used (males and females are evenly represented in all experiments). Body weights ranged from 16.9 to 27.7 g. Litters were culled to 8 pups within 3 days after birth (day of birth = Day 0). Mothers and their litters were housed in standard laboratory cages (48 × 20 × 26 cm) in the animal colony at the University of Iowa where food and water were available ad libitum. All animals were maintained on a 12:12 hour light and dark schedule with lights on at 07:00 hours. All experiments were conducted during the lights-on phase.
Electrical and chemical stimulation of the medullary inhibitory area
Surgery
A pre-collicular decerebration was performed under isoflurane anesthesia, as described elsewhere (Karlsson et al., 2004) and a small opening was drilled in the skull above the medullary area. Unipolar stainless steel electrodes (50 μm; California Fine Wire, Grover Beach, CA) were inserted bilaterally into the nuchal muscle for EMG recording (Karlsson and Blumberg, 2002).
Procedure
After surgery, the pup was wrapped in gauze to inhibit movement (Corner and Kwee, 1976) and transferred to a stereotax equipped such that body temperature could be controlled at approximately 37°C (Karlsson et al., 2004). When secured in the stereotax, the pup's head was leveled between bregma and lambda. All experiments were performed using unanesthetized pups.
Electrical stimulations were performed using 30 animals from 15 litters. Decerebrated infant rats do not exhibit rigidity as do adults (Kreider and Blumberg, 2000, Karlsson et al., 2004); they do, however, exhibit a post-surgical period of elevated and stable tone that lasts approximately 10 to 30 min (after which time sleep-wake cyclicity commences). When stable tone was attained, a concentric, bipolar, stimulating tungsten electrode (1 MΩ, 3-4 μm at the tip, Model TM33CCINS, World Precision Instruments, Sarasota, FL) connected to a stimulus isolator (Model A395, World Precision Instruments, Sarasota, FL) and a stimulus generator (Model 48, Grass, Quincy, MA) was lowered into the medulla. Trains of electrical pulses (.2 ms, 30-90 μA, 100 Hz) were delivered for 500 ms every 5 s. When a site was identified in which stimulation produced nuchal atonia, 3 min of EMG data were collected to hard disk. After data collection was complete, the stimulation site was marked by passing 50 μA anodal current through the electrode for 3 s. Because the goal of the present study was to investigate the medullary inhibitory area, no attempt was made to mark those sites associated with motor facilitation (although their approximate location was noted).
Chemical stimulations were performed using 16 animals from 8 litters. The surgical methods were identical to those described above. For infusions, a .5 μl 33 gauge microsyringe (Model 7000.50C; Hamilton Co., Reno, NV) was lowered stereotactically into the medullary inhibitory site that was previously identified using electrical stimulation (coordinates relative to lambda AP: −2.5 mm; ML: 0.0 mm; DV: −12.3 mm). Three neurotransmitter agonists were used (N = 4 per group): the acetylcholine agonist carbachol, the glutamate agonist quisqualic acid, and corticotropin-releasing factor (CRF) (Sigma, St. Louis, MO). All agonists were dissolved in Ringers solution at the following concentrations: 100 mM for carbachol, 5 mM for quisqualic acid, and 0.1 mM for CRF. Control animals received infusions of Ringers solution (n=4). All chemicals were infused at the same volume and rate (200 nL over 10 s). The concentration, volume, and infusion rate used were determined from pilot experiments and previously published work (Lai and Siegel, 1988, Lai and Siegel, 1992, Hajnik et al., 2000). Finally, in two additional infants, EMG electrodes were implanted in the left nuchal muscle, bilaterally in the lower back (i.e., ilias muscle), and in the right thigh (i.e., vastus lateralis); these pups received infusions of quisqualic acid as described above.
For all tests, EMG electrodes were connected to a differential amplifier (A-M Systems, Carlsborg, WA), and the signal amplified (×10k) and filtered (300-3000 Hz), sampled using a digital interface at 5000 Hz (Model 1401, Cambridge Electronic Design, Cambridge, England), and visualized using Spike2 software (Cambridge Electronic Design, Cambridge, England).
Neural activity within the medullary inhibitory area
Surgery
Under isoflurane anesthesia, a precollicular decerebration was performed and bipolar nuchal EMG electrodes were implanted bilaterally in 6 pups from 6 litters. In addition, the infant's fragile skull was bleached, dried, and then coated with Vetbond (3M, St. Paul, MN) for added strength. Next, a custom-built, T-shaped, stainless steel head-plant, designed to attach to the earbar and nosebar holders of a standard Kopf stereotax, was glued to the skull over the pretreated area using cyanoacrylate adhesive gel. This device made it possible to level and stabilize the pup's head within the stereotax for recording of neural activity. After securing the head-plant, a small hole was drilled over the recording site. Finally, the pup was wrapped in gauze as described above.
Procedure
The recording was performed in a stereotax with body temperature maintained at approximately 37°C. Within 1-3 h after surgery, pups began exhibiting sleep-wake cycles as judged by oscillations in muscle tone as well as twitches of the tail against a background of nuchal atonia (Karlsson et al., 2004). As soon as cyclicity was observed, an 8-site stainless steel recording electrode (1-2 MΩ; ALA Science, Westbury, New York, NY), connected to a unity gain headstage and digital amplifier (Tucker-Davies Technologies, Alachua, FL), was lowered into the medial medulla while neurophysiological activity was monitored. The signals were amplified (10k) and filtered (500-5 kHz band-pass) before being sampled at 12.5 kHz. When stable units were observed and at least one of these units appeared to exhibit state-dependent activity, the electrode was left in place for 5-10 minutes before data collection began. Neurophysiological and EMG data were recorded synchronously for 10 min (encompassing 6-10 sleep-wake cycles) to hard disk for off-line analysis. At the end of the experiment, which lasted 2 to 4 h, the recording site was marked by passing a 50 μA anodal current through all electrodes for 3 s.
Lesions of the medullary inhibitory area
Surgery
Eight non-decerebrated pups from 4 litters were used in this experiment. Under isoflurane anesthesia, a .5 μl 33 gauge microsyringe (Model 7000.50C; Hamilton Co., Reno, NV) was lowered stereotactically into the medullary inhibitory site that was previously identified using electrical stimulation (coordinates relative to lambda AP: −2.5 mm; ML: 0.0 mm; DV: −12.3 mm). Lesions were produced by infusing a high concentration of quisqualic acid (50 mM; 150 nl over 15 s) (n=4). Control animals received identical treatment but received infusion of Ringers solution only (n=4). Next, bipolar nuchal EMG electrodes were implanted bilaterally. The pup was placed on a felt pad in a supine position (to enable observation of twitching in the individual limbs) and lightly restrained, as described previously (Karlsson and Blumberg, 2002). Finally, the pup recovered in an incubator (35°C) for 3-4 h.
Procedure
After recovery, pups were intubated with 0.3 ml of warm cream (half-and-half) and transferred to a humidified recording chamber maintained at 35°C. A microcamera placed above the lid of the recording chamber allowed recording of sleep-wake behaviors, as described elsewhere (Karlsson and Blumberg, 2002). Video data were recorded using a digital video system (Vetron, Rebersburg, PA). After 20 min of acclimation to the chamber, behavioral and EMG data were collected for 30 min and recorded synchronized to digital tape for off-line scoring and analysis.
Data Analysis
Electrical and chemical stimulation
Data were analyzed off-line using Spike2 software. For electrical stimulation, EMG recordings were rectified and amplitudes were measured over three 500 ms periods: before, during, and after stimulation. Averages from 10 stimulations were calculated for each pup. For chemical stimulation, EMG recordings were rectified and amplitudes were measured over the 30-s period immediately before infusion and the period from 30 to 60 s after infusion (this post-infusion period was chosen after visual inspection of the data). Group differences were tested using a single-factor ANOVA and the post hoc test was Fisher's PLSD. Alpha was set at .05. For both electrical and chemical stimulation experiments, a site was considered atonia-producing if stimulation resulted in a decrease in EMG amplitude of at least 2 standard deviations from the mean.
Neural activity
Using Spike2, 4 of the 8 recorded channels that yielded the most heterogeneous unit activity were selected for analysis. Once the channels were selected, principal components analysis was used for spike sorting (Lewicki, 1998). Periods of nuchal atonia (indicative of sleep) and high tone (indicative of wakefulness) were identified from the EMG record, as described elsewhere (Karlsson et al., 2004). Subsequently, the firing rates of individual sorted units were compared with the behavioral state of the animal across 6-10 pairs of high-tone and atonia periods. State-related differences in mean firing rates were tested using the Wilcoxon matched-pairs signed-rank test (Mileykovskiy et al., 2002); for all tests, alpha was set at .05. Neurons that significantly increased or decreased their firing rate during high-tone periods were designated “tone-on” and “atonia-on,” respectively. Neurons that exhibited no significant change during these periods were designated “indifferent.”
Chemical lesions of the ventromedial medulla
Two kinds of analyses were performed. First, EMG signals were digitized at 5 kHz using a data acquisition system (BioPac Systems Inc., Santa Barbara, CA). Next, the EMG signals were summed, integrated, and full-wave rectified, and inspected for duration of tone or atonia, as described previously (Karlsson and Blumberg, 2002). The mean duration of tone and atonia periods over the 30-min recording period was measured. In addition, changes in nuchal EMG amplitude were tested by measuring 10 1-s artifact-free samples from each pup. Second, a trained observer, blind to the experimental conditions, scored a 15-min segment of EMG and behavioral data for the presence of myoclonic twitching and high-amplitude movements (Karlsson and Blumberg, 2002). Myoclonic twitches are phasic, rapid, and independent movements of the limbs and tail; high-amplitude movements comprise such wake-related behaviors as stretching, kicking, and yawning (Gramsbergen et al., 1970, Blumberg and Stolba, 1996). We and others have used similar scoring procedures in the past and have found them to be highly reliable, with inter- and intrarater reliability coefficients typically exceeding 0.85 (Smotherman and Robinson, 1991, Blumberg and Lucas, 1994).
In all cases, means are presented with their standard errors.
Histology
After each experiment, the pup was killed with an overdose of sodium pentobarbital and perfused transcardially with physiological saline followed by 3% formalin. Brains were post-fixed in the skull for 1-2 days in a formalin-sucrose solution and then removed from the skull and fixed for at least 2 more days in a fresh solution. After fixation the brains were sliced in 50 μm sections with a sliding microtome (Model SM 2000 R, Leica, Bensheim, Germany) and stained with cresyl violet. The location of the marking lesions, the tracks from the infusion syringe, and the extent of the chemical lesions were determined by examining serial sections.
RESULTS
Electrical stimulation of the medullary inhibitory area
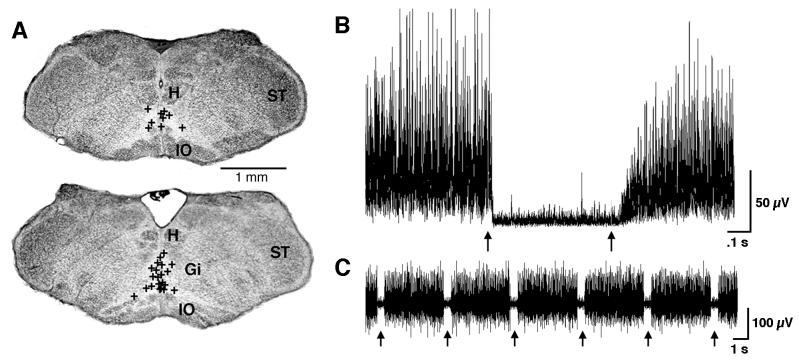
Exploration of the infant medulla revealed that nuchal atonia was readily produced with placement of the stimulating electrode on or near the midline within a restricted region of the ventromedial medulla (Figure 1A). Specifically, inhibition was consistently obtained on or near the midline dorsal to the inferior olive. This area comprises the ventromedial parts of the Gi, PM, and raphe obscurus (RO). Figure 1B depicts the averaged EMG from 10 stimulation pulses and Figure 1C depicts, for a representative infant, the raw EMG responses to 6 stimulation pulses. The mean atonia onset latency, calculated from 10 randomly selected non-littermates, was 43.9 ± 9.0 ms (range: 16.0 – 96.0 ms).
Figure 1.
Inhibitory sites in the ventromedial medulla identified using electrical stimulation in P8-10 rats. (A) Coronal sections of the medullary inhibitory area. Each symbol (+) represents one inhibitory site from one pup (H, hypoglossal nucleus; ST, spinal trigeminal nucleus; Gi, nucleus gigantocellularis; IO, inferior olive). (B) Averaged nuchal EMG trace from 10 stimulus pulses applied to the inhibitory area of one pup; the arrows depict pulse onset and offset. (C) Representative sample of raw nuchal EMG responses to stimulation pulses; the arrows indicate periods of stimulation-induced atonia.
Whereas muscle tone inhibition was typically observed on or near the midline, our observations indicated that muscle tone facilitation typically occurred at more lateral sites. This was consistently found within subjects; for example, sequential advances of the electrode within the coronal plane indicated muscle excitation laterally, inhibition medially, and excitation again on the other side of midline.
Chemical stimulation of the medullary inhibitory area
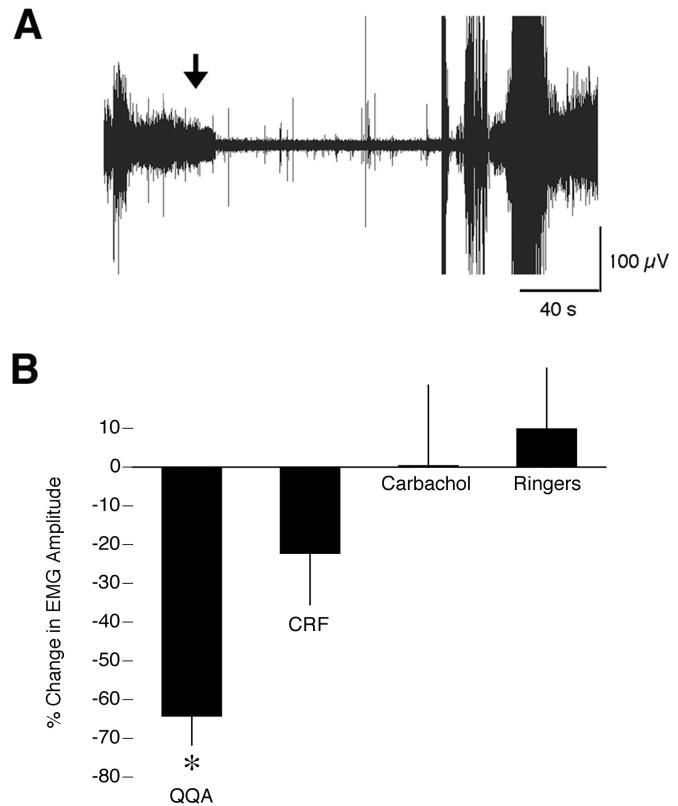
To investigate which neurotransmitters might mediate atonia in infants, three agonists (quisqualic acid, carbachol, and CRF) that have been previously demonstrated to mediate atonia in adult rats and/or cats were infused into the medullary inhibitory area (Lai and Siegel, 1988, Lai and Siegel, 1992, Hajnik et al., 2000). ANOVA revealed a significant effect of group on the percent change in EMG amplitude from baseline within 30-60 s after infusion (P < 0.05; F = 4.8; df = 3,12; Figure 2B). Of the 12 infants infused with carbachol (n = 4), CRF (n = 4), or Ringers solution (n = 4), none exhibited a significant reduction in nuchal muscle tone (as defined as a reduction in EMG amplitude exceeding 2 standard deviations from the mean). In contrast, infusion with quisqualic acid produced atonia in all 4 infants tested (a representative response is depicted in Figure 2A). For these subjects, the mean atonia onset latency was 16.1 + 2.9 s (range: 8.0 – 21.7 s); mean duration of atonia was 120.5 + 17.1 s (range: 75.7 – 157.0 s).
Figure 2.
Quisqualic acid induces nuchal atonia in P8-10 rats. (A) Representative nuchal EMG response to infusion of quisqualic acid (5 mM). The arrow indicates the onset of the infusion (200 nL over 10 s). (B) Percentage change in EMG amplitude from baseline after infusion of quisqualic acid (QQA), corticotropin-releasing factor (CRF), carbachol, or Ringers. Amplitudes were averaged over the 30-s period immediately before infusion and the period from 30 to 60 s after infusion. * significant difference from carbachol and Ringers. N = 4 per group. Mean +/− s.e.
Two additional pups were infused with quisqualic acid into the medullary inhibitory area while EMGs were measured in the left nuchal muscle, bilaterally in the lower back (i.e., ilias muscle), and in the right thigh (i.e., vastus lateralis). Atonia ensued concurrently in all three muscle groups, indicating that stimulation of cell bodies within the medullary inhibitory area can produce atonia bilaterally throughout the body.
Atonia-related neural activity within the medullary inhibitory area
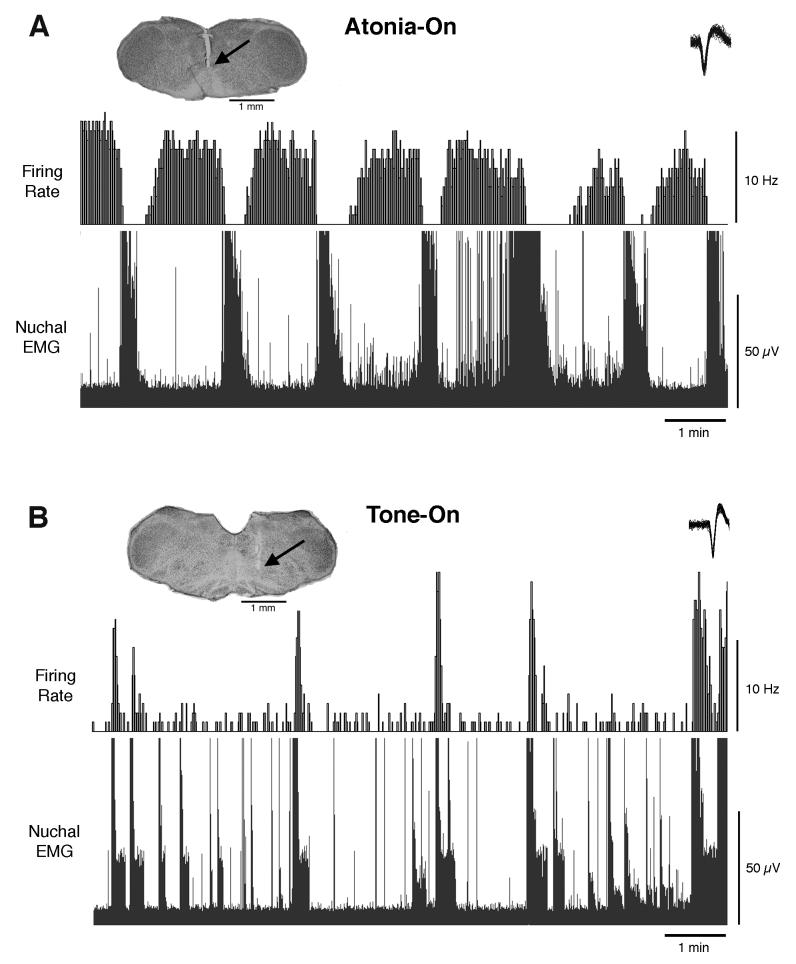
A total of 20 neurons from 6 infants were recorded in the medullary inhibitory area (1-4 units were recorded from each pup). Discharge rates were analyzed and the units classified as atonia-on, tone-on, or indifferent. Of the 20 recorded units, 6 exhibited increased activity during periods of nuchal atonia; these units were located at sites within ventral Gi, PM, and RO. Six additional units exhibited increased activity during periods of high tone; these units were located within Gi and PM.
Figure 3A depicts discharge rates and nuchal EMG of a representative atonia-on unit. The mean discharge rates for all atonia-on units was 3.3 ± 0.7 Hz (range: 1.9 – 5.8 Hz) during periods of muscle atonia and 0.7 ± 0.1 Hz (range: 0.2 – 1.1 Hz) during periods of high muscle tone.
Figure 3.
State-dependent unit discharges in the ventromedial medulla of P7-10 rats. The discharge profile of (A) a representative atonia-on unit and (B) a representative tone-on unit are presented above their associated nuchal EMG traces. Within each photomicrograph, the arrow indicates the tip of the electrode. The inset in each of the upper-right corners depicts 50 superimposed action potentials of the sorted unit. For purposes of illustration, the EMG traces are full-wave rectified.
Figure 3B depicts discharge rates and nuchal EMG for 1 of the 6 tone-on units. The mean discharge rates for the tone-on units was 0.6 ± 0.7 Hz (range: 0.1 – 2.0) during periods of muscle atonia and 3.9 ± 3.0 Hz (range: 0.8 – 10.3 Hz) during periods of high muscle tone.
Chemical lesions of the medullary inhibitory area disrupt nuchal atonia
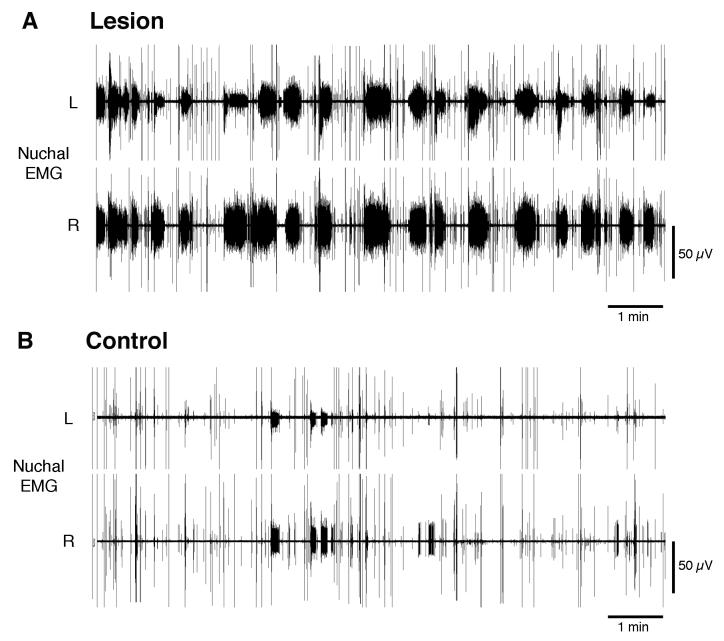
Chemical lesions of the ventromedial medulla produced significant changes in the expression of atonia. Figure 4A presents 10 min of data from a lesioned pup, showing prolonged durations of high-tone periods and reduced durations of atonia periods in both the left and right nuchal muscles as compared with the control pup depicted in Figure 4B. Mean high-tone durations doubled in the lesion group (6.1 ± 1.2 s) in relation to the control group (3.0 ± 0.6 s), and this difference was statistically significant (P < 0.05; t = 4.7; df = 3). Conversely, mean atonia durations were halved in the lesion group (11.6 ± 2.4 s) in relation to the control group (28.3 ± 7.1 s), but this difference only approached statistical significance (P < 0.06; t = 3.0; df = 3). In addition, as Figures 4 and 5 suggest, mean nuchal EMG amplitude was significantly greater in the lesioned group (P < 0.05; t = 2.8; df = 6).
Figure 4.
Lesions of the medullary inhibitory area of P8-10 rats disrupt expression of nuchal atonia. Representative EMG traces after an infusion of (A) highly concentrated quisqualic acid (50 mM) or (B) Ringers solution into the medullary inhibitory area showing bilateral responses in the left (L) and right (R) nuchal muscles. Each recording is 10 min long.
Figure 5.
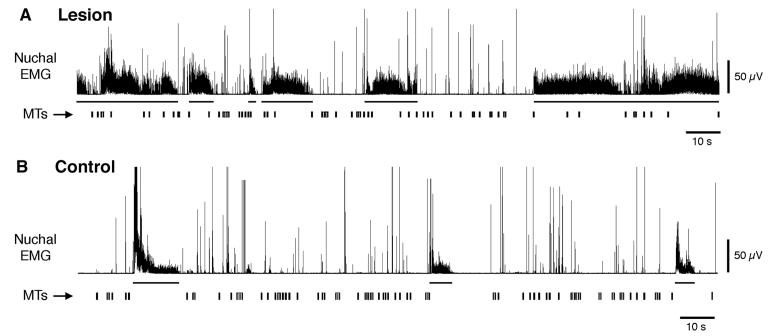
Myoclonic twitches (MTs) and atonia are decoupled after lesions of the medullary inhibitory area in P8-10 rats. Representative nuchal EMG trace after an infusion of (A) highly concentrated quisqualic acid (50 mM) or (B) Ringers solution into the medullary inhibitory area (150 nl over 15 s). The horizontal bars beneath the EMG traces indicate periods of high muscle tone; vertical bars indicate instances of myoclonic twitching.
Lesions within the ventromedial medulla also disrupted the tight coupling normally seen between nuchal atonia and myoclonic twitching (Figure 5). In the control group, only 1.2 ± 0.8 twitches occurred during periods of high muscle tone, representing just 5 total twitches across all 4 pups (the mean number of twitches per 15-min period was 541.0 ± 41.3). In contrast, in the lesioned group, a mean of 20.8 ± 2.8 twitches occurred during periods of high muscle tone; although this represents only a small proportion of all myoclonic twitches recorded (< 5%; the mean number of twitches was 542.2 ± 73.6), the 20-fold increase is significant (P < 0.01; t = 9.3; df = 3). Figure 5A shows, for a representative lesioned pup, many instances of twitching against a background of high nuchal muscle tone.
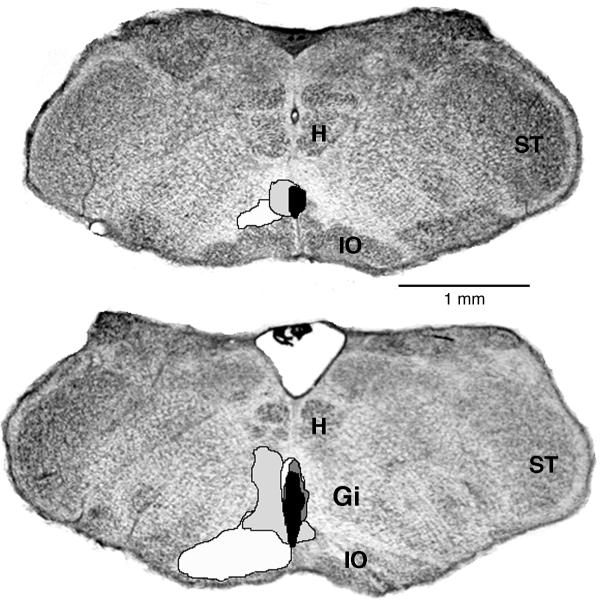
Finally, the reconstructed chemical lesions are shown in Figure 6. All lesions were within the region that was previously found to produce atonia in response to electrical stimulation (see Figure 1). Interestingly, the 2 largest lesions produced greater increases in the expression of mean high-tone duration (large: 7.6-8.6 s; small: 3.6-4.7 s) and greater decreases in mean atonia duration (large: 7.7-8.9 s; small: 11.4-18.6 s).
Figure 6.
Reconstruction of the medullary lesions produced by quisqualic acid in Experiment 3. The lesions from each of 4 P8-10 pups are designated by different shades of gray. The coronal sections are 50 μm thick and 250 μm apart; the upper section is caudal to the lower section. See abbreviations in Figure 1.
Discussion
We describe here for the first time an area in the ventromedial medulla that is instrumental for the expression of atonia in infant rats. This area, which includes the Gi, PM, and RO, (i) causes atonia when stimulated; (ii) contains neurons that exhibit atonia-related discharge profiles; and (iii) when lesioned, results in the partial loss of atonia and decoupling of the components of infant sleep. All together, these findings demonstrate that sleep atonia in infant rats is an active process that is generated within a region of the medulla that is identical to that previously identified in adults (Hajnik et al., 2000).
Electrical stimulation activates both fibers of passage and cell bodies. Therefore, we also performed chemical stimulations. Quisqualic acid effectively produced atonia when infused into the ventromedial medulla; the short latencies to produce atonia (i.e., 8-22 s), and the fact that midline infusions produced bilateral atonia in multiple muscle groups in two of our subjects, supports the notion that cell bodies located on or near the midline mediate muscle atonia in infant rats. This notion is consistent with a previous study in young adult rats that found that sites producing bilateral (as opposed to only contralateral) inhibition of atonia were located medially within the medulla and were more prominent in younger subjects (i.e., 8- vs. 16-week-olds; Hajnik et al., 2000).
In contrast with quisqualic acid, neither CRF nor carbachol were effective. The failure to find effects of CRF and carbachol may or may not indicate developmental differences in the neuropharmacological control of atonia in rats. First, thus far, CRF has only been shown to be effective in adult cats (Lai and Siegel, 1992). Second, even in adult cats, distinct regions of the medulla are differentially responsive to infusions of glutamate and carbachol (Lai and Siegel, 1988). Finally, failure of infant rats to respond to carbachol may reflect species differences in the role of the cholinergic system in activating REM sleep or one of its components (Boissard et al., 2002).
Only a few previous experiments have documented state-dependent unit activity in unanesthesized pups (Tamásy et al., 1980, Corner and Bour, 1984). The inherent difficulties associated with recording from neurons in infants have led most researchers to rely on anesthetized or in vitro preparations in which behavioral states are, of course, absent (Leinekugel et al., 2002). Thus, an in vivo preparation that combines the control of anesthetized recordings while preserving the spontaneous rapid cycles of sleep and wakefulness was developed and is introduced in the present study. The fact that decerebrated infant rats, soon after surgery, begin cycling rapidly between sleep and wakefulness makes this preparation ideal for studies aimed at exploring neural population patterns across the sleep-wake cycle (Karlsson et al., 2004). A similar procedure was recently reported in non-decerebrated adult rats, but extensive training was required for these restrained animals to exhibit sleep-wake cycles (Lee et al., 2004).
Here, we report clear instances of state-dependent unit activity within the ventromedial medulla. Three types of discharge profiles were described: atonia-on, tone-on, and indifferent. The discharge profiles of the atonia-on neurons compare well with ventromedial medullary tonic REM-on neurons that have previously been described in adult cats (Siegel et al., 1979, Kanamori et al., 1980, Steriade et al., 1984, Sakai, 1988). In addition, REM-on units in the medullary inhibitory area of narcoleptic dogs discharge vigorously during cataplexy (Siegel et al., 1991).
The stimulation and recording studies described above suggest the presence of neurons that govern sleep atonia in infants. However, to rule out the possibility that the discharge profile of the neurons is merely correlated with - but not causal to – atonia, the cells of the inhibitory area were lesioned using highly concentrated quisqualic acid. It was found that the expression of atonia was significantly reduced in the lesioned pups. Moreover, EMG amplitude increased in the lesioned pups, consistent with a previous report in adult cats (Holmes and Jones, 1994).
Rat pups exhibit two tightly coupled sleep components as early as P2: myoclonic twitching and atonia (Karlsson and Blumberg, 2002). As shown here, lesions of the medullary inhibitory area result in the partial decoupling of myoclonic twitches and atonia (i.e., twitches were occasionally detected against a background of high muscle tone). This decoupling of the two sleep components is reminiscent of “REM without atonia” as described previously (Mirmiran, 1982, Morrison, 1988, Siegel, 2000).
Experiments in adult rats and cats indicate that the medullary inhibitory area alone is not sufficient to generate atonia (Siegel et al., 1986, Kohyama et al., 1998). Consistent with these findings, we have observed that rats as young as P2 also do not exhibit atonia when decerebrated between the medulla and mesopontine region (Karlsson and Blumberg; unpublished observations). In conjunction with previous findings in decerebrated and intact infants of different ages (Karlsson et al., 2004), it appears then that sleep-wake cyclicity during infancy depends upon integration of activity between the mesopontine and medullary areas.
The present study adds to a growing body of evidence indicating coherent organization of a sleep state in infant rats that depends upon coordination within brainstem and forebrain structures. First, as in adults during REM sleep, myoclonic twitching occurs only against a background of nuchal atonia (Karlsson and Blumberg, 2002). Second, hippocampal theta activity at P2 is also expressed predominantly during periods of nuchal atonia (Karlsson and Blumberg, 2003). Third, myoclonic twitching is disrupted by decerebrations that are caudal, but not rostral, to the mesopontine region (Kreider and Blumberg, 2000). Fourth, over the first postnatal week, cyclicity between periods of high muscle tone and atonia is increasingly modulated by structures within the anterior hypothalamus (Karlsson et al., 2004), perhaps including the ventrolateral preoptic area (Saper et al., 2001). Importantly, all of these sleep processes have been demonstrated in pups well before the onset of a differentiated EEG at P12 (Gramsbergen et al., 1970, Corner and Kwee, 1976). Finally, the present findings demonstrate that a well-established component of REM sleep in adults, nuchal atonia, is produced by activation of neurons within the ventromedial medulla, providing perhaps the strongest and most direct evidence to data of common neural substrates for sleep in infants and adults. This finding, in conjunction with those discussed above, is difficult to reconcile with the view that sleep in infant rats before P12 is “not controlled by central executive sleep mechanisms” (p. 29) (Frank and Heller, 2003).
In summary, the medullary inhibitory area in infant rats appears functionally identical to the corresponding adult inhibitory area. We hypothesize that, early in ontogeny, the medullary inhibitory area is modulated by mesopontine circuits and that this circuit is sufficient to generate sleep-wake cyclicity (Karlsson et al., 2004). We further hypothesize that this neural circuit forms the foundation for all REM sleep processes upon which further elaborations occur during development.
Acknowledgements
Supported by grants from the National Institute of Mental Health (MH50701, MH66424) and the National Institute of Child Health and Human Development (HD38708). We thank Cynthia Shaw and Jessica Middlemis-Brown for technical assistance.
References
- Blumberg MS, Karlsson KÆ, Seelke AMH, Mohns EJ. The ontogeny of mammalian sleep: A response to Frank and Heller (2003) J Sleep Res. doi: 10.1111/j.1365-2869.2004.00430_1.x. in press-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Lucas DE. Dual mechanisms of twitching during sleep in neonatal rats. Behav Neurosci. 1994;108:1196–1202. doi: 10.1037//0735-7044.108.6.1196. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Middlemis-Brown JE, Johnson ED. Sleep homeostasis in infant rats. Behav Neurosci. doi: 10.1037/0735-7044.118.6.1253. in press-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Stolba MA. Thermogenesis, myoclonic twitching, and ultrasonic vocalization in neonatal rats during moderate and extreme cold exposure. Behav Neurosci. 1996;110:305–314. doi: 10.1037//0735-7044.110.2.305. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Tobler I. Animal sleep: A review of sleep duration across phylogeny. Neuroscience & Biobehavioral Reviews. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. Control of Motoneurons During Sleep. In: Dement WC, editor. Principles and Practice of Sleep Medicine. Second Edition Saunders; New York: 2000. [Google Scholar]
- Corner MA, Bour HL. Postnatal development of spontaneous neuronal discharges in the pontine reticular formation of free-moving rats during sleep and wakefulness. Exp Brain Res. 1984;54:66–72. doi: 10.1007/BF00235819. [DOI] [PubMed] [Google Scholar]
- Corner MA, Kwee P. Cyclic EEG and motility patterns during sleep in restrained infant rats. Electroencephalogr Clin Neurophysiol. 1976;41:64–72. doi: 10.1016/0013-4694(76)90215-7. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. J Sleep Res. 2003;12:25–34. doi: 10.1046/j.1365-2869.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. The neurophysiology of sleep and waking: intracerebral connections, functioning and ascending influences of the medulla oblongata. Prog Neurobiol. 1999;59:1–54. doi: 10.1016/s0301-0082(98)00094-x. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Dev Psychobiol. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Hajnik T, Lai YY, Siegel JM. Atonia-related regions in the rodent pons and medulla. J Neurophysiol. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Sehgal A, Pack A. The need for a simple animal model to understand sleep. Prog Neurobiol. 2000;61:339–351. doi: 10.1016/s0301-0082(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1179–1200. doi: 10.1016/0306-4522(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Kanamori N, Sakai K, Jouvet M. Neuronal activity specific to paradoxical sleep in the ventromedial medullary reticular formation of unrestrained cats. Brain Res. 1980;189:251–255. doi: 10.1016/0006-8993(80)90024-4. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Blumberg MS. The union of the state: myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behav Neurosci. 2002;116:912–917. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Blumberg MS. Hippocampal theta in the newborn rat is revealed under conditions that promote REM sleep. J Neurosci. 2003;23:1114–1118. doi: 10.1523/JNEUROSCI.23-04-01114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Kreider JC, Blumberg MS. Hypothalamic contribution to sleep-wake cycle development. Neuroscience. 2004;123:575–582. doi: 10.1016/j.neuroscience.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Kohyama J, Lai YY, Siegel JM. Inactivation of the pons blocks medullary-induced muscle tone suppression in the decerebrate cat. Sleep. 1998;21:695–699. doi: 10.1093/sleep/21.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider JC, Blumberg MS. Mesopontine contribution to the expression of active ‘twitch’ sleep in decerebrate week-old rats. Brain Res. 2000;872:149–159. doi: 10.1016/s0006-8993(00)02518-x. [DOI] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Corticotropin-releasing factor mediated muscle atonia in pons and medulla. Brain Res. 1992;575:63–68. doi: 10.1016/0006-8993(92)90423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Manns ID, Alonso A, Jones BE. Sleep-wake related discharge properties of basal forebrain neurons recorded with micropipettes in head-fixed rats. J Neurophysiol. 2004;92:1182–1198. doi: 10.1152/jn.01003.2003. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsaki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- Lewicki MS. A review of methods for spike sorting: the detection and classification of neural action potentials. Network. 1998;9:R53–78. [PubMed] [Google Scholar]
- Magoun HW. Bulbar inhibition and facilitation of motor activity. Science. 1944;100:549–550. doi: 10.1126/science.100.2607.549. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Cessation of activity in red nucleus neurons during stimulation of the medial medulla in decerebrate rats. J Physiol. 2002;545:997–1006. doi: 10.1113/jphysiol.2002.028985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran M. ‘Oneiric’ behavior during active sleep induced by bilateral lesions of the pontine tegmentum in juvenile rats. In: Koella WP, editor. Sleep: Sixth European Congress of Sleep Research. Karger; Basel: 1982. pp. 236–239. [Google Scholar]
- Morrison AR. Paradoxical sleep without atonia. Arch Ital Biol. 1988;126:275–289. [PubMed] [Google Scholar]
- Sakai K. Executive mechanisms of paradoxical sleep. Arch Ital Biol. 1988;126:239–257. [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Schenkel E, Siegel JM. REM sleep without atonia after lesions of the medial medulla. Neurosci Lett. 1989;98:159–165. doi: 10.1016/0304-3940(89)90503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AM, Blumberg MS. Sniffing in infant rats during sleep and wakefulness. Behav Neurosci. 2004;118:267–273. doi: 10.1037/0735-7044.118.2.267. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Brainstem mechanisms generating REM sleep. In: Kryger MH, et al., editors. Principles and practice of sleep medicine. W. B. Saunders Company; Philadelphia: 2000. pp. 112–133. [Google Scholar]
- Siegel JM, Lai YY. Brainstem systems mediating the control of muscle tone. In: Mallick BN, Singh R, editors. Environment and Physiology. Narosa Publishing House; New Delhi: 1994. pp. 62–78. [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science. 1991;252:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS, Nienhuis R. Behavioral states in the chronic medullary and midpontine cat. Electroencephalogr Clin Neurophysiol. 1986;63:274–288. doi: 10.1016/0013-4694(86)90095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Wheeler RL, McGinty DJ. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res. 1979;179:49–60. doi: 10.1016/0006-8993(79)90488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Accessibility of the rat fetus for psychobiological investigation. In: Shair HN, et al., editors. Developmental psychobiology: New methods and changing concepts. Oxford University Press; New York: 1991. pp. 148–164. [Google Scholar]
- Steriade M, Sakai K, Jouvet M. Bulbo-thalamic neurons related to thalamocortical activation processes during paradoxical sleep. Exp Brain Res. 1984;54:463–475. doi: 10.1007/BF00235472. [DOI] [PubMed] [Google Scholar]
- Tamásy V, Korányi L, Lissák K. Early postnatal development of wakefulness-sleep cycle and neuronal responsiveness: A multiunit activity study on freely moving newborn rat. Electroencephalogr Clin Neurophysiol. 1980;49:102–111. doi: 10.1016/0013-4694(80)90356-9. [DOI] [PubMed] [Google Scholar]