Abstract
The ovulatory gonadotropin surge regulates expression of plasminogen activator (PA) family members within the ovarian follicle, which are implicated in follicle wall degradation at ovulation. Gonadotropin also stimulates follicular prostaglandin E2 (PGE2) production, which is required for follicle rupture. To determine whether the ovulatory gonadotropin surge regulates PA-mediated proteolysis via PGE2 in the primate follicle, monkeys received gonadotropins to stimulate follicle development. Follicular aspirates or whole ovaries were obtained before (0 h) and after human chorionic gonadotropin (hCG) administration to span the periovulatory interval. Granulosa cell levels of tissue-type PA (tPA) and PA inhibitor type 1 (PAI-1) proteins were low at 0 h hCG and higher after hCG administration. In situ zymography showed no ovarian tPA activity 0 h after hCG; tPA activity was present in granulosa cells obtained after hCG treatment. Importantly, tPA and PAI-1 proteins and tPA activity were low/nondetectable in granulosa cells obtained after treatment with hCG and the PG synthesis inhibitor celecoxib. To determine whether hCG stimulation of tPA and PAI-1 requires PGE2, granulosa cells obtained at 0 h were cultured with hCG plus indomethacin to inhibit PG production; some cells also received PGE2 or an agonist selective for one PGE2 receptor (EP). PGE2, an EP2 agonist, and an EP3 agonist increased tPA protein, whereas PGE2, an EP1 agonist, and an EP3 agonist increased PAI-1 protein. Therefore, gonadotropin increases granulosa cell tPA and PAI-1 protein levels and tPA-dependent proteolytic activity. PGE2 also increases tPA and PAI-1 protein levels in granulosa cells, suggesting that elevated PGE2 late in the periovulatory interval acts to stimulate proteolysis and follicle rupture.
Different prostaglandin E2 receptors increase granulosa cell expression of tissue type plasminogen activator (PA) and PA inhibitor type 1, so receptor-selective agonists may be useful to promote or prevent proteolysis needed for ovulation.
Oocyte release at ovulation requires extensive breakdown and remodeling of the connective tissue matrix that surrounds the mammalian follicle. The plasminogen activator (PA) system may play a crucial role in follicle wall rupture during ovulation (1). Tissue-type PA (tPA) and urokinase-type PA (uPA) are serine proteases that convert plasminogen into the active proteolytic enzyme plasmin (2,3). Fibrin is the preferred substrate for plasmin activity, but most matrix proteins, including collagen IV, fibronectin, laminin, and proteoglycans, are targets for plasmin activity. In addition, plasmin can activate matrix metalloproteinases, which degrade a variety of extracellular matrix proteins (3). The PA inhibitors type 1 (PAI-1) and type 2 (PAI-2) regulate PA-dependent proteolysis by limiting proteolysis both temporally and spatially (4).
A role for PAs in ovulation is well established. PAs are expressed and secreted within periovulatory follicles of many species, including rat, cow, pig, ewe, and monkey (5,6,7,8,9,10,11,12). Several studies provide evidence that the PA/plasmin system is regulated by the ovulatory gonadotropin surge within the periovulatory follicle (1,5,9,13). Follicular fluid contains considerable levels of plasminogen, and activated plasmin has been shown to weaken the follicle wall in vitro (14,15). In rats and sheep, ovulation was suppressed by ovarian administration of anti-tPA antibodies, anti-uPA antibodies, or α2-antiplasmin (11,16). Furthermore, mice lacking expression of both tPA and uPA have reduced ovulation rates (17), again supporting a key role for PA family members in follicle rupture.
Prostaglandins (PGs) produced in the follicle are required for successful ovulation. Follicular levels of PGs increase markedly in response to an ovulatory dose of gonadotropin, with granulosa cells being the primary site of synthesis (18,19,20,21). Inhibition or deletion of the cyclooxygenase-2 (COX-2) enzyme (also known as PTGS2) results in low follicular PG levels and impaired or delayed ovulation in many mammals (19,22,23,24,25) including monkeys and women (26,27). Importantly, follicle rupture can be restored by administration of PGE2 (22,25,26). These data suggest a key role for PGE2 in ovulation as a mediator of proteolytic degradation of the follicle wall. PGE2 alone or in combination with LH increased fibrinolytic activity in the medium of cultures of rat granulosa cells, whereas the PG synthesis inhibitor indomethacin blocked gonadotropin-induced fibrinolysis (5,13,28). Human chorionic gonadotropin (hCG) increased tPA-dependent fibrinolytic activity in rhesus monkey follicular fluid and cultured granulosa cells (9). However, the role of PGE2 in this process is unclear in primates.
The study presented here was conducted to determine whether PGE2 is an essential mediator of gonadotropin-stimulated PA proteolytic activity within the primate periovulatory follicle. Both in vivo and in vitro experiments provide evidence that PGs enhance PA-mediated proteolysis within the monkey follicle. Specific PGE2 receptors (EPs) are identified as essential mediators of gonadotropin-stimulated PA system protein levels, consistent with a key role for PGE2 as an intrafollicular regulator of PA-mediated proteolysis in the rupture of the primate follicle at ovulation.
Materials and Methods
Animals
Granulosa cells and whole ovaries were obtained from adult female cynomolgus macaques at Eastern Virginia Medical School (Norfolk, VA). All animal protocols and experiments were approved by the Eastern Virginia Medical School Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal husbandry and sample collection were performed as previously described (29). Blood samples were obtained under ketamine chemical restraint (10 mg/kg body weight) by saphenous or femoral venipuncture, and serum was stored at −20 C. Aseptic surgeries were performed in a dedicated surgical suite under isoflurane anesthesia.
A controlled ovarian stimulation model developed for the collection of multiple oocytes for in vitro fertilization was used to obtain monkey granulosa cells (30,31). Beginning within 3 d of initiation of menstruation, recombinant human (r-h) FSH [90 IU daily; Organon Pharmaceuticals, a part of Schering-Plough Corp. (Roseland, NJ), and Serono Reproductive Biology Institute, Rockland, MA] was administered for 6–8 d, followed by daily administration of 90 IU r-hFSH plus 60 IU r-hLH (Serono) for 2 d to stimulate the growth of multiple preovulatory follicles. The GnRH antagonist antide (0.5 mg/kg body weight; Serono) was also administered daily to prevent an endogenous ovulatory LH surge. Adequate follicular development was monitored by serum estradiol levels and ultrasonography (32,33). Follicular aspiration was performed during aseptic surgery before (0 h) or 12, 24, or 36 h after administration of 1000 IU r-hCG (Serono) (34). To inhibit follicular PG production during the periovulatory interval, additional animals were treated with gonadotropins and Antide as described above; these animals also received the COX-2 selective inhibitor celecoxib (Celebrex; Pfizer, New York, NY) 32 mg orally every 12 h beginning with hCG administration until follicles were aspirated 36 h later. This treatment was previously shown to decrease follicular fluid PGE2 to very low levels (35). To obtain granulosa cells, each follicle was pierced with a 22-gauge needle, and the aspirated contents of all follicles larger than 4 mm in diameter were pooled. Periovulatory follicles in cynomolgus macaques are typically 4–6 mm in diameter as assessed by ultrasonography and confirmed by direct measurement at surgery. Whole ovaries were also obtained from monkeys experiencing ovarian stimulation as described above.
Tissue preparation
Monkey granulosa cells and oocytes were pelleted from the follicular aspirates by centrifugation at 300×g. The supernatant (follicular fluid) was removed and stored at −80 C. After oocyte removal, a granulosa cell-enriched population of the remaining cells was obtained by Percoll gradient centrifugation (36). Granulosa cells were either used immediately for cell culture or were frozen in liquid nitrogen and stored at −80 C for preparation of total RNA. Viability of granulosa cell-enriched preparations was assessed by trypan blue exclusion and averaged 76%. Whole ovaries were bisected, maintaining at least two periovulatory follicles greater than 4 mm in diameter on each piece. The pieces were covered with O.C.T. Compound (Sakura, Tokyo, Japan), frozen in liquid propane, and stored at −80 C.
Granulosa cell culture
Monkey granulosa cells obtained before hCG administration were plated on LabTek glass chamber slides (Nalgene Nunc, Rochester, NY) at a concentration of 20,000 cells/200 μl medium or 48-well culture plates (Corning Inc., Corning, NY) at a concentration of 100,000 cells/400 μl medium. The cells were cultured on fibronectin-coated surfaces in serum-free DMEM-Ham’s F12 medium containing insulin, transferrin, selenium, aprotinin, and human low-density lipoprotein as previously described (37). At the initiation of culture, hCG (Serono; 100 ng/ml) and the nonselective COX inhibitor indomethacin (Sigma Chemical Co., St Louis, MO; 100 nm) were added to all except the control wells. After 24 h in vitro, one of the following treatments was added: PGE2 (1 μm), the EP1 agonist 17 phenyl-trinor PGE2 (10 μm), the EP2 agonist butaprost (10 μm), or the EP3 agonist sulprostone (1 μm). Each of these agonists is selective for the indicated EP by at least one order of magnitude at the concentration used in this study (38,39). PGE2 and all EP-selective agonists were obtained from Cayman Chemical (Ann Arbor, MI). Cells were then maintained in vitro for an additional 4 or 12 h, after which the cells were either lysed with Trizol reagent (Invitrogen, Rockville, MD) for RNA extraction or fixed with 10% buffered formalin for 30 min and then stored at 4 C in PBS for immunofluorescent detection of tPA and PAI-1 proteins. Additional granulosa cells were cultured on chamber slides with hCG plus indomethacin for 24 or 36 h and then fixed and stored as described above for immunofluorescent detection of EP.
Real-time RT-PCR
Levels of mRNA for tPA, uPA, and PAI-1 were assessed by real-time RT-PCR using a Roche LightCycler (Roche Diagnostics, Indianapolis, IN). Total RNA was obtained from granulosa cells using Trizol reagent, treated with deoxyribonuclease and reverse transcribed as previously described (40). PCR was performed using the FastStart DNA Master SYBR Green I kit (Roche) following manufacturer’s instructions and using 0.5 nm primers and 4 mm MgCl2. Primers were designed using LightCycler Probe Design software (Roche) based on the human or monkey sequences (Table 1) and span an intron to prevent undetected amplification of genomic DNA. PCR products were sequenced (Microchemical Core Facility, San Diego State University, San Diego, CA) to confirm amplicon identity. At least five log dilutions of the sequenced PCR product were included in each assay and used to generate a standard curve. For each sample, the content of tPA, uPA, PAI-1, and β-actin mRNA was determined in independent assays. No amplification was observed when monkey cDNA was omitted. All data are expressed as the ratio of mRNA of interest to β-actin mRNA for each sample.
Table 1.
Reaction conditions for real-time RT-PCR
| Target | Primer sequences (5′–3′) | Amplified cDNA (bp) | Published human sequence | % Identity between monkey and human sequence | Monkey amplified fragment sequence |
|---|---|---|---|---|---|
| tPA | |||||
| Forward | GCTACGTCTTTAAGGCG | 365 | NM_000930 | 96 | EU427935 |
| Reverse | GATGCGAAACTGAGGC | ||||
| uPA | |||||
| Forward | ACCACAACGACATTGC | 244 | NM_002658 | 97 | EU427936 |
| Reverse | GAGCCGTAGTAGTGGG | ||||
| PAI-1 | |||||
| Forward | GCCATTACTACGACATCCT | 347 | NM_000602 | 97 | EU427934 |
| Reverse | CGTGCCACTCTCGTTC | ||||
| β-Actin | |||||
| Forward | ATCCGCAAAGACCTGT | 270 | NM_001101 | 97 | AY765990 |
| Reverse | GTCCGCTAGAAGCAT |
Immunofluorescent protein detection
Frozen ovarian tissues were sectioned at 10 μm and fixed with buffered 10% formalin. After antigen retrieval with 10 mm sodium citrate (pH 6), sections were blocked with 5% nonimmune goat serum (Vector Laboratories, Burlingame, CA) in PBS containing 0.1% Triton X-100 (PBS-Triton). Sections were then incubated for 1 h at room temperature either with primary antibody generated against tPA (rabbit antihuman polyclonal antibody 4 μg/ml; Santa Cruz Biotechnologies, Santa Cruz, CA), uPA (mouse antihuman monoclonal antibody, 15 μg/ml; Calbiochem, San Diego, CA), PAI-1 (mouse antihuman monoclonal antibody, 10 μg/ml; Calbiochem), or no primary antibody, followed by incubation with Alexa Fluor 488-conjugated antirabbit or antimouse secondary antibody (4 μg/ml; Molecular Probes, Eugene, OR). After incubation with 1% Sudan Black in 70% methanol, the slides were coverslipped using Vectashield medium (Vector).
Formalin-fixed cultured granulosa cells were blocked with 5% nonimmune goat serum and incubated for 1 h at room temperature with either the tPA or PAI-1 primary antibody and Alexa Fluor 488 secondary antibody as described above. Additional formalin-fixed cultured granulosa cells were blocked with 5% nonimmune goat serum and incubated for 1 h at room temperature with a rabbit antihuman polyclonal antibody against one of the following PGE2 receptors: EP1 (2 μg/ml), EP2 (10 μg/ml), or EP3 (4 μg/ml); all EP antibodies were obtained from Cayman Chemical. In some experiments, the anti-EP primary antibody was preincubated with the respective blocking peptide at a 1:2 ratio before use in the primary antibody incubation step. Immunofluorescent protein detection proceeded as described above.
All images were obtained using an Olympus BX41 fluorescent microscope fitted with a DP70 digital camera and associated software (Olympus, Melville, NY). For semiquantitative analysis of ovarian tissue sections, images were analyzed using MetaMorph software (Molecular Devices, Sunnyvale, CA). Images were color separated into red, green, and blue, with information representing protein detection in the green channel. A lower threshold was set to 90 for the green channel in all images. Two rectangular areas, or regions, were created and saved for use on each image. For each image, these two regions were placed over areas containing granulosa cells but not ovarian stroma or other parts of the ovary. Pixel area was determined for each region. Cell nuclei were counted within each region by viewing the red channel. Pixel area was divided by cell nuclei for each region, and the average pixel area per cell was calculated for each tissue section.
In situ zymography
PA activity was detected using modifications of previously reported methods (41,42). Frozen ovarian sections, 10 μm thick, were rapidly thawed at 37 C and stained with propidium iodide (100 ng/ml; Sigma-Aldrich, St. Louis, MO) in PBS for 1 min. Carnation nonfat milk powder (Nestle, Glendale, CA) was dissolved in PBS (20% wt/vol), heated to 95 C for 30 min, and centrifuged for 5 min at 3000 × g. The supernatant was maintained at 55 C. A 1.2% solution of agar in water was boiled for 10 min and then chilled to 55 C. Four parts of 1.2% agar were mixed with one part of milk solution, and plasminogen (75 μg/ml; Molecular Innovations, Inc., Southfield, MI) was added. Tissue sections were overlaid with the agar-milk-plasminogen mixture, coverslipped, and incubated at 37 C in a humidified chamber for 90 min. Proteolytic areas appeared as clear, transparent zones in the opaque and cloudy overlay mixture. Ovarian sections were then stored at 4 C until images were taken. Dark-field and fluorescent images were obtained using an Olympus BX41 fluorescent microscope as described above.
To determine whether plasminogen was required for proteolysis, some ovarian sections were overlaid with an agar-milk mixture lacking plasminogen. Additional ovarian sections were preincubated with the anti-tPA antibody (8 μg/ml) or anti-uPA antibody (20 μg/ml) for 10 min at room temperature, after which in situ zymography was performed as described above. In preliminary experiments, proteolysis was monitored periodically every 30 min for 4 h and then at 5, 7, and 20 h to determine the optimal incubation time. Proteolysis over areas containing cells was apparently complete by 90 min, so all data were collected after 90 min incubation. After 3 h incubation, proteolytic zones were visible distant from the tissue section, indicating spontaneous degradation of milk casein.
tPA and PAI-1 measurements in follicular fluid
Follicular fluid levels of total tPA and total PAI-1 were determined using AssayMax Human tPA and AssayMax Human PAI-1 ELISA kits (AssayPro, St. Charles, MO) according to the kit instructions. Intraassay coefficients of variation for tPA and PAI-1 kits were 5.7 and 4.7% respectively; all samples were tested within one tPA or PAI-1 assay. The lower limit of detection (0.031 ng/ml for tPA) was used for statistical analysis of one granulosa cell sample obtained at 0 h hCG because tPA was not detectable by ELISA.
Data analysis
All data were assessed for heterogeneity of variance and were log-transformed when Bartlett’s test yielded a significance of less than 0.05. Data presented in Figs. 1 (A–C), 3 (A, B, and D), and 4 (B and C) were log-transformed before further analysis. In all figures, untransformed data are presented. Data presented in Figs. 1 and 3 were assessed by ANOVA. Levels of tPA and PAI-1 mRNA in cultured granulosa cells (Fig. 4, B and C) were assessed by ANOVA with one repeated measure. For all ANOVAs, post hoc analyses were performed using Duncan’s multiple-range test. Statistical analyses were performed using StatPak version 4.12 software (Northwest Analytical, Portland, OR). Data are presented as mean ± sem, and significance was assumed at P < 0.05.
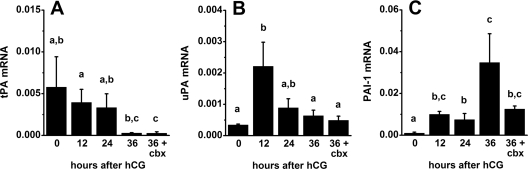
Figure 1.
tPA, uPA, and PAI-1 mRNA levels in monkey granulosa cells. Granulosa cells were obtained from monkeys experiencing controlled ovarian stimulation before (0 h) and 12, 24, and 36 h after hCG administration; additional animals received hCG and celecoxib (36+cbx) for 36 h. Granulosa cell levels of mRNAs were determined by real-time RT-PCR; all mRNA levels are expressed relative to β-actin mRNA. Data are presented as mean ± sem (n = 4–6 animals per time point). In A–C, groups with no common letters are different by ANOVA and Duncan’s; P < 0.05.
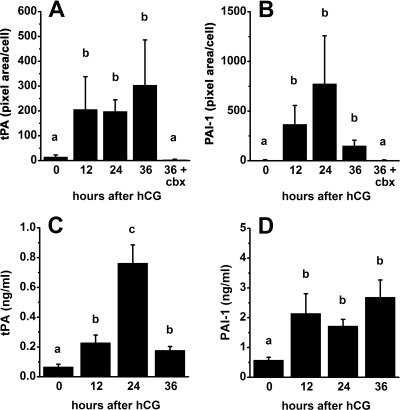
Figure 3.
tPA and PAI-1 protein levels in granulosa cells and follicular fluid obtained from monkeys experiencing controlled ovarian stimulation and treated with hCG for 0, 12, 24, and 36 h or with hCG plus celecoxib for 36 h. A and B, Images shown in Fig. 2, A–J, and replicate experiments were assessed for tPA (A) and PAI-1 (B) proteins using MetaMorph analysis of green fluorescence (pixel area) and normalized to cell number (n = 3 animals per time point). C and D, tPA and PAI-1 proteins were measured by ELISA. Data are presented as mean ± sem (n = 4–5 animals per time point). In each panel, groups with no common letters are different by ANOVA and Duncan’s; P < 0.05.
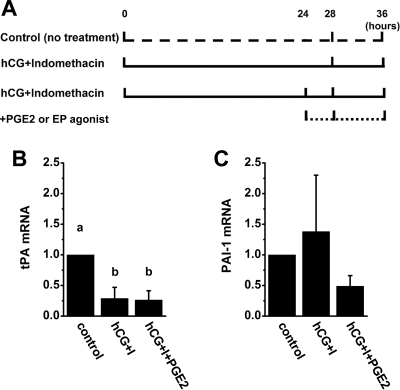
Figure 4.
A, Cell culture model to mimic in vivo periovulatory events. Cells obtained from large, preovulatory follicles before hCG (0 h hCG) were maintained in vitro either with no treatment (control) or with an ovulatory dose of hCG plus the nonselective COX inhibitor indomethacin included in culture medium to inhibit endogenous PG production (hCG plus indomethacin). After 24 h culture, PGE2 or an EP-selective agonist was added to some cultures (hCG plus indomethacin plus PGE2 or EP agonist) to simulate the rise in follicular fluid PGE2, which occurs 24 h after hCG administration in vivo. Cells were harvested after an additional 4 or 12 h culture, for a total of 28 or 36 h in vitro, to examine the effects of short-term or long-term PGE2 exposure, respectively. B and C, Using this experimental design, tPA (B) and PAI-1 (C) mRNA levels were measured in granulosa cells maintained in vitro for 36 h with no treatment (control), hCG plus indomethacin (hCG+I), or hCG plus indomethacin plus PGE2 (hCG+I+PGE2). Granulosa cell levels of mRNAs were determined by real-time RT-PCR and expressed relative to β-actin mRNA; within each animal, mRNA levels in treated cells are shown as a ratio of control levels. Data are presented as mean ± sem (n = 4–5 animals per treatment). In B, groups with no common letters are different by ANOVA and Duncan’s; P < 0.05.
Results
tPA, uPA, and PAI-1 in granulosa cells
tPA, uPA, and PAI-1 mRNA was present in monkey granulosa cells before and after administration of an ovulatory dose of hCG in vivo (Fig. 1). Levels of tPA mRNA were not different 0–24 h after hCG administration, but tPA expression decreased 36 h after hCG to levels below those measured after 12 h hCG exposure (Fig. 1A). Levels of uPA mRNA were low at 0 h hCG, increased transiently 12 h after hCG administration, and returned to low levels 36 h after hCG administration (Fig. 1B). PAI-1 mRNA was low at 0 h hCG, increased 12–24 h after hCG administration, and remained high for the rest of the periovulatory period, peaking 36 h after hCG administration (Fig. 1C).
The ability of hCG-stimulated PGs to modulate levels of PA family member mRNAs was also examined. Administration of the COX-2-selective inhibitor celecoxib with hCG for 36 h did not alter mRNA levels for tPA, uPA, or PAI-1 when compared with mRNA levels after treatment with hCG alone (Fig. 1). Although treatment with hCG and celecoxib for 36 h was previously shown to reduce follicle PG levels (35), celecoxib treatment did not alter levels of mRNAs for PA family members present in granulosa cells just before ovulation.
PA family member proteins were localized to monkey ovarian cells using immunofluorescence. Before hCG administration, tPA protein was low or undetectable in both granulosa cells and in the ovarian stroma (Fig. 2A). After treatment with hCG for 12, 24, or 36 h, tPA protein was easily detected in the cytoplasm of granulosa cells and, to a lesser extent, in the ovarian stroma (Fig. 2, B–D). uPA protein was low or undetectable in granulosa cells and the ovarian stroma (data not shown). However, uPA was present in the endothelial cells of large blood vessels located in the ovarian stroma (Fig. 2V). PAI-1 protein was low to undetectable in granulosa cells and the ovarian stroma before hCG administration (Fig. 2F). However, PAI-1 protein was easily detected in the cytoplasm of granulosa cells of ovaries obtained 12, 24, and 36 h after hCG administration (Fig. 2, G–I). Treatment with hCG plus celecoxib for 36 h reduced both tPA and PAI-1 proteins to very low levels when compared with tPA and PAI-1 protein detection in granulosa cells of monkeys treated for 36 h with hCG only (Fig. 2, E and J vs. D and I). Immunofluorescence was absent when the primary antibodies were omitted, serving as a negative control (data not shown). Therefore, tPA and PAI-1, but not uPA, proteins were present in the granulosa cells of monkey periovulatory follicles.
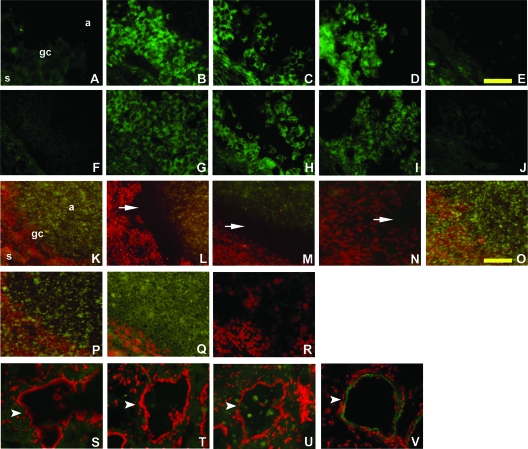
Figure 2.
Detection of tPA and PAI-1 proteins and PA proteolytic activity in monkey periovulatory follicles. Ovaries were obtained from monkeys experiencing controlled ovarian stimulation as described in the legend for Fig. 1. Immunodetection (bright green) of tPA (A–E) and PAI-1 (F–J) is shown after treatment with hCG for 0 (A and F), 12 (B and G), 24 (C and H), or 36 (D and I) hours or with hCG plus celecoxib for 36 h (E and J). Sections are not counterstained. Scale bar in E, 100 μm A–J). In situ zymography was performed using ovarian sections obtained after treatment with hCG for 0 (K), 12 (L), 24 (M, P, Q, and R), or 36 (N, S, T, and U) hours or with hCG plus celecoxib for 36 h (O). K–U are composites of dark-field (milk casein) and fluorescent (propidium iodide) images. In all panels, propidium iodide staining of cell nuclei appears red. Undegraded casein is detectable as greenish cloudy spots; casein is absent in the areas of proteolysis (black or spot-free areas indicated by white arrows). Blood vessels are indicated by white arrowheads. In each periovulatory follicle shown, the antrum (a) is in the upper right, granulosa cells (gc) are central, and the ovarian stroma (s) is in the lower left portion of the image. No proteolysis was observed when plasminogen was omitted from the overlay mixture (P). Ovarian tissues containing periovulatory follicles (Q and R) and blood vessels (T and U) were preincubated with the anti-tPA antibody (Q and T) or the anti-uPA antibody (R and U) before overlay for zymography. Immunofluorescent detection (green) of uPA in an ovarian blood vessel is also shown; nuclei are counterstained red (V). Scale bar in O, 400 μm (K–V). Ovarian images in A–O are representative of three animals per time point.
Computer-aided analysis of these images confirmed that protein levels of both tPA and PAI-1 were low before hCG and elevated 12–36 h after hCG administration (Fig. 3, A and B). Similar analysis confirmed that administration of hCG plus celecoxib for 36 h yielded lower levels of tPA and PAI-1 proteins when compared with protein levels in granulosa cells of monkeys treated with hCG only for 36 h.
In situ zymography for PA activity in the monkey periovulatory follicle
Detection of PA activity used the concept that PA family members convert inactive plasminogen to the active protease plasmin, which can digest the milk protein casein. To identify regions of ovarian follicles that contained PA activity, tissue sections were overlaid with a mixture containing both plasminogen and casein. Intact casein appeared as a greenish spotty substance (Fig. 2K); proteolysis was detected as dark regions that were cleared of the spotty material (Fig. 2, L–N). Omission of plasminogen from the overlay mixture deprived the reaction of this essential proenzyme, and no proteolysis was observed (Fig. 2P), demonstrating that this technique specifically detected PA-dependent proteolytic activity.
Ovarian tissues obtained before administration of an ovulatory dose of hCG did not contain sufficient PA proteolytic activity to degrade the casein present in the overlay mixture (Fig. 2K). In contrast, vast zones of proteolysis were observed over and around granulosa cells of ovaries obtained 12, 24, and 36 h after hCG (Fig. 2, L–N). Proteolytic activity was absent over ovarian sections obtained from animals treated for 36 h with hCG and celecoxib (Fig. 2O). Importantly, preincubation of tissue sections with the anti-tPA antibody essentially eliminated proteolysis around granulosa cells (Fig. 2Q), whereas preincubation with the anti-uPA antibody did not reduce proteolysis (Fig. 2R). These data support the conclusion that PA activity in the monkey periovulatory follicle is catalyzed mainly by tPA. In contrast, proteolytic activity observed over ovarian vessels was reduced by preincubation with the anti-uPA antibody (Fig. 2U) but not the anti-tPA antibody (Fig. 2T), demonstrating that this in situ zymography technique can detect uPA, as well as tPA, activity. Therefore, exposure to an ovulatory dose of hCG increased granulosa cell tPA activity. Coadministration of hCG and celecoxib for 36 h yielded little or no tPA activity, comparable to the low level of tPA activity present before hCG administration.
tPA and PAI-1 protein levels in follicular fluid
Secreted tPA and PAI-1 proteins were examined by assessment of PA family members in monkey follicular fluid. Levels of tPA in follicular fluid were low before hCG, increased to reach peak levels 24 h after hCG administration, and returned to intermediate levels 36 h after hCG (Fig. 3C). PAI-1 levels in follicular fluid were low before hCG, increased 12 h after hCG administration, and remained high for the rest of the periovulatory period (Fig. 3D). Therefore, levels of tPA and PAI-1 proteins in follicular fluid increased in response to hCG administration, similar to hCG-regulated changes in tPA and PAI-1 proteins detected in granulosa cells by immunofluorescence (Figs. 2 and 3, A and B).
tPA and PAI-1 expression in cultured granulosa cells
Inhibition of PG synthesis within the periovulatory follicle prevented follicle rupture, whereas intrafollicular injection of PGE2 restored follicle rupture in inhibitor-treated monkeys (26). PGE2 levels in monkey follicular fluid were very low 0 and 12 h after hCG administration; PGE2 levels increased 24 h after hCG and peaked at 36 h after hCG (29). Therefore, key actions of PGE2 are anticipated to occur late in the periovulatory interval. Although celecoxib treatment in vivo reduces intrafollicular PGE2 to low levels (35), this COX-2 inhibitor likely blocks production of many eicosanoids within the monkey follicle. Additional experiments were necessary to identify the unique role of PGE2 in the regulation of tPA and PAI-1 in monkey granulosa cells.
To determine whether hCG alone or hCG-stimulated PGE2 regulates granulosa cell tPA and PAI-1 expression, a cell culture model was developed to mimic in vivo periovulatory events (Fig. 4A). Cells obtained from large, preovulatory follicles before hCG (0 h hCG) were maintained in vitro with an ovulatory dose of hCG; the general COX inhibitor indomethacin was also included in culture medium to inhibit endogenous PG production. After 24 h culture, PGE2 or an EP-selective agonist was added to some cultures to simulate the rise in follicular fluid PGE2 that occurs 24 h after hCG administration in vivo. Cells were then cultured for an additional 4 or 12 h, for a total of 28 or 36 h in vitro, to examine the effects of short-term or long-term PGE2 exposure, respectively.
Granulosa cells cultured for a total of either 28 or 36 h with no treatment (control), hCG plus indomethacin, hCG plus indomethacin plus PGE2, or hCG plus indomethacin plus an EP-selective agonist were assessed for tPA and PAI-1 mRNA levels. The addition of PGE2 or any of the EP-selective agonists did not alter tPA or PAI-1 mRNA levels when compared with treatment with hCG plus indomethacin only (Fig. 4, B and C, and data not shown). This is similar to our findings obtained from monkey granulosa cells treated with hCG alone or hCG plus celecoxib in vivo, where no differences were observed between tPA or PAI-1 mRNA levels in granulosa cells obtained late in the periovulatory interval in the absence or presence of this COX-2-selective inhibitor (Fig. 1).
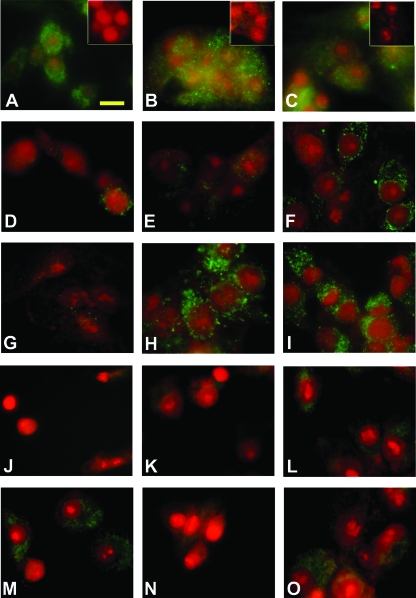
Levels of tPA protein were assessed by immunofluorescence in granulosa cells cultured as described above. Granulosa cells cultured for 28 h with no treatment (Fig. 5D) or with hCG plus indomethacin (Fig. 5E) showed very low levels of tPA protein. In granulosa cells that received PGE2 for the final 4 h during a total of 28 h in vitro with hCG plus indomethacin (Fig. 5F), tPA protein was apparently more abundant. tPA protein detection was very low in granulosa cells that received EP1 agonist 17 phenyl-trinor PGE2 instead of PGE2 (Fig. 5G). In contrast, intense tPA immunofluorescence was present in granulosa cells treated with either the EP2 agonist butaprost or the EP3 agonist sulprostone (Fig. 5, H and I). PGE2, butaprost, and sulprostone (but not 17 phenyl-trinor PGE2) also increased immunofluorescent detection of tPA protein in granulosa cells cultured for a total of 36 h (data not shown).
Figure 5.
Immunofluorescent detection of EP1, EP2, EP3, tPA, and PAI-1 proteins in cultured granulosa cells. For EP protein detection, granulosa cells obtained from animals undergoing controlled ovarian stimulation before hCG administration were maintained in vitro with hCG plus indomethacin for 36 h. The cells were formalin fixed and stained (bright green) for EP1 (A), EP2 (B), and EP3 (C) proteins. Immunofluorescence was reduced or eliminated when primary antibodies were preincubated with the respective blocking peptide (A–C, insets). For immunofluorescent detection of tPA and PAI-1 proteins, granulosa cells were cultured as described in Fig. 4A with no treatment (D and J) or with hCG plus indomethacin (E–I and K–O). After 24 h, some cultures received additional treatment with PGE2 (F and L), 17 phenyl-trinor PGE2 (G and M), butaprost (H and N), or sulprostone (I and O). Cells were cultured for a total of 28 h for immunodetection of tPA (D–I) or 36 h for immunodetection of PAI-1 (J–O). Immunodetection appears green; nuclei are counterstained red. Images in A–C are representative of two animals. Images in D–O are representative of three to four animals per treatment. Scale bar in A, 20 μm (all images).
PAI-1 protein was low/nondetectable in granulosa cells cultured for 28 h with no treatment, hCG plus indomethacin, hCG plus indomethacin plus PGE2, or hCG plus indomethacin plus any EP-selective agonist (data not shown). Cells cultured for 36 h with no additional treatment (Fig. 5J) or with hCG plus indomethacin (Fig. 5K) showed very low levels of PAI-1 protein. PAI-1 immunofluorescence was more abundant in granulosa cells that received PGE2 during the final 12 h during a total of 36 h in vitro (Fig. 5L). Treatment with either the EP1 agonist 17 phenyl-trinor PGE2 or the EP3 agonist sulprostone increased PAI-1 immunofluorescence (Fig. 4, M and O) similar to PAI-1 levels observed in granulosa cells treated with PGE2. In contrast, PAI-1 immunofluorescence was very low or absent in granulosa cells treated with the EP2 agonist butaprost (Fig. 5N). Neither tPA nor PAI-1 was detected when the primary antibody was omitted (data not shown).
To determine whether EPs were available to mediate the effects of PGE2 or EP-selective agonists in vitro, immunofluorescent detection of EP proteins was performed. EP1, EP2, and EP3 proteins were present in granulosa cells cultured with hCG plus indomethacin for 24 and 36 h (data not shown and Fig. 5, A–C). No EP immunostaining was observed when the primary antibody was preincubated with the respective blocking peptide (Fig. 5, A–C, insets).
Discussion
This study is the first to demonstrate that gonadotropin regulation of PA activity in primate periovulatory follicle is mediated via PGE2. In vivo, granulosa cell tPA and PAI-1 protein levels as well as PA proteolytic activity increased in response to administration of an ovulatory dose of gonadotropin. Because increased tPA and PAI-1 occurred in response to hCG but before the documented elevation in follicular PG production, the initial increase in tPA and PAI-1 protein and activity likely occurred independent of PGs. However, these higher levels of tPA and PAI-1 proteins as well as PA activity were not present late in the periovulatory period when a COX-2 inhibitor was administered. PGE2 was previously identified as the key COX product involved in ovulation in many species (23,24), including monkeys (26). Although defects in follicle rupture consistently occurred when follicular PG synthesis was inhibited, the specific mechanism by which elevated intrafollicular PGE2 promotes ovulation is unknown. This investigation presents novel data that suggest that PGs are needed for elevated PA proteolytic activity late in the periovulatory interval. Granulosa cell levels of tPA and PAI-1 proteins were increased via stimulation of EPs, confirming that PGE2 is involved in periovulatory regulation of PA family components. Different EPs mediated the ability of PGE2 to increase levels of tPA and PAI-1 proteins. EP-selective agonists and antagonists may be useful to promote or inhibit follicular proteolysis by independently controlling the expression and activities of members of the PA system.
Protein levels of components of the PA system increased in response to the ovulatory gonadotropin surge. In the present study, we demonstrated that hCG increased levels of tPA and PAI-1 proteins in monkey granulosa cells and follicular fluid. In previous studies, follicular fluid and conditioned media from cultures of follicular cells were assessed for secreted PA family member proteins, but gonadotropin regulation of these proteins in vivo was not directly addressed (5,8,13,43,44). Studies of primary cultures of follicular cells from rhesus monkeys suggested that theca, but not granulosa, cells produce PAI-1 in response to hCG exposure (9). In the present study, ovarian tissue sections and immunodetection were used to identify granulosa cells as the primary site of tPA and PAI-1 synthesis in periovulatory follicles of cynomolgus macaques. Although tPA was consistently detected in monkey follicles, uPA protein was not detected, despite the presence of uPA mRNA in monkey granulosa cells. Therefore, tPA is likely the predominant PA enzyme involved in proteolysis of the follicle wall. The present study demonstrates that hCG increases granulosa cell tPA and PAI-1 protein levels, implicating hCG as a key stimulus for initiation of synthesis of these important proteins.
Earlier studies have shown that the ovulatory gonadotropin surge alters mRNA levels of PAs and their inhibitors in ovarian follicles (7,8,45,46). Although only PAI-1 mRNA was previously detected in human periovulatory granulosa cells (47), our study used a more sensitive real-time RT-PCR technique and detected tPA, uPA, and PAI-1 mRNA in monkey granulosa cells throughout the periovulatory period. Granulosa cells from women undergoing in vitro fertilization are routinely collected late in the periovulatory period, when, as we have shown, uPA and tPA mRNAs are very low, whereas PAI-1 mRNA levels were highest. Reports of gonadotropin regulation of ovarian mRNA for tPA, uPA, and PAI-1 vary widely. An ovulatory dose of hCG increased mRNA for tPA, uPA, and PAI-1 in bovine granulosa cells (8). Administration of hCG to rats increased tPA mRNA in granulosa cells, but hCG has been reported to both increase and decrease mRNAs for uPA and PAI-1 in rat granulosa cell or ovarian homogenates (7,46). Most importantly, mRNA levels for tPA and PAI-1 did not correlate with changing protein levels in the present study. In addition, uPA mRNA was detected in monkey granulosa cells, whereas immunofluorescence was unable to confirm the presence of uPA protein in these same cells. Interestingly, few previous reports include assessment of both mRNA and protein for PA family members in ovarian cells. In a single study of rat granulosa cells, an ovulatory dose of hCG increased, then decreased, PAI-1 mRNA, whereas PAI-1 protein levels were easily detected before hCG administration and did not appear to change throughout the study period (7). Assessment of protein and, ideally, activity levels will be required to understand how PA family members contribute to the proteolytic events necessary for ovulation.
The ovulatory gonadotropin surge induced changes in proteolytic activity in the follicle wall during the periovulatory period. In situ zymography was used to demonstrate the presence of proteolytic activity primarily around granulosa cells in monkey periovulatory follicles. This proteolysis correlated with changes in tPA protein observed in granulosa cells by immunofluorescence, with little protein or proteolysis detected in ovaries obtained before (0 h) hCG and abundant protein and proteolysis observed at all times after hCG administration. Pretreatment of ovarian tissues with the anti-tPA antibody (but not the anti-uPA antibody) essentially eliminated plasminogen-dependent proteolytic activity over granulosa cells, supporting identification of tPA, but not uPA, as the active PA produced by granulosa cells of the primate periovulatory follicle. Similarly, tPA activity was present in bovine follicular fluid, but uPA activity was not detected (48), supporting the hypothesis that granulosa cells produce primarily, if not exclusively, tPA protein in these species. Previous studies provide indirect evidence of tPA regulation by gonadotropin. In rat and monkey granulosa cells, gonadotropin exposure in vivo increased tPA activity in conditioned medium obtained from granulosa cell cultures (5,9). Perhaps most interesting is a study by Dow and colleagues, where the apex and base of periovulatory follicles were dissected free, and homogenates were subjected to gel zymography for tPA and uPA activity (48). An ovulatory dose of gonadotropin increased tPA and uPA activity in both apical and basal homogenates, and PA-mediated proteolysis was not restricted to the follicle apex. Work by Murdoch and colleagues (49) has suggested that the ovarian surface epithelium secretes uPA via the basolateral membrane, so PA activity provided by the surface epithelium may determine the actual location of the follicle apex via PA proteolytic activity. The presence of PA-mediated proteolysis around the entire follicle also suggests that the PA system may be involved in the structural remodeling and neovascularization necessary for both ovulation and corpus luteum formation.
Data from the present study, coupled with reports of studies in other species, suggest that PA activity is not regulated primarily by control of mRNA levels for PA family members. In the present study, hCG increased tPA protein levels in follicular fluid and granulosa cells, and increased tPA protein correlated with increased tPA proteolytic activity. High tPA protein and activity levels occurred 12 h after hCG, when tPA mRNA levels were high; however, tPA protein and activity were also high 36 h after hCG, when tPA mRNA levels were low. In the rat ovary, an ovulatory dose of hCG increased tPA protein throughout the 24-h study interval, but tPA activity peaked 12 h after hCG administration before dropping to lower levels thereafter (7). These data support the general observation (2) that PA family members can be regulated at both transcriptional and posttranscriptional levels.
The gonadotropin-stimulated increase in tPA activity occurred despite a concomitant increase in PAI-1 protein expression. In cynomolgus macaques, PAI-1 protein was present at low levels in ovaries obtained before hCG but increased after hCG administration. Plasminogen- and tPA-dependent proteolysis began as early as 12 h after hCG administration and continued throughout the periovulatory period, so hCG administration increased follicular tPA activity in parallel with elevated levels of tPA and PAI-1 proteins. As a member of the serpin family, PAI-1 inhibits PA activity by mimicking the substrate and trapping the enzyme in an inactive stable 1:1 complex (50). The observed gonadotropin-stimulated increase in proteolytic activity indicates that at least a portion of the tPA present after gonadotropin stimulation was unopposed by PAI-1. Parallel increases of proteolytic enzymes and inhibitors as described in this study have been reported for matrix metalloproteinases and their tissue inhibitors (51,52,53). This type of parallel regulation may prevent uncontrolled proteolysis by limiting the site and/or extent of proteolytic degradation.
Gonadotropin stimulation of PA-mediated proteolysis in the follicle likely requires ovarian PG synthesis. The general COX inhibitor indomethacin inhibited gonadotropin-stimulated fibrinolytic activity in rat granulosa cells in vitro, whereas PGs alone or in combination with LH induced fibrinolytic activity in granulosa cell conditioned media (5,13,28,43). Indomethacin also inhibited tPA activity in bovine follicular fluid and homogenates of the follicle apex (8). These studies support a role for PGs to regulate PA-mediated proteolytic activity in ovarian tissues, but the specific PG involved has not been identified.
Our in vitro studies support the hypothesis that PGE2 is the PG responsible for ovulatory PA activity. In monkeys, follicular PGE2 increased 24 h after hCG administration to peak just before the expected time of ovulation (29). In the present study, treatment with celecoxib reduced granulosa cell levels of PA family member proteins as well as tPA-dependent proteolytic activity. For in vitro studies, cultures of granulosa cells were treated to simulate this window of PGE2 exposure, which occurs late in the periovulatory period in vivo. To inhibit the production of all PGs in cultured granulosa cells, indomethacin was included with an ovulatory dose of hCG, and 24 h later, PGE2 or an EP-selective agonist was added to examine the effect on tPA and PAI-1 protein levels. Similar to our results in vivo, treatment with hCG in the absence of PGs did not result in elevated granulosa cell levels of tPA and PAI-1 proteins after 28 or 36 h in vitro. In contrast, hCG plus PGE2 resulted in elevated tPA and PAI-1 protein levels, confirming a key role for this specific PG in plasminogen-dependent proteolysis of the follicle wall.
Furthermore, our in vitro studies demonstrate that different subsets of PGE2 receptors were required to increase tPA and PAI-1 protein levels. PGE2 can act through four G protein-coupled receptors: EP1, EP2, EP3, and EP4 (54,55). In a previous study, we demonstrated that monkey mural granulosa cells express functional EP1, EP2, and EP3 (37). Therefore, receptor-selective agonists were used to stimulate individual EPs in cultured granulosa cells. Activation of EP2 and EP3 increased tPA protein levels. In contrast, PAI-1 protein levels were increased by stimulation of EP1 and EP3. Further studies will be required to identify the specific intracellular signaling pathways responsible for the increase in tPA and PAI-1 proteins.
These studies confirm that PGE2 is the bioactive PG that regulates tPA and PAI-1 proteins in granulosa cells. Ovulation failure in COX inhibitor-treated animals may be explained by blockade of PGE2 production and the resulting deficit in PA-mediated proteolysis. These novel studies indicate that levels of tPA and PAI-1 proteins are regulated via different PGE2 receptors. Drugs that bind to a single EP may be useful to selectively stimulate proteolysis of the follicle wall via EP2 or selectively inhibit follicular proteolysis via EP1. By identifying EPs that regulate specific proteins involved in ovarian proteolysis, this study suggests new targets for drug therapies to either promote or prevent ovulation.
Acknowledgments
We thank Brandy Dozier and Kim Hester for their role in animal training, animal protocols, and surgeries. Recombinant human gonadotropins were generously provided by Organon Pharmaceuticals, a part of Schering-Plough Corp. (Roseland, NJ) and Serono Reproductive Biology Institute (Rockland, MA). Antide was also provided by Serono. These studies were supported by HD54691 to D.M.D. This research was supported by grant funding from Virginia’s Commonwealth Health Research Board.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 25, 2008
Abbreviations: COX-2, Cyclooxygenase-2; EP, PGE2 receptor; hCG, human chorionic gonadotropin; PA, plasminogen activator; PAI-1, PA inhibitor type 1; PG, prostaglandin; r-h, recombinant human; tPA, tissue-type PA; uPA, urokinase-type PA.
References
- Liu YX 1999 Regulation of the plasminogen activator system in the ovary. Biol Signals Recept 8:160–177 [DOI] [PubMed] [Google Scholar]
- Vassalli JD, Sappino AP, Belin D 1991 The plasminogen activator/plasmin system. J Clin Invest 88:1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CS, Wilhelm SM, Pentland AP, Marmar BL, Grant GA, Eisen AZ, Goldberg GI 1989 Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci USA 86:2632–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen PA, Georg B, Lund LR, Riccio A, Stacey SN 1990 Plasminogen activator inhibitors: hormonally regulated serpins. Mol Cell Endocrinol 68:1–19 [DOI] [PubMed] [Google Scholar]
- Strickland S, Beers WH 1976 Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem 251:5694–5702 [PubMed] [Google Scholar]
- Liu YX, Cajander SB, Ny T, Kristensen P, Hsueh AJ 1987 Gonadotropin regulation of tissue-type and urokinase-type plasminogen activators in rat granulosa and theca-interstitial cells during the periovulatory period. Mol Cell Endocrinol 54:221–229 [DOI] [PubMed] [Google Scholar]
- Liu YX, Peng XR, Ny T 1991 Tissue-specific and time-coordinated hormone regulation of plasminogen-activator-inhibitor type I and tissue-type plasminogen activator in the rat ovary during gonadotropin-induced ovulation. Eur J Biochem 195:549–555 [DOI] [PubMed] [Google Scholar]
- Li Q, Jimenez-Krassel F, Kobayashi Y, Ireland JJ, Smith GW 2006 Effect of intrafollicular indomethacin injection on gonadotropin surge-induced expression of select extracellular matrix degrading enzymes and their inhibitors in bovine preovulatory follicles. Reproduction 131:533–543 [DOI] [PubMed] [Google Scholar]
- Liu YX, Liu K, Feng Q, Hu ZY, Liu HZ, Fu GQ, Li YC, Zou RJ, Ny T 2004 Tissue-type plasminogen activator and its inhibitor plasminogen activator inhibitor type 1 are coordinately expressed during ovulation in the rhesus monkey. Endocrinology 145:1767–1775 [DOI] [PubMed] [Google Scholar]
- Ohnishi J, Ohnishi E, Shibuya H, Takahashi T 2005 Functions for proteinases in the ovulatory process. Biochim Biophys Acta 1751:95–109 [DOI] [PubMed] [Google Scholar]
- Colgin DC, Murdoch WJ 1997 Evidence for a role of the ovarian surface epithelium in the ovulatory mechanism of the sheep: secretion of urokinase-type plasminogen activator. Anim Reprod Sci 47:197–204 [DOI] [PubMed] [Google Scholar]
- Politis I, Srikandakumar A, Turner JD, Tsang BK, Ainsworth L, Downey BR 1990 Changes in and partial identification of the plasminogen activator and plasminogen activator inhibitor systems during ovarian follicular maturation in the pig. Biol Reprod 43:636–642 [DOI] [PubMed] [Google Scholar]
- Canipari R, Strickland S 1986 Studies on the hormonal regulation of plasminogen activator production in the rat ovary. Endocrinology 118:1652–1659 [DOI] [PubMed] [Google Scholar]
- Beers WH 1975 Follicular plasminogen and plasminogen activator and the effect of plasmin on ovarian follicle wall. Cell 6:379–386 [DOI] [PubMed] [Google Scholar]
- Ohnishi J, Yokota J, Rajapakse RG, Ohinishi E, Kudo T, Wada S, Takahashi T 2004 Activity of an enzyme converting single-chain tissue-type plasminogen activator to the two-chain form in preovulatory human follicular fluid. Mol Reprod Dev 67:178–185 [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Bicsak TA, Cajander SB, Ny T, Hsueh AJ 1989 Suppression of ovulation rate by antibodies to tissue-type plasminogen activator and α2-antiplasmin. Endocrinology 124:415–421 [DOI] [PubMed] [Google Scholar]
- Leonardsson G, Peng XR, Liu K, Nordstrom L, Carmeliet P, Mulligan R, Collen D, Ny T 1995 Ovulation efficiency is reduced in mice that lack plasminogen activator gene function: functional redundancy among physiological plasminogen activators. Proc Natl Acad Sci USA 92:12446–12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL 2001 The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod 7:731–739 [DOI] [PubMed] [Google Scholar]
- Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Dore M 2004 Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update 10:373–385 [DOI] [PubMed] [Google Scholar]
- Boerboom D, Sirois J 1998 Molecular characterization of equine prostaglandin G/H synthase-2 and regulation of its messenger ribonucleic acid in preovulatory follicles. Endocrinology 139:1662–1670 [DOI] [PubMed] [Google Scholar]
- Wong WY, DeWitt DL, Smith WL, Richards JS 1989 Rapid induction of prostaglandin endoperoxide synthase in rat preovulatory follicles by luteinizing hormone and cAMP is blocked by inhibitors of transcription and translation. Mol Endocrinol 3:1714–1723 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R 1999 Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology 140:2685–2695 [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK 1997 Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197–208 [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Hansen TR, McPherson LA 1993 A review: role of eicosanoids in vertebrate ovulation. Prostaglandins 46:85–115 [DOI] [PubMed] [Google Scholar]
- Peters MW, Pursley JR, Smith GW 2004 Inhibition of intrafollicular PGE2 synthesis and ovulation following ultrasound-mediated intrafollicular injection of the selective cyclooxygenase-2 inhibitor NS-398 in cattle. J Anim Sci 82:1656–1662 [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL 2002 Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod 17:2825–2831 [DOI] [PubMed] [Google Scholar]
- Pall M, Friden BE, Brannstrom M 2001 Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: a randomized double-blind study. Hum Reprod 16:1323–1328 [DOI] [PubMed] [Google Scholar]
- Espey L, Shimada H, Okamura H, Mori T 1985 Effect of various agents on ovarian plasminogen activator activity during ovulation in pregnant mare’s serum gonadotropin-primed immature rats. Biol Reprod 32:1087–1094 [DOI] [PubMed] [Google Scholar]
- Duffy DM, Dozier BL, Seachord CL 2005 Prostaglandin dehydrogenase and prostaglandin levels in periovulatory follicles: implications for control of primate ovulation by prostaglandin E2. J Clin Endocrinol Metab 90:1021–1027 [DOI] [PubMed] [Google Scholar]
- Vandevoort CA, Baughman WL, Stouffer RL 1989 Comparison of different regimens of human gonadotropins for superovulation of rhesus monkeys: ovulatory response and subsequent luteal function. J In Vitro Fert Embryo Transf 6:85–91 [DOI] [PubMed] [Google Scholar]
- Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB 1996 Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA 93:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DP, Alexander M, Zelinski-Wooten M, Stouffer RL 1996 Maturity and fertility of rhesus monkey oocytes collected at different intervals after an ovulatory stimulus (human chorionic gonadotropin) in in vitro fertilization cycles. Mol Reprod Dev 43:76–81 [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Tarantal AF 1991 The macaque model for in vitro fertilization: superovulation techniques and ultrasound-guided follicular aspiration. J Med Primatol 20:110–116 [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL, Duffy DM 1999 Gonadotropin and steroid regulation of steroid receptor and aryl hydrocarbon receptor messenger ribonucleic acid in macaque granulosa cells during the periovulatory interval. Endocrinology 140:4753–4760 [DOI] [PubMed] [Google Scholar]
- Seachord CL, VandeVoort CA, Duffy DM 2005 Adipose differentiation-related protein: a gonadotropin- and prostaglandin-regulated protein in primate periovulatory follicles. Biol Reprod 72:1305–1314 [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL 1999 Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in macaque periovulatory granulosa cells: time course and steroid regulation. Biol Reprod 61:14–21 [DOI] [PubMed] [Google Scholar]
- Markosyan N, Dozier BL, Lattanzio FA, Duffy DM 2006 Primate granulosa cell response via prostaglandin E2 receptors increases late in the periovulatory interval. Biol Reprod 75:868–876 [DOI] [PubMed] [Google Scholar]
- Lawrence RA, Jones RL, Wilson NH 1992 Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br J Pharmacol 105:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S 1994 International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46:205–229 [PubMed] [Google Scholar]
- Duffy DM, Seachord CL, Dozier BL 2005 Microsomal prostaglandin E synthase-1 (mPGES-1) is the primary form of PGES expressed by the primate periovulatory follicle. Hum Reprod 20:1485–1492 [DOI] [PubMed] [Google Scholar]
- Bartlett SE, Menino Jr AR 1993 Partial characterization of the plasminogen activator produced by ovine embryos in vitro. Biol Reprod 49:381–386 [DOI] [PubMed] [Google Scholar]
- de Vries TJ, Kitson JL, Silvers WK, Mintz B 1995 Expression of plasminogen activators and plasminogen activator inhibitors in cutaneous melanomas of transgenic melanoma-susceptible mice. Cancer Res 55:4681–4687 [PubMed] [Google Scholar]
- Canipari R, Strickland S 1985 Plasminogen activator in the rat ovary. Production and gonadotropin regulation of the enzyme in granulosa and thecal cells. J Biol Chem 260:5121–5125 [PubMed] [Google Scholar]
- Jones PB, Vernon MW, Muse KN, Curry Jr TE 1989 Plasminogen activator and plasminogen activator inhibitor in human preovulatory follicular fluid. J Clin Endocrinol Metab 68:1039–1045. [DOI] [PubMed] [Google Scholar]
- Shen X, Minoura H, Yoshida T, Toyoda N 1997 Changes in ovarian expression of tissue-type plasminogen activator and plasminogen activator inhibitor type-1 messenger ribonucleic acids during ovulation in rat. Endocr J 44:341–348 [DOI] [PubMed] [Google Scholar]
- Li M, Karakji EG, Xing R, Fryer JN, Carnegie JA, Rabbani SA, Tsang BK 1997 Expression of urokinase-type plasminogen activator and its receptor during ovarian follicular development. Endocrinology 138:2790–2799 [DOI] [PubMed] [Google Scholar]
- Jones PB, Muse KN, Wilson EA, Curry Jr TE 1988 Expression of plasminogen activator (PA) and a PA inhibitor in human granulosa cells from preovulatory follicles. J Clin Endocrinol Metab 67:857–860 [DOI] [PubMed] [Google Scholar]
- Dow MP, Bakke LJ, Cassar CA, Peters MW, Pursley JR, Smith GW 2002 Gonadotropin surge-induced up-regulation of the plasminogen activators (tissue plasminogen activator and urokinase plasminogen activator) and the urokinase plasminogen activator receptor within bovine periovulatory follicular and luteal tissue. Biol Reprod 66:1413–1421 [DOI] [PubMed] [Google Scholar]
- Murdoch J, Van Kirk EA, Murdoch WJ 1999 Hormonal control of urokinase plasminogen activator secretion by sheep ovarian surface epithelial cells. Biol Reprod 61:1487–1491 [DOI] [PubMed] [Google Scholar]
- Schulze AJ, Huber R, Bode W, Engh RA 1994 Structural aspects of serpin inhibition. FEBS Lett 344:117–124 [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA 1993 Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4:197–250 [DOI] [PubMed] [Google Scholar]
- Curry Jr TE, Osteen KG 2003 The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 24:428–465 [DOI] [PubMed] [Google Scholar]
- Curry Jr TE, Smith MF 2006 Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med 24:228–241 [DOI] [PubMed] [Google Scholar]
- Coleman RA, Eglen RM, Jones RL, Narumiya S, Shimizu T, Smith WL, Dahlen SE, Drazen JM, Gardiner PJ, Jackson WT 1995 Prostanoid and leukotriene receptors: a progress report from the IUPHAR working parties on classification and nomenclature. Adv Prostaglandin Thromboxane Leukot Res 23:283–285 [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD 2001 Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41:661–690 [DOI] [PubMed] [Google Scholar]