Abstract
KiSS-1 gene expression has been shown to increase as puberty approaches, and its peptide products, kisspeptins, are involved in LHRH secretion at puberty. Factors contributing to increased KiSS-1 expression, however, have not been identified; thus, the purpose of this study was to assess whether IGF-I could induce transcription of this gene in prepubertal female rats. IGF-I or saline was centrally administered to immature rats that were killed 2, 4, and 6 h later. Real-time PCR revealed that IGF-I induced (P < 0.01) KiSS-1 gene expression at 6 h in a tissue fragment that contained both the anteroventral periventricular (AVPV) and arcuate (ARC) nuclei. Subsequently, the AVPV and ARC nuclei were separated to assess whether region-specific effects could be identified. IGF-I stimulated (P < 0.01) KiSS-1 gene expression in the AVPV nucleus at 6 h after injection, with no change observed in the ARC nucleus. Serum estradiol (E2) levels were not altered at any time point after IGF-I, demonstrating that the increased KiSS-1 expression observed was not caused by an elevation in E2. Additionally, the IGF-I action to induce KiSS-1 gene expression in the AVPV nucleus was further demonstrated when the IGF-I was administered systemically. E2 appears to play an important permissive role because 1-d ovariectomized rats responded to IGF-I with increased (P < 0.01) KiSS-1 expression, whereas, 20 d after ovariectomy, when the E2 levels had fallen below assay sensitivity, the IGF-I was unable to induce KiSS-1 expression. The IGF-I effect was further demonstrated by showing that the IGF-I receptor antagonist, JB-1, blocked the IGF-I-induced increase in KiSS-1 expression. Collectively, these data indicate that IGF-I is an activator of the KiSS-1 gene in the prepubertal female rat.
IGF-1 is an early activator of the KiSS-1 gene at the time of female puberty.
Kisspetins are products of the KiSS-1 metastasis suppressor gene and ligands of the G protein-coupled receptor 54 (GPR54) and play a key role in the timing of puberty (1,2). The importance of the KiSS-1/GPR54 system to reproduction was revealed by the discovery that a mutation of GPR54 in human (3,4) and a deletion of GPR54 locus in mice (4) caused hypogonadotropic hypogonadism and delayed puberty. Expression of the KiSS-1 and GPR54 genes have been shown to increase during pubertal development (5) and change depending on the steroid milieu (6,7). The KiSS-1-derived peptide products, kisspeptin-54 (metastin; named for its ability to suppress tumor metastasis) and kisspeptin-10, have been shown to act at the hypothalamic level to stimulate LH release in immature rats and rhesus monkeys (5,8,9) and advance vaginal opening in rats (2). Although these studies demonstrate that KiSS-1 and GPR54 play important roles in the onset of puberty, the signals that control the increase in KiSS-1 gene expression during prepubertal development have not been identified.
In recent years, IGF-I has been considered one of the components contributing to early signaling processes controlling LHRH secretion and the timing of female puberty. Circulating IGF-I increases before puberty in rodents (10,11), ruminants (12), subhuman primates (13), and humans (14,15). Central administration of IGF-I stimulates the LHRH/LH-releasing system and can advance puberty in female rats (11). IGF-I replacement in GH-deficient mice restores normal puberty (16), and in the primate, IGF-I can advance the time of first ovulation (17). IGF-I can cross the blood-brain barrier (18) and bind to its receptors that are located on neurons and glia throughout the brain, including the preoptic area (POA) and medial basal hypothalamus (MBH) (19,20,21,22). Additionally, IGF-I can induce expression of the hypothalamic Oct-2 gene (23), a downstream gene associated with the glial component of the LHRH-releasing pathway.
We propose that IGF-I may be an upstream regulator of the KiSS-1 gene at the time of female puberty. Thus, the present study was conducted to determine whether centrally administered IGF-I can increase KiSS-1 gene expression in specific regions of the brain controlling LHRH secretion in prepubertal female rats and, if so, to determine any role E2 may have on this action. A preliminary report of these findings has been presented in abstract form (24).
Materials and Methods
Animals
Eighteen-day pregnant female rats of the Sprague Dawley line were purchased from Charles River (Boston, MA) and allowed to deliver pups normally in the Texas A&M University lab animal facility. Female pups were weaned at 21 d of age and housed three per cage under controlled conditions of light (lights on at 0600 h and off at 1800 h) and temperature (23 C), with ad libitum access to food and water. All procedures performed on the animals were approved by the University Animal Care and Use Committee and in accordance with the National Academy of Sciences National Research Council Guidelines for the Care and Use of Laboratory Animals. Surgical anesthesia was an ip injection of 2.5% tribromoethanol (0.5 ml/ 60 g body weight).
Experimental design
Experiment 1: IGF-I induction of KiSS-1 mRNA expression
Female rats (22 d old) were stereotaxically implanted with a stainless steel cannula (23 gauge) in the third ventricle (3V) of the brain. After moving 1.5 mm caudally from bregma, the cannula was lowered 8.2 mm ventrally into the 3V and secured with dental cement (23). After 4 d recovery, the 26-d-old rats were administered sterile saline or 20, 100, or 200 ng IGF-I in 3 μl saline. It has been shown previously that both 3V and systemic delivery of IGF-I are capable of stimulating LH release in prepubertal female animals (11,25). Because the site of this action is at the hypothalamic level (11), the 3V approach is useful, especially because it bypasses the IGF-binding proteins present in blood. The rat IGF-I was purchased from Novozymes Biopharma AU Ltd. (Adelaide, Australia). The peptide was delivered during a 1-min period of time into the 3V at 0900 h, and the animals were killed 2, 4, and 6 h after IGF-I/saline. These doses are in the low to moderate range of the dose response used previously to stimulate an increase in LH (11). These doses and time points were also used previously to demonstrate IGF-I’s ability to stimulate Oct-2 gene expression (23). Juvenile (26 d old) female rats were used to precociously induce expression of the KiSS-1 gene before the elevation known to occur in female rats at 30 d of age (5). Animals were killed by decapitation and proper placement of the cannula was verified. The POA/MBH region was dissected from each brain, divided into two blocks of tissue as detailed below, and then frozen on dry ice. For the first block, we made an initial cut approximately 1 mm rostral to the optic chiasm (OC), and then a second cut was made caudally, which included the rostral one third of the OC. The block was then formed by making cuts along the borders of the OC laterally and along the border of the anterior commissure (AC) dorsally. Thus, this block consists of the rostral POA and contained only the rostral-most extent of the anteroventral periventricular (AVPV) nucleus. The second block contained the remaining two thirds of the OC rostrally and included the mammillary bodies caudally. This block was formed by making cuts along the borders of the OC laterally and along the border of the thalamus dorsally. Thus, this block consists of the middle and caudal portions of the POA as well as the rostral hypothalamus. Because this block contained most of the AVPV nucleus, along with the arcuate (ARC) nucleus, the two principal sites of KiSS-1/kisspeptin production, it is referred to as AVPV/ARC. KiSS-1 gene expression was then assessed in these tissues by real-time PCR. Other studies have included the POA and ARC tissues together in one block to determine changes in KiSS-1 gene expression (2,26,27).
Experiment 2: regional IGF-I induction of KiSS-1 mRNA expression
Female rats (22 d old) were implanted with 3V cannulae as described above. After 4 d recovery, 26-d-old rats were administered either sterile saline or 200 ng IGF-I/3 μl saline. This dose was determined to be the most effective in experiment 1. The peptide was delivered during a 1-min period of time into the 3V at 0900 h, and the animals were killed 1, 2, 4, and 6 h after IGF-I/saline. Animals were killed by decapitation, and proper placement of the cannula was verified. To determine whether the IGF-I-induced expression of KiSS-1 gene is site specific, two different blocks, one containing the entire AVPV nucleus and the other containing the ARC nucleus, were dissected from each brain and frozen separately on dry ice. For the block containing the AVPV nucleus, a cut was made approximately 1 mm rostral to the OC, and then a second cut was made at the caudal border of the OC. The block was then formed by making cuts along the borders of the OC laterally and along the border of the AC dorsally. The block containing the ARC nucleus extended from the previous cut to the mammillary bodies caudally. This block was formed by making cuts along the borders of the hypothalamic sulci laterally and along the border of the thalamus dorsally. KiSS-1 gene expression was then assessed in these tissues by real-time PCR. Trunk blood samples were analyzed for serum LH and estradiol (E2) by RIA.
Experiment 3: effect of systemic administration of IGF-I on KiSS-1 gene expression
Because the serum levels of IGF-I rise before puberty, we conducted this experiment to determine whether the systemic administration of the peptide, and thus prematurely elevating its circulating levels, would induce a similar increase in KiSS-1 gene expression in the AVPV nucleus as we observed after the peptide was administered centrally. Twenty-five-day-old rats were implanted with a SILASTIC brand cannula (Dow Corning, Midland, MI) inserted into the right external jugular vein according to a previously described method (28). On the following day, 26-d-old rats were attached to an extension of tubing to each cannula and then flushed with heparinized saline (100 IU/ml). At 0900 h, a basal blood sample was drawn from each freely moving animal, and then an iv injection of saline or IGF-I (200 ng/100 μl) was delivered into the jugular cannula. Six hours after the iv injection, the animals were killed by decapitation, and trunk blood was taken along with the AVPV nucleus, which was dissected as above and then frozen on dry ice for real-time PCR.
After confirmation that systemic delivery of the peptide could induce an increase in KiSS-1 gene expression, the experiment was repeated to assure that elevated circulating levels of IGF-I occurred after the iv infusion of the peptide. In this case, the experiment was conducted as above except that additional blood samples were drawn via the jugular cannula at 0.5, 1, 2, and 4 h after the administration of IGF-I. As above, the final blood sample and tissues were collected after decapitation 6 h after IGF-I was infused. All blood samples were centrifuged at 4 C and the serum stored at −70 C until assayed for IGF-I and E2.
Experiment 4A: effect of IGF-I on KiSS-1 gene expression 1 d after ovariectomy
Female rats (23 d old) were implanted with 3V cannulae and then were bilaterally ovariectomized when 29 d of age. Twenty-four hours later, IGF-I (200 ng/3 μl) or an equal volume of saline was delivered during a 1-min period of time into the 3V at 1000 h. All animals were killed by decapitation at 6 h after IGF-I/saline, and proper placement of each cannula was verified. The same AVPV and ARC tissue blocks as in experiment 2 were dissected from each brain and frozen on dry ice. KiSS-1 gene expression was then assessed in these tissues by real-time PCR. Serum E2 was assessed in trunk blood samples.
Experiment 4B: effect of IGF-I on KiSS-1 gene expression 20 d after ovariectomy
Rats were bilaterally ovariectomized when 29 d old and then implanted with 3V cannulae 14 d later. The effect of IGF-I stimulation was assessed at 20 d after ovariectomy using the same protocol as described in experiment 4A.
Experiment 5: inhibition of KiSS-1 gene expression by an IGF-I antagonist
Rats were implanted with 3V cannulae when 22 d old. After 4 d recovery, the animals were divided into three groups. At 0900 h and again at 1000 h, animals in groups 1 and 2 received a 3V injection of saline (4 μl), whereas animals in group 3 received a 3V injection of JB-1, a specific IGF-I receptor (IGF-IR) antagonist (4 μg/4 μl; Bachem, Inc., Palo Alto, CA). JB-1 is an IGF-I analog that acts specifically on the type 1 IGF receptor to block IGF-I-induced autophosphorylation and cell proliferation (29). This specific dose of JB-1 has been used to block E2-induced LHRH release (30). At 1030 h, a 3V injection of IGF-I (200 ng/3 μl saline) was administered to animals in groups 2 and 3. Animals in group 1 received a 3V injection of saline. All animals were killed 6 h after the 3V injection of IGF-I or saline. The 6-h time point was chosen because we observed in experiment 2 that KiSS-1 gene transcription in the AVPV nucleus increased significantly at this time. The brains were removed, cannulae placement was verified, and the brain was dissected as above in experiment 2 and frozen on dry ice for real-time PCR.
Isolation of total RNA
Total cellular RNA was initially isolated from the tissue blocks by homogenizing in TRIzol reagent (Invitrogen Life Technologies, Carsbad, CA). Each homogenate was further extracted for RNA using the QIAGEN RNeasy kit and treated with ribonuclease-free deoxyribonuclease I according to the manufacturer’s instructions (QIAGEN Inc., Valencia, CA). The integrity of the RNA was checked by the visualization of the ethidium bromide-stained 28S and 18S rRNA bands. The concentration of RNA was measured spectrophotometrically by absorbance at 260 nm in a Model Smartspec 3000 spectrophotometer (Bio-Rad Laboratories, Hercules, CA).
Reverse transcription and real-time quantitative PCR
Total RNA (1 μg) from each preparation was denatured at 65 C for 5 min, and then samples were used for RT with oligo (deoxythymidine) using SuperScript III First-Strand Synthesis System (Invitrogen). Real-time PCR was performed in 25-μl reactions containing 2 μl cDNA, 500 nm primer pairs, and 1× SYBR green PCR master mix in 96-well plates on an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA). PCR primers for the analysis were designed according to the guidelines of Applied Biosystems with help of the Primer Express 2.0 software (Applied Biosystems, Foster City, CA), and each primer was checked by BLAST search for the absence of any cross-reactivity. To check the integrity of the RNA and to normalize for the quantity of RNA in the initial RT reaction, the housekeeping gene β-actin was run in all reactions separately under the same experimental conditions. A reaction without reverse transcriptase was also performed to rule out the possibility of any contamination of genomic DNA. The primers for the PCR are rat KiSS-1 (GenBank accession no. AY196983; forward 5′-GCTGCTGCTTCTCCTCTGTGT-3′ and reverse 5′-CTGTTGGCCTGTGGGTTCA-3′; product size 88 bp) and rat β-actin (GenBank accession NM_031144; forward 5′-TCTGTGTGGATTGGTGGCTCTA-3′ and reverse 5′-CTGCTTGCTGATCCACATCTG-3′; product size 69 bp). The PCR cycling conditions were 95 C for 10 min, followed by 40 cycles at 95 C for 15 sec and 60 C for 1 min. Dissociation curve analysis was also done for each gene at the end of the PCR. In this regard, each amplicon generated a single peak and did not show any peak when the template was not included in the PCR. Additionally, each PCR product was electrophoresed onto a 2% agarose gel containing 0.5 μg/ml ethidium bromide, which showed a single band of the expected size. This confirms the specificity of the primers and shows that there was no formation of primer-dimers. The raw data from each experiment was used to determine the relative levels of expression for each gene by the Δ-Δ CT method as described by Hettinger et al. (31).
RIAs
Serum E2, LH, and IGF-I levels were measured as previously described (11,32,33). The E2 assay kit was purchased from Diagnostic Products Corp. (Los Angeles, CA). Reagents for the LH assay were obtained from Dr. A. F. Parlow and the National Hormone and Pituitary Program. Serum IGF-I was measured by a specific rat IGF-I RIA kit p’?urchased from Diagnostic Systems Laboratories, Webster, TX (Beckman Coulter, Inc.). The sensitivities for the E2, LH, and IGF-I assays were 5 pg/ml, 0.07 ng/ml, and 150 ng/ml, respectively. The inter- and intraassay variations for all assays were less than 5%.
Statistical analysis
Differences between two groups were analyzed by Student’s t test. Multiple comparisons were performed using ANOVA, with post hoc testing using the Student-Newman-Keuls multiple range test. All statistical tests were conducted using INSTAT and Prism software for the IBM PC (GraphPad, Inc., San Diego, CA). P values <0.05 were considered to be statistically significant.
Results
Experiment 1: IGF-I induction of KiSS-1 mRNA expression
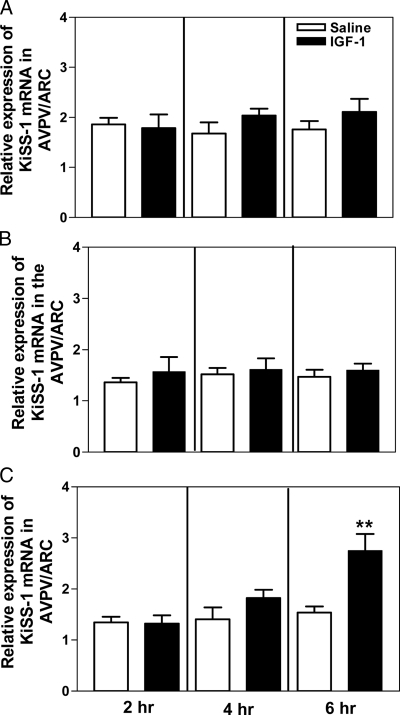
IGF-I did not alter the level of gene expression for KiSS-1 at any dose or time point studied in the tissue block representing the most rostral extent of the POA (not shown). In contrast, dose- and time-related changes were observed in the POA/Hypothalamus block of tissue that contained the majority of the AVPV nucleus as well as the ARC nucleus. Specifically, the 20- and 100-ng doses of IGF-I administered into the 3V did not alter KiSS-1 gene expression after 2, 4, or 6 h compared with the expression observed in the tissues obtained from saline control animals (Fig. 1, A and B). The 200-ng dose of IGF-I, however, induced a marked increase (P < 0.01) in the expression of KiSS-1 mRNA at the 6-h time point (Fig. 1C).
Figure 1.
Effect of IGF-I on KiSS-1 gene expression in a tissue block containing both the AVPV and ARC nuclei. IGF-I was injected into the 3V at doses of 20 (A), 100 (B), and 200 (C) ng, and KiSS-1 gene expression in the AVPV/ARC block was assessed by real-time PCR in all groups at 2, 4, and 6 h after injection. Note that the 200-ng dose stimulated KiSS-1 gene expression at 6 h. Open bars represent the mean (±sem) of the saline-treated control animals, and solid bars represent the mean (±sem) of the IGF-I-treated animals. Each bar represents six to 12 animals. **, P < 0.01 vs. saline-treated controls.
Experiment 2: regional IGF-I induction of KiSS-1 mRNA expression
Two separate tissue blocks, one containing the entire AVPV nucleus and the other containing the ARC nucleus, were used to assess any site-specific effects of IGF-I on the KiSS-1 gene. Figure 2A shows that IGF-I (200 ng) administered into the 3V did not significantly alter KiSS-1 gene expression in the tissue block containing the AVPV nucleus between 1 and 4 h after injection; however, the level of gene expression in this region was increased (P < 0.01) at 6 h. Conversely, KiSS-1 gene expression did not change in the block containing the ARC nucleus at any of the time points (not shown). Figure 2B shows that compared with control levels, IGF-I induced an increase (P < 0.05) in serum LH only at the 1-h after injection time point and not thereafter. Figure 2C demonstrates that E2 levels were not different between saline control animals and animals that received IGF-I at any of the time points.
Figure 2.
Effect of IGF-I on KiSS-1 gene expression in the AVPV nucleus. IGF-I was injected into the 3V at a dose of 200 ng, and KiSS-1 gene expression (A), LH (B), and E2 (C) were assessed at 2, 4, and 6 h after IGF-I injection. Note that real-time PCR revealed that IGF-I induced an increase in KiSS-1 mRNA at 6 h. LH levels were modestly elevated only at 1 h, and E2 levels were not altered at any time point. Open bars represent the mean (±sem) of the saline-treated control animals, and solid bars represent the mean (±sem) of the IGF-I-treated animals. Each bar represents five to 12 animals. *, P < 0.05; **, P < 0.01 vs. saline-treated controls.
Experiment 3: Effect of systemic administration of IGF-I on KiSS-1 gene expression
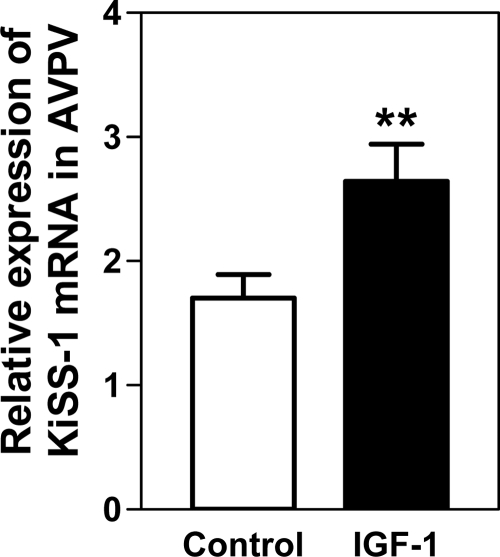
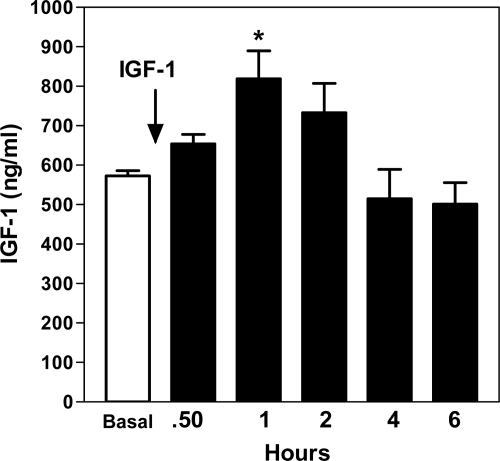
Figure 3 demonstrates that the systemic administration of IGF-I (200 ng) was capable of inducing an increase (P < 0.01) in KiSS-1 gene expression in the AVPV nucleus in a manner similar to that observed after the central administration of the peptide. Figure 4 shows that the serum levels of IGF-I were elevated (P < 0.05) over the basal concentration after infusion into the jugular vein, peaking by 1 h and then declining. The basal levels of serum E2 were 15.6 ± 3.3 pg/ml and showed no change after IGF-I administration (not shown).
Figure 3.
Effect of systemic administration of IGF-I on KiSS-1 gene expression in the AVPV nucleus. Saline or IGF-I (200 ng) was delivered into the jugular vein and KiSS-1 assessed by real-time PCR at 6 h after saline/IGF-I injection. Note that IGF-I induced an increase (P < 0.01) in KiSS-1 gene expression. The open bar represents the mean (±sem) of the saline-treated control rats. The solid bar represents the mean (±sem) of the rats receiving IGF-I. Each bar represents 13 for saline control and 17 for IGF-I-treated animals. **, P < 0.01 vs. control.
Figure 4.
Effect of systemic administration of IGF-I on serum levels of the peptide. After a basal blood sample was taken, IGF-I (200 ng) was delivered into the jugular vein and blood samples were withdrawn at 0.5, 1, 2, and 4 h before a final blood sample was collected after decapitation at 6 h after IGF-I. Note that by 1 h after infusion, the serum levels of IGF-I had increased and then declined gradually to basal levels by 4 h. The open bar represents the mean (±sem) basal serum levels of IGF-I, and the solid bars depict the mean (±sem) IGF-I levels after infusion of the peptide. Each bar represents six animals. *, P < 0.05 vs. basal.
Experiment 4: IGF-I induction of KiSS-1 mRNA expression in ovariectomized rats
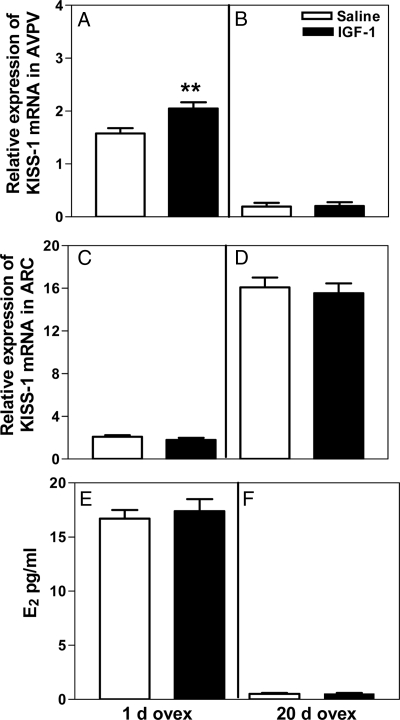
Figure 5A demonstrates that 1 d after ovariectomy, IGF-I is capable of stimulating (P < 0.01) KiSS-1 gene expression in the AVPV nucleus 6 h after injection. Conversely, IGF-I did not induce KiSS-1 gene expression in the ARC nucleus at this time (Fig. 5C). Figure 5E demonstrates that the ability of IGF-I to induce the KiSS-1 gene in the AVPV nucleus, as shown in Fig. 5A, was associated with E2 levels of 17 pg/ml.
Figure 5.
Effect of ovariectomy on IGF-I stimulated KiSS-1 gene expression in the AVPV and ARC nuclei. IGF-I (200 ng) was injected into the 3V 1 and 20 d after ovariectomy, and KiSS-1 expression was assessed in both nuclei after 6 h by real-time PCR. Note that 1 d after ovariectomy (ovex), the IGF-I stimulated (P < 0.01) KiSS-1 gene expression in the AVPV nucleus (A); however, in the ARC nucleus, there was no change in KiSS-1 gene expression (C). The ability of IGF-I to induce the KiSS-1 gene at 1 d after ovariectomy was associated with serum E2 levels of 17 pg/ml (E). Note that 20 d after ovariectomy, there was a marked decline in basal KiSS-1 expression in the AVPV nucleus, and the IGF-I was ineffective in inducing KiSS-1 expression (B). In the ARC nucleus, there was a marked increase in the basal expression of KiSS-1 gene expression, and again no stimulation by IGF-I (D). At 20 d after ovariectomy, when IGF-I was ineffective at stimulating the gene, the serum E2 levels were below assay sensitivity of 5 pg/ml (F). Open bars represent the mean (±sem) of the saline-treated control animals, and solid bars represent the mean (±sem) of the IGF-I-treated animals. Each bar represents 11 1-d ovariectomized rats and seven 20-d ovariectomized rats. **, P < 0.01 vs. saline-treated controls.
Figure 5B shows that 20 d after ovariectomy, there was a marked reduction in basal KiSS-1 mRNA in the AVPV region and that the IGF-I peptide was ineffective at this time in stimulating KiSS-1 gene expression. In the ARC nucleus, there was a marked ovariectomy-induced increase in KiSS-1 gene expression and, again, no stimulation of the gene in this region by IGF-I (Fig. 5D). Figure 5F depicts that 20 d after ovariectomy, when IGF-I was ineffective in stimulating the KiSS-1 gene, the circulating levels of E2 were below the assay sensitivity of 5 pg/ml.
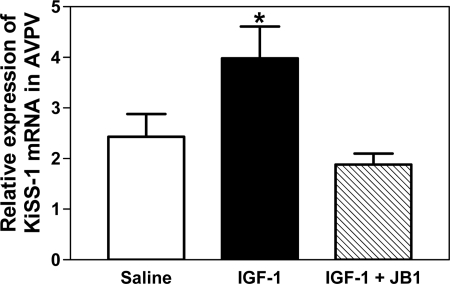
Experiment 5: Inhibition of KiSS-1 gene expression by an IGF-I antagonist
Specificity of the effect of IGF-I to induce transcription of the KiSS-1 gene was assessed by centrally administering the selective IGF-IR antagonist, JB-1, before the IGF-I stimulation. Figure 6 shows that the IGF-I-induced increase (P < 0.05) in KiSS-1 gene expression in the AVPV nucleus was blocked by JB-1.
Figure 6.
Effect of IGF-IR inhibition on IGF-I-induced KiSS-1 gene expression in the AVPV nucleus. The IGF-IR blocker JB-1 was injected into the 3V 1.5 h and then again 30 min before the administration of IGF-I (200 ng). KiSS-1 mRNA was assessed by real-time PCR at 6 h after IGF-I injection. Note that the IGF-I-induced increase in KiSS-1 gene expression was blocked in the rats that received the JB-1. The open bar represents the mean (±sem) of the saline-treated control rats. The solid bar represents the mean (±sem) of the IGF-I-treated rats, and the hatched bar represents the mean (±sem) of the rats treated with both JB-1and IGF-I. Each bar represents six animals. *, P < 0.05 vs. the saline-treated control and JB-1/IGF-I groups.
Discussion
It is well known that the KiSS-1 gene, its receptor, and its protein products are involved in the culmination of events that results in sexual maturation (for review see Ref. 1). The KiSS-1 gene and its receptor, GPR54, increase in the reproductive control center of the brain before pubertal onset in primates (9) and rats (2). These genes respond to changes in sex steroid levels, in that gonadectomy decreases expression of the KiSS-1 gene in the AVPV nucleus but increases its expression in the ARC nucleus, whereas steroid replacement causes the opposite effects (6,7). Changes in environmental influences such as light cycle (34) and nutrition (26) also affect the expression of the KiSS-1 gene. However, the factors that control the prepubertal changes in KiSS-1 gene expression and the precise mechanisms whereby kisspeptins activate LHRH neurons have yet to be identified.
Several lines of evidence suggest that IGF-I is a potential candidate to activate the KiSS-1 gene at puberty. Serum IGF-I increases before puberty and can cross the blood-brain barrier (18) and bind to its receptors located on neurons and glia throughout the brain, including the POA and MBH (19,20,21,22). Immunoreactive levels of IGF-I increase in the glia of the median eminence before the first preovulatory surge (35), along with an increase in IGF-IR mRNA (11). Central administration of IGF-I stimulates the LHRH/LH-releasing system and advances puberty in female rats (11), can restore puberty in GH-deficient female mice (16), and advances first ovulation in female rhesus monkeys (17). Previously, we demonstrated that IGF-I can stimulate the transcription factors Oct 2a and 2c before their normal elevation during the peripubertal period (23). These POU homeodomain genes are components of the glial-neuronal regulatory process that controls LHRH secretion by trans-activating the gene encoding TGFα at the time of puberty (36). IGF-I is also involved in the positive feedback effect of E2 on reproductive receptivity and the LH surge (30), and a minimum threshold of E2 is necessary for IGF-I to stimulate LH release (32) before pubertal onset. Given the actions of IGF-I and kisspeptins at the time of puberty, we felt it important to determine whether IGF-I can induce prepubertal KiSS-1 gene expression.
Previous studies have used a block of tissue that included both the AVPV and ARC nuclei to determine the changes in the level of KiSS-1 gene expression, i.e. during pubertal development (2,5), as a result of undernutrition (26) and throughout the estrous cycle (27). We noted initially that the block of tissue representing the rostral POA and containing only the rostral most extent of the AVPV nucleus, did not show any change in KiSS-1 gene expression after stimulation with IGF-I. Conversely, IGF-I stimulated the KiSS-1 gene in the tissue block that contained the middle and caudal portions of the AVPV nucleus as well as the ARC nucleus. These results infer that IGF-I is capable of inducing the KiSS-1 gene and that the responsive neurons were in the caudal two thirds of the AVPV nucleus and/or within the ARC nucleus. As a result, we subsequently separated the AVPV and ARC nuclei into two distinct blocks to determine whether these regions respond differently to IGF-I stimulation. We found that KiSS-1 gene expression responded to the IGF-I stimulation in the brain fragment containing the AVPV nucleus but not the block containing the ARC nucleus. Our demonstration of regional differences in KiSS-1 gene responsiveness to IGF-I stimulation are in line with other reports showing region-specific responses of this gene under different experimental conditions (6,7,33,37,38). It should be noted that a study using a hypothalamic mouse cell line revealed that IGF-I stimulated LHRH gene expression but caused only a modest increase in KiSS-1 gene expression (39). The lack of a marked KiSS-1 response to IGF-I in that study, compared with the significant response observed in the present study, is most likely due to experimental differences between the in vivo and cell culture approaches.
The fact that we showed an acute IGF-I induction of the KiSS-1 gene in 26-d-old female rats after both central and systemic administration is important, because this occurs before the normal rise in the expression of this gene at d 30 (5). Serum levels of IGF-I rise during juvenile development (10,11) and continue to rise throughout peripubertal development and are associated with increases in circulating gonadotropins and E2 (11). Under the conditions of the present study, the 200-ng dose of IGF-I administered systemically to 26-d-old rats caused an increase in serum levels of the peptide from 578 ng/ml basally to 819 ng/ml 1 h after the peptide was administered into the jugular vein. It is possible that the peak serum IGF-I levels could have occurred between 30 min and 1 h; however, this time interval was not assessed. Regardless of the exact time that the peak occurred, it is not surprising that some time is required for the bolus peptide infusion to evenly disperse throughout circulation. Importantly, the resulting elevation in serum IGF-I apparently increased the IGF-I content recognized by the brain to a threshold that precociously induced KiSS-1 expression in the AVPV nucleus.
During normal pubertal development, hepatic IGF-I gene expression increases, whereas changes in hypothalamic expression of this gene were not observed (11), thus indicating that IGF-I of peripheral origin is capable of acting within the hypothalamus to activate the prepubertal LHRH/LH axis in female rats (11). We have shown previously that IGF-I can directly stimulate LHRH release from the nerve terminals in the median eminence (40) and now show that the peptide can also act to induce expression of the KiSS-1 gene in the brain after its administration either centrally or systemically. Hence, the present study demonstrates a duel central action of the peptide. One action was the expected early IGF-I stimulation of LH release, which was neither robust enough nor sustained long enough to alter the serum levels of E2. The other action noted was the IGF-I induction of the KiSS-1 gene, resulting in a significant increase in the expression of KiSS-1 6 h after IGF-I. The fact that there was no change in serum E2 levels indicates further that it is IGF-I and not E2 that acutely induces the prepubertal KiSS-1 gene.
Chronic administration of IGF-I, however, is known to cause sustained elevations in serum LH and E2 and advances female puberty (11); hence, we suggest this may be due to an early, then continued induction of the KiSS-1 production of kisspeptin products. Interestingly, the kisspeptins are more potent stimulators of prepubertal LHRH/LH secretion than IGF-I; therefore, this indicates that IGF-I may be activating the KiSS-1 gene in the AVPV nucleus, which is more sensitive at the time of puberty (41), to help drive LH and E2 secretion during the pubertal process. Additionally, serum levels of IGF-I and IGF-IR expression rise during the peripubertal period, peaking at late proestrus (11), and KiSS-1 gene expression in the AVPV nucleus also increases at proestrus (42). Collectively, these results further suggest an IGF-I/KiSS-1 interrelationship during pubertal development.
The actions and interactions between IGF-I and E2 in prepubertal animals are interesting and worthy of further discussion. Previously, we showed that the central administration of IGF-I stimulates prepubertal LH secretion and that this effect is dependent on the presence of E2 (32). Others have reported that IGF-IR gene and protein levels in the hypothalamus also increase along with E2 at puberty (11,21) and together are necessary for reproductive receptivity and lordosis before the LH surge (30). The present study demonstrates that IGF-I can activate the prepubertal KiSS-1 gene in the AVPV nucleus in the absence of elevated serum E2. Our subsequent results from ovariectomized animals demonstrated that the presence of E2 is an important factor in that IGF-I was still able to stimulate the KiSS-1 gene 1 d after ovariectomy, when E2 levels were approximately 17 pg/ml. Conversely, 20 d after ovariectomy, when E2 levels had fallen below the level of the assay sensitivity, the basal expression of KiSS-1 in the AVPV nucleus was markedly reduced and the levels in the ARC nucleus were high, which is in agreement with other results after gonadectomy (6,33,37). Importantly, the IGF-I peptide was ineffective in stimulating KiSS-1 gene expression in both of these areas of the brain after steroid removal. Hence, as with LH (32), we have shown that IGF-I induction of the KiSS-1 gene is dependent on the presence of E2. The positive influence of E2 on the actions of IGF-I also raises the possibility that as E2 levels rise during pubertal development, the magnitude of the IGF-I effect may increase.
During development, the number of immunoreactive kisspeptin cells increases in the female mouse AVPV/periventricular nucleus from postnatal d 25 to d 31, as does the number of LHRH perikarya with kisspeptin neuronal contacts, whereas the number of kisspeptin cells did not change in the ARC (41) nucleus. The authors suggest that the AVPV area contains the kisspeptin neurons that are more sensitive to changes in hormone milieu at the time of puberty. Additionally, it has been shown that the activation of the LHRH-releasing system during pubertal development involves not only the increase in kisspeptins but also an enhancement of this protein’s ability to stimulate LHRH neurons. Han et al. (43) demonstrated that LHRH neurons responded better to kisspeptin as the animals mature sexually, but GPR54 expression in LHRH neurons did not change, thus indicating that the KiSS-1 neuron, not its receptor, is changing during pubertal development. Additionally, brain IGF-I fluctuates in accordance with changes in ovarian steroid levels (35), similar to the response of KiSS-1 neurons in the AVPV nucleus (6,7). Therefore, IGF-I not only has the capacity to stimulate prepubertal LHRH release directly (11,37), but our present results support the hypothesis that IGF-I promotes increased LHRH responsiveness by causing an up-regulation of kisspeptin transcription in the AVPV nucleus, thus further increasing prepubertal LHRH secretion in female rats. Whether prepubertal male rats respond to IGF-I in a similar fashion as described here for females has not been studied.
IGF-I can act via its own type 1 receptor or possibly by acting on the estrogen receptor (ER) via a ligand-independent signaling pathway (for review see Ref. 44). In neuroblastoma cell lines, it has been shown that IGF-I can activate ER transcriptional activity via the Ras/MAPK signaling pathway independent of E2 (45) and that the neurotrophic effects of IGF-I on hypothalamic neurons are blocked by either the estrogen antagonist or an antisense oligonucleotide to ERs (46). Interestingly, it seems that if IGF-I was able to activate the ER independent of E2, then KiSS-1 gene expression should have been elevated by IGF-I in the 20-d ovariectomized animals assessed in the present study, yet no KiSS-1 induction was observed. Experiments using the IGF-I antagonist JB-1 have been successful at identifying specific actions on the IGF-IR (29,30,47,48). JB-1 can specifically inhibit cellular proliferation in a dose-dependent manner and blocks the autophosphorylation of the IGF-IR by IGF-I (29). Other studies have shown in adult rats that JB-1 prevents the accumulation of IGF-I in the ARC and inhibits the decrease of inhibitory synaptic contacts that occurs between proestrus and estrus (47) and disrupts estrous cyclicity (48). Quesada and Etgen (30) administered JB-1 centrally and blocked the estrogen/progesterone-induced LH surge in ovariectomized rats. In the present study, by blocking the IGF-IR with JB-1, we observed that the IGF-I-induced increase in KiSS-1 gene expression was blocked. Thus, our study suggests that IGF-I can activate KiSS-1 transcription by binding to the type 1 IGF receptor. Whether this action occurs directly on the kisspeptin-containing neuron or via an interneuron or astroglial cell that also expresses IGF-IR (19,49) remains to be determined.
In summary, the present study shows for the first time that IGF-I is capable of stimulating KiSS-1 gene expression in prepubertal female rats. Thus, our results demonstrate there is an interrelationship between IGF-I and the KiSS-1 gene before puberty; however, more research is needed to further determine the cellular mechanisms by which IGF-I stimulates the KiSS-1 gene.
Footnotes
This work was supported by National Institutes of Health Grant AA-07216 (to W.L.D.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 14, 2008
Abbreviations: AC, Anterior commissure; ARC, arcuate; AVPV, anteroventral periventricular; E2, estradiol; ER, estrogen receptor; GPR54, G protein-coupled receptor 54; IGF-IR, IGF-I receptor; MBH, medial basal hypothalamus; OC, optic chiasm; POA, preoptic area; 3V, third ventricle.
References
- Tena-Sempere M 2006 KiSS-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology 83:275–281 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M 2004 Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GRP54. J Physiol 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel J, Matsuda F, Chaussin J, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GRP54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki E, Thresher A, Acierno J, Shagoury J, Bo-Abbas Y, Kuohung W, Schwinof K, Hendrick A, Zahn D, Dixon J, Kaiser U, Slaugenhaupt S, Gusella J, O'Rahilly S, Carlton M, Crowley W, Aparicio S, Colledge W 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barriero ML, Roa J, Sanchez-Criado JE, Agilar E, Dieguez C, Pinilla L, Tena-Sempere M 2004 Developmental and hormonally regulated mRNA expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent LH-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ 2007 KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR 2004 Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16:850–858 [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara S, Crowley WF, Ojeda SR, Plant TM 2005 Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman DJ, Spaliviero JA, Scott CD, Baxter RC 1987 Hormonal regulation of the peripubertal surge of insulin-like growth factor-I in the rat. Endocrinology 120:491–496 [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL 1996 Insulin-like growth factor-I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology 137:3717–3727 [DOI] [PubMed] [Google Scholar]
- Roberts CA, McCutcheon SN, Blair HT 1990 Developmental patterns of plasma insulin-like growth factor-1 in sheep. Domest Anim Endocrinol 4:457–463 [DOI] [PubMed] [Google Scholar]
- Copeland KC, Kuehl TJ, Castracane VD 1982 Pubertal endocrinology of the baboon: elevated somatomedin-C/insulin-like growth factor-I at puberty. J Clin Endocrinol Metab 55:1198–1201 [DOI] [PubMed] [Google Scholar]
- Anders J, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE 1994 Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab 78:744–752 [DOI] [PubMed] [Google Scholar]
- Tam CS, deZegher F, Garnett SP, Baur LA, Cowell CT 2006 Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab 91:4369–4373 [DOI] [PubMed] [Google Scholar]
- Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A 1999 Deficits in female reproductive function in GH-R-KO mice: role of IGF-I. Endocrinology 140:2637–2640 [DOI] [PubMed] [Google Scholar]
- Wilson ME 1998 Premature elevation in serum insulin-like growth factor-1 advances first ovulation in rhesus monkeys. J Endocrinol 158:247–257 [DOI] [PubMed] [Google Scholar]
- Reinhardt RR, Bondy CA 1994 Insulin-like growth factors cross the blood-brain barrier. Endocrinology 135:1753–1761 [DOI] [PubMed] [Google Scholar]
- Lesniak MA, Hill JM, Kiess W, Rojeski M, Pert CR, Roth J 1988 Receptors for insulin-like growth factors I and II: autoradiographic localization in rat brain and comparison to receptors for insulin. Endocrinology 123:2089–2099 [DOI] [PubMed] [Google Scholar]
- Bondy C, Werner H, Roberts Jr CT, Leroith D 1992 Cellular pattern of type-1 insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience 46:909–923 [DOI] [PubMed] [Google Scholar]
- Daftary SS, Gore AC 2004 The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing gormones neurones during postnatal development. J Neuroendocrinol 16:160–169 [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Doncarlos L, Garcia-Segura LM 2000 Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience 99:751–760 [DOI] [PubMed] [Google Scholar]
- Dees WL, Srivastava VK, Hiney JK 2005 Alcohol alters insulin-like growth factor-1 activated Oct 2 POU gene expression in the immature female hypothalamus. J Studies Alcohol 66:35–45 [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Pine MD, Dees WL, Actions and intereactions of insulin-like growth factor-1 and alcohol on KiSS-1 gene expression in the juvenile female rat. Joint Scientific Meeting of the Research Society on Alcoholism and the International Society for Biomedical Research on Alcoholism, Washington, DC, 2008, p 20A [Google Scholar]
- Adam CL, Findlay PA, Moore AH 1998 Effects of insulin-like growth factor-1 on luteinizing hormone secretion in sheep. Anim Reprod Sci 50:45–56 [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar L, Pinilla L, Dieguez C, Tena-Sempere M 2005 Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146:3917–3925 [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M 2006 Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of the kisspeptin in different reproductive states of the female rat. Endocrinology 147:2864–2878 [DOI] [PubMed] [Google Scholar]
- Harms PG, Ojeda SR 1974 A rapid simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol 36:391–392 [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z, Wernicke D, Porcu P, Jameson BA, Baserga R 1992 Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res 52:6447–6451 [PubMed] [Google Scholar]
- Quesada A, Etgen AM 2002 Functional interactions between estrogen and insulin-like growth factor-1 in the regulation of α1B-adrenoceptors and female reproductive function. J Neurosci 22:2401–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger AM, Allen MR, Zhang BR, Goad DW, Malayer JR, Geisert RD 2001 Presence of the acute phase protein, bikunin, in the endometrium of gilts during estrous cycle and early pregnancy. Biol Reprod 65:507–513 [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Dearth RK, Dees WL 2004 Influence of estradiol on insulin-like growth factor-1-induced luteinizing hormone secretion. Brain Res 1012:91–97 [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Dearth RK, Hiney JK, Chandrashekar V, Mattison JA, Bartke A, Dees WL 2002 Alcohol suppresses insulin-like growth factor-1 gene expression in prepubertal transgenic female mice overexpressing the bovine growth hormone gene. Alcoholism Clin Exp Res 11:1697–1702 [DOI] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti ML, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE 2007 Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology 148:1158–1166 [DOI] [PubMed] [Google Scholar]
- Duenas M, Luquin S, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM 1994 Gonadal hormone regulation of insulin-like growth factor-1-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology 59:528–538 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Hill J, Hill DF, Costa ME, Tapia V, Cornea A, Ma YJ 1999 The Oct-2 POU-domain gene in the neuroendocrine brain: a transcriptional regulator of mammalian puberty. Endocrinology 140:3774–3789 [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll Ea, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA 2006 Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147:1154–1158 [DOI] [PubMed] [Google Scholar]
- Luque RM, Kineman RD, Tena-Sempere M 2007 Regulation of hypothalamic expression of Kiss-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology 148:4601–4611 [DOI] [PubMed] [Google Scholar]
- Hiney JK, Ojeda SR, Dees WL 1991 Insulin-like growth factor (IGF-I) stimulates LHRH release from the prepubertal female median eminence in vitro. Neuroendocrinology 54:420–423 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus: sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors from generating the preovulatory luteinizing hormone surge. J Neuroscience 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neuroscience 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Wandosell F, Garcia-Segura LM 2006 Cross-talk between estrogen receptors and insulin-like growth factor-1 in the brain: cellular and molecular mechanisms. Front Neuroendocrinol 27:391–403 [DOI] [PubMed] [Google Scholar]
- Patrone C, Gianazza E, Santagati S, Agrati P, Maggi A 1998 Divergent pathways regulate ligand-independent activation of ERα in SK-N-BE neuroblastoma and COS-1 renal carcinoma cells. Mol Endocrinol 12:835–841 [DOI] [PubMed] [Google Scholar]
- Duenas M, Torres-Aleman I, Naftolin F, Garcia-Segura LM 1996 Interaction of insulin-like growth factor-1 and estradiol signaling pathways on hypothalamic neuronal differentiation. Neuroscience 74:531–539 [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Naftolin F, Garcia-Segura LM 1999 Phasic synaptic remodeling of the rat arcuate nucleus during the estrous cycle depends on insulin-like growth factor-1 receptor activation. J Neurosci Res 55:286–292 [DOI] [PubMed] [Google Scholar]
- Todd BJ, Fraley GS, Peck AC, Schwartz GJ, Etgen AM 2007 Central insulin-like growth factor 1 receptors play distinct roles in the control of reproduction, food intake, and body weight in female rats. Biol Reprod 77:492–503 [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Morschl E, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM 1997 Role of astroglia and insulin-like growth factor-1 in gonadal hormone-dependent synaptic plasticity. Brain Res Bull 44:525–531 [DOI] [PubMed] [Google Scholar]