Abstract
Mitochondrial reactive oxygen species have been implicated in both diabetic complications and the progression of the underlying diabetic state. However, it is not clear whether mitochondria of diabetic origin are intrinsically altered to generate excess reactive oxygen species independent of the surrounding diabetic milieu. Mitochondria were isolated from gastrocnemius, heart, and liver of 2-wk and 2-month streptozotocin diabetic rats and controls. We rigidly quantified mitochondrial superoxide, respiration and ATP production, respiratory coupling, the expression of several proteins with antioxidant properties, and the redox state of glutathione. Both fluorescent assessment and electron paramagnetic spectroscopy revealed that superoxide production was unchanged or reduced in the 2-month diabetic mitochondria compared with controls. Kinetic analysis of the proton leak showed that diabetic heart and muscle mitochondria were actually more coupled compared with control despite an approximate 2- to 4-fold increase in uncoupling protein-3 content. Adenine nucleotide translocator type 1 expression was reduced by approximately 50% in diabetic muscle mitochondria. Catalase was significantly up-regulated in muscle and heart tissue and in heart mitochondria, whereas glutathione peroxidase expression was increased in liver mitochondria of diabetic rats. We conclude that gastrocnemius, heart, and liver mitochondria of streptozotocin diabetic rats are not irrevocably altered toward excess superoxide production either by complex I or complex III. Moreover, gastrocnemius and heart mitochondria demonstrate increased, not decreased, respiratory coupling. Mitochondria of insulin-deficient diabetic rats do show signs of adaptation to antecedent oxidative stress manifested as tissue-specific enzyme and uncoupling protein expression but remain remarkably robust with respect to superoxide production.
Mitochondria of streptozotocin-diabetic rats do not generate excess superoxide, manifest decreased or unchanged respiratory coupling, and show adaptation to antecedent in vivo oxidative stress.
The mitochondrial electron transport system (ETS) generates oxygen radicals through electron leaks as substrates are metabolized (1). Unfortunately, these trigger oxidative damage manifest in ways such as peroxidation of lipid membranes (2), protein modification (3), damage to mitochondrial DNA (4), and cytochrome C release from mitochondria associated with apoptosis (5). It is theorized that the hyperglycemia and elevated circulating free fatty acids, as seen in diabetes, induce mitochondrial ROS, which may contribute to the complications of diabetes (6,7,8,9) or worsen the diabetic state (10,11).
On the other hand, diabetic cells and tissues have the capacity to invoke adaptive mechanisms that evolved to defend against oxidative stress (11). One putative mechanism is a physiological or mild uncoupling of oxidative phosphorylation that would reduce superoxide generation by reducing mitochondrial membrane potential (12). In addition to uncoupling, several enzymatic means may protect against reactive oxygen species (ROS) in mitochondria. These include conversion of superoxide to H2O2 by manganese superoxide dismutase (MnSOD) and scavenging of H2O2 by catalase, glutathione (GHS) peroxidase (GPX), or peroxiredoxin III (PRX III) (11).
An important and unresolved issue concerns the function of mitochondria per se, independent of the diabetic milieu. Do mitochondria from the diabetic milieu, fueled in equivalent fashion to control mitochondria, generate excess ROS, or do protective mechanisms compensate? If protective mechanisms do remain intact, is there a bioenergetic cost? For example, if respiratory uncoupling is invoked as a defensive means, then the capacity to generate ATP might be restricted. On the other hand, mitochondria might function in normal or near-normal fashion when studied in vitro, suggesting that any excess mitochondrial ROS depends on interactions between mitochondria and the surrounding in vivo environment. These are important issues if we are to fully understand ROS production in diabetes and may have therapeutic impact regarding where to best target antioxidant drugs.
Little is known in this regard. Very recently, Bugger et al. (13) reported the absence of mitochondrial uncoupling and decreased ROS production despite increased uncoupling protein-3 (UCP3) expression in heart mitochondria of Akita mice, a model of type 1 diabetes.
Here, we hypothesized that the insulin-deficient diabetic environment induces changes in mitochondria leading to the intrinsic generation of excess superoxide. To address this, we examined ROS production, mitochondrial respiratory parameters, respiratory uncoupling, ATP production, and enzyme defense in mitochondria isolated from insulin-sensitive target tissues (muscle, heart, and liver) of control and insulin-deficient diabetic rats of differing durations. ROS were assessed by methods relatively specific for superoxide produced by complex I, by complex III, or through reverse transport of electrons from complex II back to complex I. Respiratory uncoupling was assessed as mitochondrial proton conductance determined from the kinetic relationship between respiration and inner membrane potential, methodology felt to provide an optimal assessment of the proton leak or uncoupling activity (14,15). Finally, we measured the expression of certain proteins important in the regulation of ROS and/or in mitochondrial respiratory coupling and the redox state of GHS.
Materials and Methods
Materials
Reagents were purchased as indicated or from standard sources. Antibodies are described in supplemental material (published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
Animal studies
Male Sprague Dawley rats were obtained from Harlan, Inc. (Indianapolis, IN). Animals were fed standard rat chow (Harlan Teklad 7001) and maintained according to standard National Institutes of Health guidelines. The protocol was approved by our institutional Animal Care Committee. Rats were fed standard chow ad libitum and euthanized by ip injection of 25 mg/kg pentobarbital followed by incision of the left ventricle. Data show that doses of pentobarbital up to 4-fold more than we used did not affect mitochondrial respiration or potential (16). Moreover, euthanasia was carried out in the same way for control and diabetic groups.
Studies were carried out in 2-wk (group I) and approximately 2-month (groups II and III) diabetic rats (n = 10, 10, and 7 diabetic and controls in groups I, II, and III, respectively). Rats (age 11–12 wk) were made diabetic with streptozotocin (STZ), 60 mg/kg ip. Controls received vehicle (saline). Group I rats were killed an average of 2 wk after STZ or vehicle administration. On each study day, one control and one diabetic rat were euthanized and mitochondria prepared side by side. Group II and group III rats were studied in identical fashion except they were killed approximately 2 months after STZ or vehicle. Glucose was determined on tail vein blood using a reagent strip and meter (OneTouch Ultra). Rats were killed at approximately 1000 h, 2 h after removal of food. Gastrocnemius muscle, heart, and liver tissues were removed, washed, blotted, and weighed before preparation of mitochondria.
Isolation of mitochondria
Mitochondria were isolated and washed three times as previously described (17,18). Muscle and heart tissues were minced for 1 min before homogenization. Cytosolic contamination was assessed by the relative extent of expression of the cytosolic protein glyceraldehyde-3-phosphate dehydrogenase and the mitochondrial protein porin in whole tissue and mitochondrial extracts.
Proton leak kinetics
Respiration and mitochondrial inner membrane potential were determined simultaneously as we previously described (19,20). Potential was calculated using the Nernst equation based on the distribution (inside and external to the mitochondrial matrix), of the lipophilic cation tetraphenyl phosphonium (TPP+). The proton leak was assessed in succinate-fueled mitochondria as the kinetic relationship of H+ flux to mitochondrial membrane potential through simultaneous recording of oxygen consumption and potential. Mitochondria were incubated in ionic respiratory buffer [120 mm KCl, 5 mm KH2PO4, 2 mm MgCl2, 1 mm EGTA, 3 mm HEPES (pH 7.2) with 0.3% fatty acid-free BSA, 2 μm oligomycin to inhibit ATP synthase, 5 μm rotenone to inhibit electron entry at Complex-I, and 0.1 μm nigericin to abolish the ΔpH (21) across the mitochondrial membrane], as we previously described (19). Under these conditions, H+ flux is proton leak dependent and follows a 6:1 stoichiometry (H+/O) with oxygen consumed (15). Malonate was added in incremental amounts to final concentrations ranging from 0.5–6.0 mm to inhibit succinate dehydrogenase, thereby creating a range of membrane potentials.
Mitochondrial matrix volume and TPP+ binding correction
To calculate membrane potential, it is necessary to include values for mitochondrial matrix volume and the extent of TPP+ binding to mitochondria (22). Due to the exponential nature of the Nernst equation, differences in matrix volumes and binding corrections actually have very little effect on calculated potential, so some studies have used assumed values reported in the past. Moreover, for our purposes, the issue would be of concern only if these parameters differed between diabetic and control rats. Therefore, we measured matrix volumes in mitochondria from group III, 2-month diabetic and control rats. Because these values did not differ, we applied them to the calculations for the group I and group II animals. Workload requirements and number of mitochondria precluded measurement of matrix volumes (along with all other oxidative and bioenergetic parameters) directly in the group I and II animals. We assumed a TPP+ binding correction of 0.25 based on literature values (23,24,25) because there is no reason to suspect these should differ between control and diabetic rats.
Mitochondrial matrix volumes were determined from exclusion volumes of 3H2O and [14C]sucrose as we previously described (26).
Mitochondrial respiration and ATP production rates
State 3 and state 4 respiration were measured by polarography in a 600-μl incubation chamber with stir bar and oxygen electrode as we have accomplished in past studies (19,26). The ADP/O ratio was calculated as molar ADP added divided by molar oxygen consumed during conversion to ATP as we described previously (18). Maximal respiration was determined in the presence of 2.5 μm carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone (FCCP). ATP production rates were determined by sampling 9.5 μl respiratory medium at 15-sec intervals for 60 sec after addition of 40 μm ADP as described (27). Sample ATP concentrations were determined as luciferase catalyzed ATP-dependent oxidation of luciferin as we have carried out in the past (18).
Mitochondrial ROS production by fluorescent measurement
H2O2 production was assessed using the fluorescent probe, 10-acetyl-3,7-dihydroxyphenoxazine (Amplex Red; Invitrogen, Carlsbad, CA). Samples were prepared in 96-well plates containing 0.06 ml/well respiratory buffer. Fluorescence was measured as we previously described (20) once every 44 sec for 50 cycles. For quantification, a H2O2 standard curve ranging from 0–12 μm was prepared and included on each plate. Addition of catalase, 500 U/ml, reduced fluorescence to below the detectable limit, indicating specificity for H2O2. Addition of substrates to respiratory buffer without mitochondria did not affect fluorescence. Addition of the ETS inhibitor rotenone with mitochondria in the absence of substrate altered fluorescence only about 5%.
Electron paramagnetic resonance (EPR) spectroscopy
ROS were also measured by EPR as we previously described (17,20). Mitochondria were studied during state 4 respiration in 0.2 ml respiratory buffer with 0.069 m 5,5-dimethyl-l-pyrroline-N-oxide (DMPO), and 0.09 mg mitochondria. Respiration was initiated with the addition of substrate (succinate or glutamate plus malate) and samples incubated for 5 min at 37 C before transfer to a flat aqueous EPR cell. Spectra were then recorded at room temperature using the following instrument settings: microwave power 40 mW, modulation amplitude 2 G, receiver gain 2 × 105, conversion time 40.96 msec, time constant 81.92 msec, and scan rate 80 G/41.92 sec. Spectra shown are the average of seven scans.
Site specificity of superoxide production
As we previously described for endothelial cell mitochondria (20), our fluorescent and EPR studies measure superoxide differently. In combination, these techniques impart a degree of specificity for complex I or III superoxide. Amplex Red detects superoxide indirectly. When added to isolated mitochondria, the probe detects H2O2 generated from superoxide by matrix MnSOD. H2O2 so generated diffuses outward from mitochondria and reacts with horseradish peroxidase in the incubation medium to trigger fluorescence. H2O2 produced in this way derives largely from matrix superoxide released at complex I (28). In contrast, our EPR spin trap, DMPO, detects superoxide directly after efflux outward from mitochondria. Superoxide produced in this way should largely derive from the Q cycle at complex III (28). Because DMPO will not easily penetrate mitochondria and because matrix superoxide is rapidly converted to H2O2, our spin trap should not detect superoxide released to the matrix as occurs at complex I. To further document the site specificity of our ROS detection methods, we carried out these studies in the presence or absence of ETS inhibitors as described in Results.
Immunoblotting
Mitochondrial proteins were separated on 12.5% polyacrylamide gels for immunoblotting as we previously described (18,19). Antibody dilutions and incubation times are tabulated (see supplemental data). Densitometry data were normalized to pooled controls included in duplicate on each blot.
Primary antibody specificity was confirmed by signal ablation by competing peptide (PRX III, UCP3, and catalase), expected relative tissue distribution [UCP3, adenine nucleotide translocator type 1 (ANT1), and GPX], strong signal generation at the expected kD by adenoviral overexpression (UCP3, MnSOD, and catalase), and lack of cross-reactivity with UCP2 (UCP3) (18).
GHS/GHS disulfide redox state
GSH was determined using a modification of the method of Tietze (29). Acid-homogenized samples were centrifuged at 10,000 × g for 5 min. The supernatant (20 μl) was mixed with 100 μl 5,5′-dithio-bis 2-nitrobenzoic acid (DTNB) buffer (110 mm Na2HPO4, 40 mm NaH2PO4, 15 mm EDTA, 0.3 mm DTNB) followed by 100 μl GHS reductase buffer (50 mm imidazole, 15 mm HCl, 1 mm EDTA, 500 μm NADPH, 1 U/ml GHS reductase). Absorbance was measured at 405 nm in a plate reader (Ultramark; Bio-Rad, Hercules, CA) at 0 and 5 min. GHS disulfide (GSSG) was quantitated by blocking GSH with l-vinyl-pyridine. The supernatant (50 μl) was mixed with 5 μl l-vinyl-pyridine (50% in ethanol) for 2 h at room temperature and then analyzed for GSH as above. For quantification, we compared absorbance to standards consisting of reduced and oxidized GHS.
Statistics
Within each group of rats, parameters were compared between control and diabetic animals by two-tailed, unpaired t test. Curves depicting mitochondrial proton conductance were compared by midpoint comparisons and by two-way ANOVA as described in Results.
Results
Animal characteristics
As expected, diabetic rats lost considerable weight and manifested high glucose concentrations compared with controls. Group I rats had a mean glucose of 502 ± 57 mg/dl immediately before being euthanized, whereas group II and III rats had mean values over 600 mg/dl at euthanasia. Detailed characteristics of the control and STZ-diabetic rats are listed in supplemental data.
Mitochondrial purity
Mitochondria were highly pure as indicated by distribution of glyceraldehyde-3-phosphate dehydrogenase and porin in whole tissue and mitochondrial extracts. These characteristics did not differ between diabetic and control mitochondria (data included in supplemental data). Mitochondrial yields (milligrams per gram tissue) for control and diabetic rats were, respectively, 20 ± 1 and 21 ± 1 for liver, 5.7 ± 0.3 and 5.3 ± 0.4 for heart, and 0.98 ± 0.09 and 0.85 ± 0.10 for gastrocnemius of 2-month diabetic rats (combined groups II and III). These values were not statistically different between the diabetic and control rats. Yields were not determined for the 2-wk diabetic rats.
Superoxide production
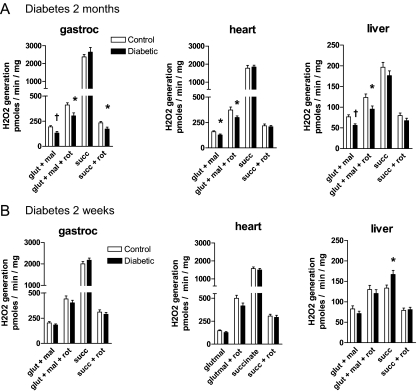
Figure 1 depicts ROS measured as Amplex Red fluorescence. As shown, diabetic mitochondria from all tissues of the 2-month STZ group generated less superoxide, compared with control, when respiring on the complex I substrates glutamate and malate. Under these conditions, this method largely detects superoxide generated by electron leaks during forward transport through complex I (30). In support of this, rotenone had the expected effect to enhance fluorescence. This is a well known phenomenon felt to result from inhibition of Q-cycle-type events within complex I probably due to prolongation of the half-life of the reactive semiquinone form of coenzyme Q (30).
Figure 1.
H2O2 production measured as Amplex Red fluorescence by mitochondria of the tissues indicated from control and 2-month (A) or control and 2-wk (B) STZ-diabetic rats incubated in respiration buffer under state 4 conditions with the additions shown. Data represent mean values (±sem) from nine to 10 mitochondrial preparations per group with each individual value determined as the mean of two or more replicate wells. Additions consisted of 5 mm succinate (succ), 5 mm glutamate plus 1 mm malate (glut + mal), and/or 5 μm rotenone (rot). *, P < 0.05; †, P < 0.01 by unpaired t test vs. control.
Diabetes had no effect on fluorescence generated by mitochondria respiring on the complex II substrate succinate, except for the liver mitochondria of the short-duration group (Fig. 1B) wherein ROS was increased. In mitochondria respiring on succinate, this fluorescent methodology predominantly detects superoxide generated during reverse electron transport backwards through complex I. Superoxide generated by reverse transport is also a well known phenomenon in isolated mitochondria (30,31,32). In support of this, rotenone had the expected effect to block ROS generated on succinate (30,31,32) (Fig. 1).
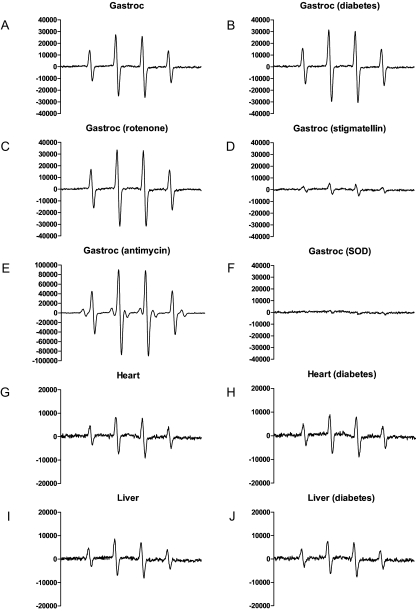
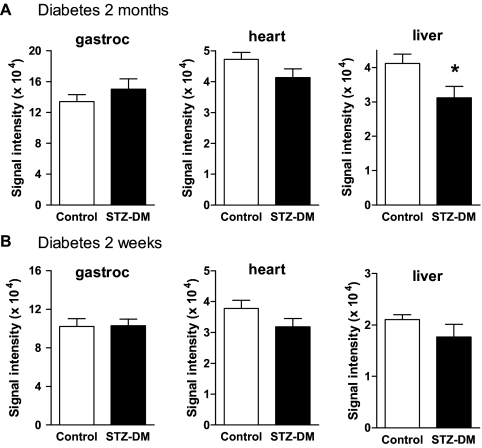
Superoxide was also measured by EPR spectroscopy (Figs. 2 and 3). Superoxide production by EPR was reduced in liver mitochondria of the longer-duration diabetic rats but unaffected in gastrocnemius and heart (Fig. 3A). Superoxide production was unaffected in mitochondria isolated from any tissue of the shorter-duration diabetic rats (Fig. 3B). As described in Materials and Methods, EPR largely detects superoxide from complex III. This was confirmed by the lack of change in signal intensity upon addition of rotenone (Fig. 2C), by the decrease in signal intensity seen upon addition of stigmatellin (Fig. 2D), which blocks electron entry to complex III (20), and by the marked increase seen with antimycin (Fig. 2E). Antimycin also blocks complex III but by inhibition of the Q-cycle with prolongation of the lifetime of the reactive semiquinone coenzyme Q·– and consequent increase in superoxide (12). Very similar findings to those observed for gastrocnemius mitochondria (Fig. 2, C–F) in the presence of rotenone, stigmatellin, antimycin, or MnSOD were noted for heart and liver mitochondria and also reproduced three times (not shown).
Figure 2.
Representative EPR spectra generated using the spin trap DMPO. Spectra were determined in the presence of mitochondria of gastrocnemius (gastroc), heart, or liver from control or 2-month diabetic rats incubated in respiratory buffer under state 4 conditions with 5 mm succinate plus the additions indicated. The signals, which could represent either superoxide or the hydroxyl radical (because both react with the spin trap to generate the same spectrum), were abolished by addition of MnSOD (F), demonstrating specificity for superoxide. Note the scale difference for gastrocnemius mitochondria incubated in the presence of antimycin A (E) and the scale differences by tissue. Additions consisted of 5 μm rotenone, 80 nm stigmatellin, 1 μm antimycin A, or 200 μg/ml manganese superoxide dismutase (SOD).
Figure 3.
Superoxide production (mean ± sem) quantified as integrated EPR spectra generated as in Fig. 2. Spectra were obtained in gastrocnemius (gastroc), heart, or liver mitochondria isolated from control or 2-month diabetic rats incubated in respiratory buffer under state 4 conditions and fueled by 5 mm succinate. Mitochondria were isolated from control and 2-month STZ-diabetic (STZ-DM) (A) or control and 2-wk STZ-diabetic (B) rats. Representative examples of these spectra are shown in Fig. 2. Values represent mean ± sem from nine to 10 mitochondrial preparations per group. *, P < 0.01 compared with control.
Mitochondrial matrix volumes
Volumes did not differ between mitochondria of control and STZ-diabetic rats. Respective values (μl ± sem) for control and diabetic rats were 1.35 ± 0.14 and 1.35 ± 0.21 (n = 7 per group) for gastrocnemius muscle, 1.37 ± 0.16 and 1.38 ± 0.18 (n = 7 per group) for heart, and 1.31 ± 0.12 and 1.40 ± 0.15 (n = 6 per group) for liver.
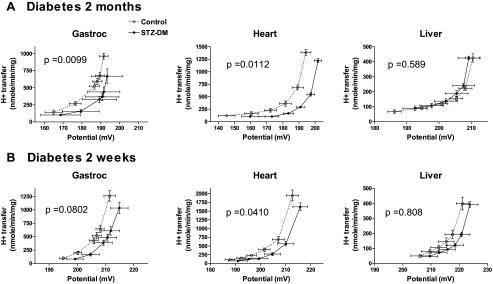
Respiration, proton conductance, and respiratory uncoupling
Mitochondrial respiration and potential were determined during incremental inhibition of succinate dehydrogenase by increasing malonate concentrations (Fig. 4). Leak-dependent proton conductance at any point on the curves is given by hydrogen transfer × potential (nanomoles H per minute per milligram per millivolt). Uncoupling is manifest as a shift upwards and to the left. To obtain a statistical measure of the difference in proton leak kinetics between STZ-diabetic and control rats, we plotted proton conductance as a function of malonate concentration and compared midpoint values between curves (supplemental data). These values differed significantly for heart and gastrocnemius mitochondria between the 2-month STZ-diabetic and controls as well as for heart mitochondria of the 2-wk STZ and control rats. A trend in the same direction was noted for gastrocnemius in the 2-wk STZ rats, but the data were not significant. There were no significant differences for liver mitochondria. As an additional measure of significance, proton conductance as a function of malonate concentration was compared by two-way ANOVA (supplemental data). The same statistical differences were observed.
Figure 4.
Kinetic relationship between inner membrane potential and hydrogen transfer as a measure of respiratory uncoupling in mitochondria isolated from the tissues indicated. Mitochondrial samples were prepared from control and diabetic rats (n = 9 or 10 per group) from 2-month (A) and 2-wk (B) STZ-diabetic rats. Data represent mean ± sem for both hydrogen transfer and potential and define proton conductance at each point on the curves. P values represent significance between diabetic and control rats by midpoint analysis of the relationship between proton conductance and malonate concentration (see text).
As shown in Table 1, respiration and proton conductance in the uninhibited state (no added malonate) were reduced in muscle and heart mitochondria of the longer-duration diabetic rats. There were very clear trends in the same direction for the 2-wk diabetic rats, although not significant. These parameters were unaffected in liver.
Table 1.
Mitochondrial respiration and proton conductance under state 4 noninhibited (no added malonate) conditions in mitochondria respiring on succinate
| Group/tissue | Respiration (nmol O/min · mg)
|
Proton conductance (nmol H+/min · /mg · mV)
|
||||
|---|---|---|---|---|---|---|
| Control | STZ-D | P | Control | STZ-D | P | |
| Group I (2-wk diabetic) | ||||||
| Gastrocnemius | 210 ± 16 | 172 ± 18 | 0.14 | 5.90 ± 0.44 | 4.73 ± 0.43 | 0.072 |
| Heart | 321 ± 29 | 265 ± 20 | 0.057 | 8.93 ± 0.70 | 7.31 ± 0.49 | 0.076 |
| Liver | 67 ± 7 | 65 ± 3 | 0.89 | 1.68 ± 0.17 | 1.64 ± 0.08 | 0.84 |
| Group II (2-month diabetic) | ||||||
| Gastrocnemius | 160 ± 7 | 112 ± 18 | 0.021 | 4.97 ± 0.22 | 3.34 ± 0.49 | 0.008 |
| Heart | 232 ± 10 | 204 ± 7 | 0.032 | 7.11 ± 0.27 | 6.02 ± 0.19 | 0.005 |
| Liver | 71 ± 5 | 71 ± 2 | 0.99 | 2.01 ± 0.12 | 2.02 ± 0.05 | 0.92 |
Data represent mean ± sem; n = 9–10. P values were determined by unpaired t test compared with control (bold values indicate P < 0.05). STZ-D, STZ-diabetic.
State 3 and state 4 respiration, ADP/O ratios, respiratory control ratio values, and maximal uncoupled (FCCP) respiration were determined in gastrocnemius, heart, and liver mitochondria isolated from group III rats (Table 2). State 4 and FCCP respiration by heart mitochondria fueled by succinate were significantly decreased in the diabetic rats compared with control. Otherwise, there were no significant differences in these parameters. However, of note is that state 3 and 4 respiration and maximal FCCP respiration were also less for all other measurements in mitochondria of all tissues respiring under either substrate condition.
Table 2.
Respiratory parameters, ADP/O ratios, and ATP production rates (mean ± sem) in mitochondria isolated from the tissues indicated of control and STZ-diabetic group III (2-month diabetic) rats
| Gastrocnemius
|
Heart
|
Liver
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Succinate
|
Glut/mal
|
Succinate
|
Glut/mal
|
Succinate
|
Glut/mal
|
|||||||
| Ctrl | STZ-D | Ctrl | STZ-D | Ctrl | STZ-D | Ctrl | STZ-D | Ctrl | STZ-D | Ctrl | STZ-D | |
| State 4 respiration (nmol O/min/mg) | 103 ± 10 | 90 ± 11 | 67 ± 6 | 64 ± 9 | 203 ± 8 | 165 ± 14a | 81 ± 6 | 69 ± 5 | 72 ± 3 | 71 ± 2 | 31 ± 4 | 28 ± 2 |
| State 3 respiration (nmol O/min · mg) | 252 ± 22 | 243 ± 30 | 463 ± 37 | 431 ± 48 | 311 ± 10 | 284 ± 20 | 402 ± 39 | 395 ± 28 | 264 ± 15 | 236 ± 13 | 177 ± 18 | 171 ± 12 |
| FCCP respiration (nmol O/min · mg) | 231 ± 28 | 210 ± 26 | 875 ± 99 | 697 ± 109 | 333 ± 9 | 288 ± 17a | 576 ± 129 | 458 ± 71 | 495 ± 49 | 414 ± 30 | 417 ± 54 | 405 ± 30 |
| RCR | 2.62 ± 0.28 | 2.71 ± 0.18 | 7.29 ± 1.03 | 7.21 ± 0.75 | 1.54 ± 0.05 | 1.75 ± 0.13 | 5.15 ± 0.55 | 5.87 ± 0.43 | 3.66 ± 0.14 | 3.38 ± 0.22 | 6.12 ± 0.93 | 6.37 ± 0.79 |
| ADP/O | 3.83 ± 0.42 | 3.79 ± 0.34 | 1.38 ± 0.09 | 1.54 ± 0.44 | 3.00 ± 0.05 | 3.11 ± 0.21 | 0.96 ± 0.08 | 1.15 ± 0.09 | 0.96 ± 0.06 | 1.00 ± 0.06 | 1.50 ± 0.14 | 1.56 ± 0.14 |
| MAPR (nmol/min/mg) | 203 ± 12 | 218 ± 27 | 181 ± 25 | 226 ± 31 | 263 ± 38 | 291 ± 31 | 226 ± 23 | 293 ± 45 | ND | ND | ND | ND |
Ctrl, Control; Glut/mal, Glutamate/malate; MAPR, mean ATP production rate; ND, not determined; RCR, respiratory control ratio (state 3/state 4 respiration); STZ-D, STZ-diabetic.
P < 0.05; n = 6–7 rats per group. Significantly different values are in bold.
ATP production
ATP production was assessed in mitochondria isolated from gastrocnemius muscle and heart of 2-month (group III) diabetic and control rats. No significant differences were observed (Table 2).
Antioxidant defense
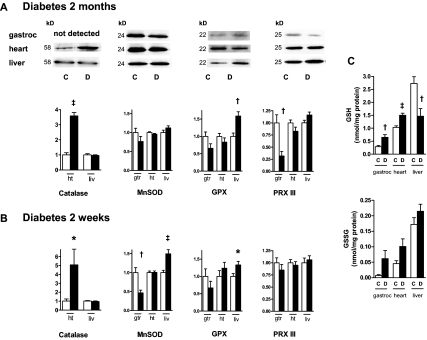
Figure 5 depicts representative immunoblot signals and quantitative data comparing the expression of antioxidant enzymes within mitochondria isolated from control and diabetic muscle, heart, and liver. The results show that catalase was markedly up-regulated in diabetic muscle and heart tissue and in heart mitochondria but not in liver mitochondria or tissue. In contrast, GHS peroxidase was up-regulated in liver mitochondria but not gastrocnemius or heart. Moreover, GSH levels were reduced in liver, but not muscle or heart, mitochondria of the longer-duration diabetic rats. This is consistent with the GHS peroxidase data, because the enzyme scavenges H2O2 by transfer of electrons from GSH through conversion to GSSG. MnSOD expression was increased but only in the liver mitochondria of the 2-wk diabetic rats and was actually decreased in muscle mitochondria of these animals. PRX III was decreased in muscle mitochondria from the 2-month diabetic rats.
Figure 5.
Expression of antioxidant enzymes (catalase, MnSOD, GPX, and PRX III) and GSH redox state. A, Representative immunoblots and quantitative expression (arbitrary units) of proteins in extracts of mitochondria isolated from 2-month control (C, open bars) and diabetic (D, dark bars) rats. Mitochondrial protein expression was quantified from gastrocnemius (gtr), heart (ht), and liver (liv) mitochondria. B, Quantitative expression in mitochondria isolated from 2-wk diabetic rats; n = 9–10 samples for each determination. *, P < 0.05; †, P < 0.01; ‡, P < 0.001 compared with control by unpaired t test. C, Reduced (GSH) and oxidized (GSSG) GHS in gastrocnemius, heart, or liver mitochondria of control and 2-month diabetic rats; n = 10 per group. *, P < 0.05; ‡, P < 0.001 compared with control by unpaired t test.
Because catalase is predominantly a cytoplasmic enzyme, we compared tissue expression of catalase in gastrocnemius muscle, heart, and liver tissues isolated from group III diabetic and control rats. Tissue catalase was up-regulated by 3.9- ± 0.9-fold in gastrocnemius (P < 0.01; n = 7) and 3.2- ± 0.7-fold in heart (P = 0.01; n = 7) and decreased 0.71 ± 0.10-fold, but not significantly, in liver.
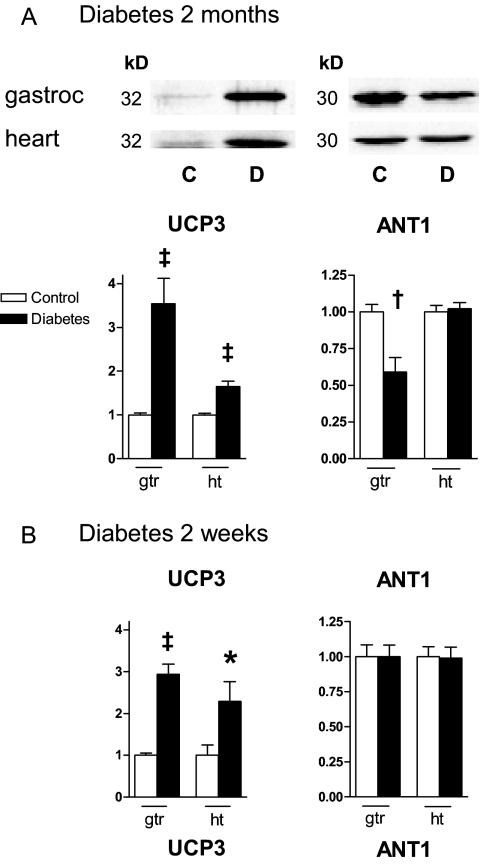
UCP3 expression was increased in diabetic gastrocnemius and heart mitochondria compared with controls (Fig. 6). ANT1 expression was reduced but only in the muscle mitochondria of the 2-month diabetic rats. As expected, UCP3 and ANT1 were not expressed in liver mitochondria (not shown).
Figure 6.
Expression of proteins with uncoupling properties (UCP3 and ANT1). A, Representative immunoblots and quantitative expression (arbitrary units) of proteins in extracts of mitochondria isolated from gastrocnemius (gtr) and heart (ht) of control (C, open bars) and 2-month diabetic (D, dark bars) rats. B, Quantitative protein expression in mitochondria from 2-wk diabetic rats; n = 9–10 samples for each determination. *, P < 0.05; †, P < 0.01; ‡, P < 0.001 compared with control by unpaired t test.
Discussion
The major changes in muscle, heart, and liver mitochondria induced by insulin-deficient diabetes are summarized in Table 3.
Table 3.
Summary depicting major effects of insulin-deficient diabetes on mitochondrial properties listed by tissue of origin
| Gastrocnemius | Heart | Liver | |
|---|---|---|---|
| Superoxidea | Decreased from complex I | Decreased from complex I | Decreased from complex I and III, less total ROS compared with muscle or heart |
| Respiratory coupling | More coupled despite increased UCP3 | More coupled despite increased UCP3 | Unaffected |
| Superoxide protection | Increased tissue catalase and mitochondrial UCP3 | Increased tissue and mitochondrial catalase and mitochondrial UCP3 | Increased mitochondrial GPX, increased MnSODb |
UCP3 is considered effective superoxide protection only in vivo (see text).
Affected only in 2-month diabetic rats.
Only in 2-wk diabetic rats.
There is considerable evidence that diabetes is associated with oxidative tissue damage (11,33,34) and that this extends to liver, skeletal muscle, and heart tissues of STZ-diabetic rodents (35,36,37,38,39,40). The crucial message reported here is that despite these findings, mitochondria per se apart from the insulin-deficient diabetic milieu remain free from unmitigated superoxide production. Our current findings agree with work that appeared while this manuscript was under review indicating that heart mitochondria isolated from insulin-deficient Akita mice produced less ROS compared with controls (13). These findings suggest that, at least in insulin-deficient states, control of the diabetic milieu may be more critical than efforts to protect mitochondria per se.
In our studies, ROS production was examined during state 4 respiration, wherein radical formation is enhanced as electron flow leads to high potential unmitigated by ATP generation (41). Hence, this approach maximizes sensitivity for detection, although we do acknowledge that mitochondria in vivo are rarely, if ever in this unmitigated state.
Our results do not imply that mitochondria did not generate excess ROS before isolation. In fact, the opposite is likely given the changes we observed in mitochondrial expression of antioxidant enzymes.
The antioxidant changes we observed were tissue specific. Catalase was up-regulated in muscle and heart tissue and heart mitochondria, whereas GPX was increased in liver mitochondria of the STZ-diabetic rats. Catalase has classically been considered a cytoplasmic enzyme. However, recent studies have documented the expression and antioxidant activity in liver (42,43), and there are reports of catalase in heart mitochondria (44,45). Our current studies showing that catalase is expressed in the mitochondrial fractions of heart and liver are in agreement, although we could not demonstrate catalase in gastrocnemius mitochondria. It is important to acknowledge the limitation that we cannot rule out some degree of contamination of our preparations by peroxisomes or microperoxisomes that express catalase. However, this limitation may largely apply only to liver, because mammalian myocytes, unlike most other tissues, do not have peroxisomes, whereas myocyte microperoxisomes are of low density and tend not to sediment with mitochondria (44).
Both catalase and GPX scavenge H2O2 and could account for the decreases in ROS seen in the diabetic heart and liver mitochondria (Fig. 1). The GPX up-regulation was associated with decreased GSH, suggesting increased activity of the enzyme with consumption of reduced GHS, a process that would scavenge H2O2 (46). Others have reported an increase in catalase expression in heart mitochondria of STZ-diabetic rats (47), and overexpression of the enzyme appears to protect cardiomyocyte function in diabetic heart (48). We could not find another report of GPX protein expression specifically within mitochondria of insulin-deficient diabetes. We observed a decrease in PRX III in gastrocnemius mitochondria of the 2-month diabetic rats (Fig. 5). We are unaware of previously reported changes in the expression of this enzyme in mitochondria of insulin-deficient diabetic animals. PRX III, in disulfide form, is activated by small mitochondrial thiol proteins (mitochondrial thioredoxins) to an active dithiol that reduces H2O2 (46,49).
Our UCP3 expression data (Fig. 6) are also consistent with antecedent in vivo adaptation to mitochondrial oxidative stress. Superoxide production by mitochondria appears to be sensitive to membrane potential, and mild uncoupling has been proposed as a means of defense against mitochondrial stress (12,50). The effect would be to reduce membrane potential and thereby counter conditions leading to ROS production, such as the half-life of the semiquinone (radical) form of coenzyme Q and the presence of reduced electron carriers whose oxidation may generate electron leaks (11,12).
We point out that the lack of uncoupling of diabetic heart and muscle mitochondria ex vivo (Fig. 4) is not discrepant with the increase we observed in UCP3 expression (Fig. 6). In fact, this apparent ex vivo discrepancy would be expected based on evidence that UCP3 requires superoxide for activation of respiratory uncoupling (51). Thus, a lack of UCP3 mediated uncoupling by diabetic mitochondria ex vivo is entirely consistent with our contention that increased superoxide production is not an intrinsic property of diabetic mitochondria but rather requires the in vivo environment.
Of note is that increased UCP3 expression in STZ-diabetic rodents has been reported before but not in conjunction with superoxide or proton conductance data (52,53). Also of note is that increased UCP3 expression without a corresponding change in the proton leak has been reported before in rodents subject to starvation (54) or lipopolysaccharide-induced free fatty acid release (55).
Our findings of increased coupling by heart and muscle of STZ-diabetic rats despite increased UCP3 protein expression beg the question of why this should be the case. Possibly, our findings represent differences between diabetic and control mitochondria involving an unknown source of proton leak, a phenomenon that does not always require protein catalysis (11). For muscle mitochondria in the 2-month diabetic rats, we did observe enhanced expression of the adenine nucleotide translocator type 1. This subtype is expressed mainly in heart and muscle and transports hydrogen along with ADP from the inner membrane to the matrix, thereby acting to enhance proton conductance and discharge membrane potential (56). However, we did not add exogenous ADP to our in vitro incubates, and our ANT1 data does not explain the increase in coupling in heart mitochondria (Fig. 4).
Although gastrocnemius and heart mitochondria of STZ-diabetic rats were more coupled compared with control, ATP production rates and ADP/O ratios did not differ significantly. This could be due to a defect anywhere in the respiratory chain linked to ATP synthesis including a component of one or more respiratory complexes or ATP synthase. A defect or defects in this regard might also explain the global reduction in respiration of heart mitochondria on succinate (FCCP respiration) and the decreases in state 4 respiration on succinate in heart and gastrocnemius mitochondria (Tables 1 and 2) as well as the strong trend toward decreased state 3 and 4 respiration and FCCP respiration in general. Although the above is somewhat speculative, defects in oxidative phosphorylation are well recognized in diabetic mitochondria (57,58).
A question may arise as to why our results show reduced ROS in diabetic mitochondria despite higher potential with less uncoupling. Two reasons follow from the discussion above. First, antecedent up-regulation of antioxidant defense may mitigate ROS, and second, as the above paragraph describes, respiration appears to be reduced, which should decrease electron flux and consequent oxygen radical leaks.
Recently Boudina et al. (27) provided direct evidence that heart mitochondria of leptin receptor-deficient db/db mice do produce excess ROS and are, in fact, mildly uncoupled. These studies differ from ours in that we examined a much different diabetic model, that is, insulin-deficient STZ-diabetic rats as opposed to the hyperinsulinemic, insulin-resistant, and leptin receptor-defective db/db model.
We acknowledge one exception to our findings of decreased or no change in ROS production by diabetic mitochondria. This involves the increase in H2O2 production by fluorescence seen in the liver mitochondria of the 2-wk diabetic rats (Fig. 1B). However, this was evident only for mitochondria respiring on succinate under conditions of reverse electron transport, not reproduced in the 2-month diabetic rats, and not seen at either time in muscle or heart mitochondria. Hence, it is difficult ascertain the meaning of this finding.
Our studies show that perturbations in mitochondrial bioenergetics (Fig. 4), superoxide production (Figs. 1 and 3), and antioxidant mechanisms (Figs. 5 and 6) are evident as early as 2 wk after induction of diabetes but appear more pronounced after 2 months. However, a limitation is that the shorter- and longer-duration diabetes groups were studied at different times, and we did not attempt to or design our studies to directly compare the animals by duration of diabetes. Another limitation is that with respect to the measurements of mitochondrial membrane potential and oxygen consumption, there is always some concern about drift in electrode sensitivity over different times of study. For each individual study group (2 wk or 2 months), this was minimized by pairing the rats so that mitochondria from one diabetic and one control animal were prepared and analyzed side by side on a given day. However, this could not be done between the groups of shorter- and longer-duration diabetes. We believe this may account for any apparent differences in membrane potential between the groups (compare the x-axis data of Fig. 4, A and B), and we make no suggestion that this indicates any real difference in physiology.
A limitation to our study is that we cannot be sure that diabetes of longer than 2 months would have resulted in additional defects in respiration or ROS production. Rosca et al. (59) noted increased superoxide production in 12-month STZ-diabetic rats as assessed by lucigenin. However, their data are somewhat difficult to compare because a different tissue was involved and because these authors used insulin to maintain the animals over the 12-month period.
In conclusion, we report two novel findings. First, mitochondria of insulin-responsive tissues, even in severe insulin-deficient diabetes, are not irrevocably altered toward excess superoxide production either from complex I or III. Second, diabetic mitochondria isolated from these tissues are not intrinsically uncoupled. Rather, muscle and heart mitochondria are more coupled despite markedly enhanced UCP3 expression. In addition, we show that insulin-deficient diabetic muscle, heart, and liver mitochondria manifest tissue-specific antioxidant protective mechanisms including altered enzyme expression and/or enhanced UCP3. Hence, despite exposure to chronic severe hyperglycemia in vivo, the organelles per se remain remarkably robust with respect to superoxide production.
Supplementary Material
Acknowledgments
We thank Brian Dake for technical assistance.
Footnotes
This work was supported by Veterans Affairs Medical Research Funds and Grant DK25295 from the National Institutes of Health.
Disclosure Statement: The authors have nothing to declare.
First Published Online September 4, 2008
Abbreviations: ANT1, Adenine nucleotide translocator type 1; DMPO, 5,5-dimethyl-l-pyrroline-N-oxide; EPR, Electron paramagnetic resonance; ETS, electron transport system; FCCP, p-[trifluoromethoxy]-phenyl-hydrazone; GHS, glutathione; GPX, GHS peroxidase; GSSG, GHS disulfide; MnSOD, manganese superoxide dismutase; PRX III, peroxiredoxin III; ROS, reactive oxygen species; STZ, streptozotocin; TPP+, tetraphenyl phosphonium; UCP3, uncoupling protein-3.
References
- Skulachev VP 1996 Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys 29:169–202 [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA 2007 Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47:629–656 [DOI] [PubMed] [Google Scholar]
- Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM 2005 Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S 1996 Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim Biophys Acta 1276:87–105 [DOI] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B 2007 Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47:143–183 [DOI] [PubMed] [Google Scholar]
- Carlsson C, Borg LA, Welsh N 1999 Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology 140:3422–3428 [DOI] [PubMed] [Google Scholar]
- Du Y, Miller CM, Kern TS 2003 Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 35:1491–1499 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M 2000 Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790 [DOI] [PubMed] [Google Scholar]
- Yamagishi SI, Edelstein D, Du XL, Brownlee M 2001 Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes 50:1491–1494 [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM 2003 Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52:1–8 [DOI] [PubMed] [Google Scholar]
- Green K, Brand MD, Murphy MP 2004 Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53:S110–S118 [DOI] [PubMed] [Google Scholar]
- Skulachev VP 1998 Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta 1363:100–124 [DOI] [PubMed] [Google Scholar]
- Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED 4 August 2008 Type 1 diabetic Akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes 10.2337/db08-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Brindle KM, Buckingham JA, Harper JA, Rolfe DF, Stuart JA 1999 The significance and mechanism of mitochondrial proton conductance. Int J Obes Relat Metab Disord 23(Suppl 6):S4–S11 [DOI] [PubMed] [Google Scholar]
- Porter RK, Joyce OJ, Farmer MK, Heneghan R, Tipton KF, Andrews JF, McBennett SM, Lund MD, Jensen CH, Melia HP 1999 Indirect measurement of mitochondrial proton leak and its application. Int J Obes Relat Metab Disord 23(Suppl 6):S12–S18 [DOI] [PubMed] [Google Scholar]
- Takaki M, Nakahara H, Kawatani Y, Utsumi K, Suga H 1997 No suppression of respiratory function of mitochondrial isolated from the hearts of anesthetized rats with high-dose pentobarbital sodium. Jpn J Physiol 47:87–92 [DOI] [PubMed] [Google Scholar]
- Fink BD, Reszka KJ, Herlein JA, Mathahs MM, Sivitz WI 2005 Respiratory uncoupling by UCP1 and UCP2 and superoxide generation in endothelial cell mitochondria. Am J Physiol Endocrinol Metab 288:E71–E79 [DOI] [PubMed] [Google Scholar]
- Hong Y, Fink BD, Dillon JS, Sivitz WI 2001 Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology 142:249–256 [DOI] [PubMed] [Google Scholar]
- Fink BD, Herlein JA, Almind K, Cinti S, Kahn CR, Sivitz WI 2007 The mitochondrial proton leak in obesity-resistant and obesity-prone mice. Am J Physiol Regul Integr Comp Physiol 293:R1773–R1780 [DOI] [PubMed] [Google Scholar]
- O'Malley Y, Fink BD, Ross NC, Prisinzano TE, Sivitz WI 2006 Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem 281:39766–39775 [DOI] [PubMed] [Google Scholar]
- Lionetti L, Iossa S, Liverini G, Brand MD 1998 Changes in the hepatic mitochondrial respiratory system in the transition from weaning to adulthood in rats. Arch Biochem Biophys 352:240–246 [DOI] [PubMed] [Google Scholar]
- Kamo N, Muratsugu M, Hongoh R, Kobatake Y 1979 Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol 49:105–121 [DOI] [PubMed] [Google Scholar]
- Brown GC, Brand MD 1988 Proton/electron stoichiometry of mitochondrial complex I estimated from the equilibrium thermodynamic force ratio. Biochem J 252:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA, Pfeiffer DR, Bradshaw PC, Moraru II, Slepchenko B, Loew LM, Hsieh CE, Buttle K, Marko M 2001 Topology of the mitochondrial inner membrane: dynamics and bioenergetic implications. IUBMB Life 52:93–100 [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Hulbert AJ, Brand MD 1994 Characteristics of mitochondrial proton leak and control of oxidative phosphorylation in the major oxygen-consuming tissues of the rat. Biochim Biophys Acta 1188:405–416 [DOI] [PubMed] [Google Scholar]
- Fink BD, Hong YS, Mathahs MM, Scholz TD, Dillon JS, Sivitz WI 2002 UCP2-dependent proton leak in isolated mammalian mitochondria. J Biol Chem 277:3918–3925 [DOI] [PubMed] [Google Scholar]
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED 2007 Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56:2457–2466 [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD 2002 Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 4277:44784–44790 [DOI] [PubMed] [Google Scholar]
- Tietze F 1969 Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522 [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD 2004 Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem 279:39414–39420 [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D 2002 Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80:780–787 [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ 2001 Δψm-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79:266–277 [DOI] [PubMed] [Google Scholar]
- Lenaz G 2001 The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life 52:159–164 [DOI] [PubMed] [Google Scholar]
- Ceriello A 2003 New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care 26:1589–1596 [DOI] [PubMed] [Google Scholar]
- Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S, Catalano MG, Danni O, Boccuzzi G 2008 Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology 149:380–388 [DOI] [PubMed] [Google Scholar]
- de Cavanagh EM, Inserra F, Toblli J, Stella I, Fraga CG, Ferder L 2001 Enalapril attenuates oxidative stress in diabetic rats. Hypertension 38:1130–1136 [DOI] [PubMed] [Google Scholar]
- Kavak S, Ayaz L, Emre M, Inal T, Tamer L, Gunay I 2008 The effects of rosiglitazone on oxidative stress and lipid profile in left ventricular muscles of diabetic rats. Cell Biochem Funct 26:478–485 [DOI] [PubMed] [Google Scholar]
- Rauscher FM, Sanders RA, Watkins 3rd JB 2001 Effects of coenzyme Q10 treatment on antioxidant pathways in normal and streptozotocin-induced diabetic rats. J Biochem Mol Toxicol 15:41–46 [DOI] [PubMed] [Google Scholar]
- Sun F, Iwaguchi K, Shudo R, Nagaki Y, Tanaka K, Ikeda K, Tokumaru S, Kojo S 1999 Change in tissue concentrations of lipid hydroperoxides, vitamin C and vitamin E in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 96:185–190 [PubMed] [Google Scholar]
- Xia Z, Kuo KH, Nagareddy PR, Wang F, Guo Z, Guo T, Jiang J, McNeill JH 2007 N-acetylcysteine attenuates PKCβ2 overexpression and myocardial hypertrophy in streptozotocin-induced diabetic rats. Cardiovasc Res 73:770–782 [DOI] [PubMed] [Google Scholar]
- Boss O, Hagen T, Lowell BB 2000 Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes 49:143–156 [DOI] [PubMed] [Google Scholar]
- Salvi M, Battaglia V, Brunati AM, La Rocca N, Tibaldi E, Pietrangeli P, Marcocci L, Mondovi B, Rossi CA, Toninello A 2007 Catalase takes part in rat liver mitochondria oxidative stress defense. J Biol Chem 282:24407–24415 [DOI] [PubMed] [Google Scholar]
- Li X, May JM 2002 Catalase-dependent measurement of H2O2 in intact mitochondria. Mitochondrion 1:447–453 [DOI] [PubMed] [Google Scholar]
- Radi R, Turrens JF, Chang LY, Bush KM, Crapo JD, Freeman BA 1991 Detection of catalase in rat heart mitochondria. J Biol Chem 266:22028–22034 [PubMed] [Google Scholar]
- Turko IV, Murad F 2003 Quantitative protein profiling in heart mitochondria from diabetic rats. J Biol Chem 278:35844–35849 [DOI] [PubMed] [Google Scholar]
- Costa NJ, Dahm CC, Hurrell F, Taylor ER, Murphy MP 2003 Interactions of mitochondrial thiols with nitric oxide. Antioxid Redox Signal 5:291–305 [DOI] [PubMed] [Google Scholar]
- Maritim AC, Sanders RA, Watkins 3rd JB 2003 Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38 [DOI] [PubMed] [Google Scholar]
- Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN 2004 Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 53:1336–1343 [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Chang TS, Jeong W, Kim K 2001 Peroxiredoxin, a novel family of peroxidases. IUBMB Life 52:35–41 [DOI] [PubMed] [Google Scholar]
- Miwa S, Brand MD 2003 Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans 31:1300–1301 [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD 2002 Superoxide activates mitochondrial uncoupling proteins. Nature 415:96–99 [DOI] [PubMed] [Google Scholar]
- Hidaka S, Kakuma T, Yoshimatsu H, Sakino H, Fukuchi S, Sakata T 1999 Streptozotocin treatment upregulates uncoupling protein 3 expression in the rat heart. Diabetes 48:430–435 [DOI] [PubMed] [Google Scholar]
- Hidaka S, Yoshimatsu H, Kakuma T, Sakino H, Kondou S, Hanada R, Oka K, Teshima Y, Kurokawa M, Sakata T 2000 Tissue-specific expression of the uncoupling protein family in streptozotocin-induced diabetic rats. Proc Soc Exp Biol Med 224:172–177 [DOI] [PubMed] [Google Scholar]
- Cadenas S, Buckingham JA, Samec S, Seydoux J, Din N, Dulloo AG, Brand MD 1999 UCP2 and UCP3 rise in starved rat skeletal muscle but mitochondrial proton conductance is unchanged. FEBS Lett 462:257–260 [DOI] [PubMed] [Google Scholar]
- Yu XX, Barger JL, Boyer BB, Brand MD, Pan G, Adams SH 2000 Impact of endotoxin on UCP homolog mRNA abundance, thermoregulation, and mitochondrial proton leak kinetics. Am J Physiol Endocrinol Metab 279:E433–E446 [DOI] [PubMed] [Google Scholar]
- Levy SE, Chen YS, Graham BH, Wallace DC 2000 Expression and sequence analysis of the mouse adenine nucleotide translocase 1 and 2 genes. Gene 254:57–66 [DOI] [PubMed] [Google Scholar]
- Boudina S, Abel ED 2007 Diabetic cardiomyopathy revisited. Circulation 115:3213–3223 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI 2006 Etiology of insulin resistance. Am J Med 119:S10–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosca MG, Monnier VM, Szweda LI, Weiss MF 2002 Alterations in renal mitochondrial respiration in response to the reactive oxoaldehyde methylglyoxal. Am J Physiol Renal Physiol 283:F52–F59 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.