Abstract
Hypophysiotropic TRH-synthesizing neurons of the hypothalamic paraventricular nucleus (PVN) have a critical role in the regulation of the energy homeostasis through control of the hypothalamic-pituitary-thyroid axis. Recently, endocannabinoids have been shown to exert inhibitory effects on TRH neurons via the type 1 cannabinoid receptor (CB1). To understand the anatomical basis for this regulatory mechanism, we determined whether CB1 is contained in axons innervating hypophysiotropic TRH neurons using a recently developed antiserum against the C-terminal portion of mouse CB1. CB1-immunoreactive axons densely innervated the parvicellular subdivisions of the PVN where the hypophysiotropic TRH neurons are located. By double-labeling immunocytochemistry, CB1-immunoreactive varicosities were observed in juxtaposition to the vast majority of TRH neurons in the PVN. At the ultrastructural level, CB1-immunoreactivity was observed in the preterminal portion of axons establishing both symmetric and asymmetric synaptic specializations with the perikarya and dendrites of TRH neurons in the PVN. These data demonstrate that CB1 is abundantly present in axons that are in synaptic association with hypophysiotropic TRH neurons, indicating an important role for endocannabinoids in the regulation of the hypothalamic-pituitary-thyroid axis. The presence of both symmetric and asymmetric type CB1 synapses on TRH neurons in the PVN suggests that endocannabinoids may influence both excitatory and inhibitory inputs of these neurons.
Type 1 cannabinoid receptor-containing axons establish both symmetric- and asymmetric-type synapses with hypophysiotropic TSH-releasing hormone neurons, suggesting that the endocannabinoid system is involved in the regulation of the hypothalamic–pituitary–thyroid axis.
Hypophysiotropic TRH-synthesizing neurons residing in the parvicellular subdivisions of the hypothalamic paraventricular nucleus (PVN) are the main central regulators of the hypothalamic-pituitary-thyroid (HPT) axis and, therefore, have a critical role in the regulation of peripheral thyroid hormone levels (1). Because thyroid hormones increase basal metabolic rate (2), fasting-induced inhibition of the hypophysiotropic TRH neurons and the resultant fall in peripheral thyroid hormone levels may be an important homeostatic response mediated by the fall in peripheral leptin levels to improve survival during the period of food restriction (1,3).
The endocannabinoid signaling system uses arachidonic acid derivatives anandamide and 2-arachidonoyl-glycerol as signaling molecules (4). These bioactive lipid mediators are widely synthesized in the central nervous system where they act primarily by binding to type 1 cannabinoid receptors (CB1) (4). Endocannabinoids are synthesized and released from postsynaptic neurons, whereas CB1 is present in presynaptic terminals (4). This retrograde signaling system enables neurons to regulate their own synaptic input by modifying transmitter release from CB1-containing axon terminals (4,5).
A large body of evidence suggests a critical role for CB1 in the modulation of food intake and energy balance (6); central administration of endocannabinoids increases food intake (7), and CB1 knockout mice eat less after food restriction and have decreased body weight (8). In addition, the hypothalamic level of endocannabinoids increases during fasting and is reduced by leptin administration (7,9).
Because the parvicellular neurons of the PVN are densely innervated by CB1-containing axon terminals (10), the CB1 agonist, Δ9-tetrahydrocannabinol (Δ9THC), inhibits the HPT axis (11), and endocannabinoids have a CB1 receptor-mediated, direct effect on the TRH neurons in the PVN (12), we determined whether CB1-containing axon terminals are in an anatomical position to directly regulate hypophysiotropic TRH neurons.
Materials and Methods
Animals
Adult male CD1 mice (n = 7) weighing 25–30 g were used in the experiments. The animals were housed under standard environmental conditions (light between 0600 and 1800 h, temperature 22 ± 1 C, rodent chow and water ad libitum). All experimental protocols were reviewed and approved by the Animal Welfare Committee at the Institute of Experimental Medicine of the Hungarian Academy of Sciences.
Tissue preparation for light microscopic immunocytochemistry
Three mice were deeply anesthetized with sodium pentobarbital (35 mg/kg body weight, ip) and treated intracerebroventricularly with 30 μg colchicine under stereotaxic control. One day later, the animals were overdosed with pentobarbital (50 mg/kg body weight, ip) and perfused transcardially with 10 ml 0.01 m PBS (pH 7.4), followed by 40 ml 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4. The brains were rapidly removed and stored in 30% sucrose in 0.01 m PBS (pH 7.4) overnight at 4 C. Serial 20-μm-thick coronal sections were cut on a freezing microtome (Leica Microsystems, Vienna, Austria). The sections were stored in an antifreeze solution (30% ethylene glycol, 25% glycerol, 0.05 m PB) at −20 C until used.
Tissue preparation for electron microscopic immunocytochemistry
For electron microscopy, three mice were treated with colchicine as described above. One day later, the animals were overdosed with pentobarbital (50 mg/kg body weight, ip) and perfused transcardially, first with 10 ml 0.01 m PBS (pH 7.4), followed by 40 ml 4% paraformaldehyde/0.2% glutaraldehyde in 0.1 m PB (pH 7.4) and then 10 ml 4% paraformaldehyde in the same buffer. The brains were rapidly removed and fixed further in 4% paraformaldehyde overnight at 4 C and then stored in PBS for 24 h at 4 C. Serial 25-μm-thick coronal sections were cut on a Vibratome through the rostrocaudal extent of the hypothalamus, collected in PBS, and stored in antifreeze solution at −20 C until used.
Light microscopic double-labeling immunocytochemistry for CB1 and TRH
Every fourth section containing the PVN was incubated in a mixture of 0.5% Triton X-100 and 0.5% H2O2 in PBS for 15 min to improve antibody penetration and remove endogenous peroxidase activity. The sections were treated with 2% normal horse serum in PBS for 20 min to reduce nonspecific antibody binding. Then, the sections were immersed (for 2 d at 4 C) in a 1:25 dilution of a rabbit antiserum directed against the C-terminal 31 amino acids (443–473) of mouse CB1 (antibody code no. CB1-2003, bleeding date July 5, 2003) (13) in antibody diluent [PBS containing 2% normal horse serum, 0.2% Kodak Photo-Flo (Eastman Kodak, Rochester, NY), and 0.2% sodium azide]. Thereafter, the sections were rinsed in PBS and incubated in biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch, West Grove, PA) for 2 h, followed by incubation in avidin-biotin-peroxidase complex (ABC, 1:1000; Vector, Burlingame, CA). The immunolabeling was visualized with a peroxidase developer consisting of 0.05% diaminobenzidine (DAB), 0.15% nickel ammonium sulfate, and 0.005% H2O2 in 0.05 m Tris buffer (pH 7.6).
The resultant reaction product was silver intensified using the Gallyas method (14) to allow identification of CB1-immunoreactive (IR) axons by its black coloration. After additional washes, the sections were incubated in rabbit serum against TRH (no. 31) (15) at 1:8000 dilution for 2 d at 4 C. The sections were then washed in PBS and incubated in biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch) at 1:500 dilution for 2 h, and after additional washes in PBS, incubated in ABC at 1:1000 dilution for 2 h. After three washes in PBS, the reaction was developed in 0.025% DAB containing 0.0036% H2O2 in 0.05 m Tris buffer (pH 7.6), to yield a contrasting brown, cytoplasmic reaction product in neuronal perikarya. The specificity of the rabbit CB1 antiserum was described elsewhere (10,13).
Electron microscopic double-labeling immunocytochemistry for CB1 and TRH
Sections prepared for electron microscopy were treated with 1% sodium borohydride in 0.1 m PB for 30 min, followed by 0.5% H2O2 in PBS for 15 min. To improve antibody penetration, the sections were cryoprotected in 15% sucrose in PBS for 15 min and in 30% sucrose in PBS overnight (4 C) and then quickly frozen on liquid nitrogen. To reduce the nonspecific antibody binding, the sections were treated with 2% normal horse serum in PBS for 20 min at room temperature. The sections were then incubated in 1:900 dilution of a goat antiserum to CB1 [directed against the C-terminal 31 amino acids (443–473) of mouse CB1 (16)] mixed with 1:7500 dilution of the rabbit antiserum to TRH for 4 d at 4 C and then incubated in biotinylated donkey anti-sheep IgG (1:500; Jackson ImmunoResearch) for 1 d at 4 C. After rinsing in PBS and 0.1% cold water fish gelatin (Electron Microscopy Sciences, Fort Washington, PA)/1% BSA in PBS, the sections were incubated in donkey anti-rabbit IgG conjugated with 0.8-nm colloidal gold (Electron Microscopy Sciences) diluted at 1:100 in PBS containing 0.1% cold water fish gelatin and 1% BSA for 2 h at room temperature. The sections were then washed in the same diluent and PBS, followed by a 10-min treatment in 1.25% glutaraldehyde in PBS. After rinsing in 0.2 m sodium citrate (pH 7.5), the gold particles were silver intensified with the IntenseM Kit (Amersham, Buckinghamshire, UK). Finally, the sections were incubated in ABC Elite working solution (1:1000; Vector) for 2 h at room temperature. Immunoreactivity of CB1 was detected with 0.05% DAB, 0.15% nickel ammonium sulfate, and 0.005% H2O2 in 0.05 m Tris buffer (pH 7.6). Sections were then osmicated for 30 min in 1% osmium tetroxide, treated with 2% uranyl acetate in 70% ethanol for 30 min, dehydrated in an ascending series of ethanol followed by propylene oxide, and flat-embedded in Durcupan ACM epoxy resin (Fluka Chemika, Buchs, Switzerland) between glass microscope slides precoated with a liquid release agent (Electron Microscopy Sciences). The resin was allowed to polymerize at 56 C for 2 d. Ultrathin sections (50–60 nm) were cut from a layer of embedded Vibratome sections 2–4 μm below the surface with a Leica ultracut UCT ultramicrotome (Leica Microsystems), collected in ribbons onto Formvar-coated single-slot grids, contrasted with 2% lead citrate, and examined with a Jeol-100C transmission electron microscope. The specificity of the goat rabbit CB1 antiserum was described elsewhere (16).
Ultrastructural examination of CB1-IR innervation of TRH neurons in the PVN
TRH neurons from the medial parvicellular subdivision of the PVN at the midlevel of the nucleus, where the majority of the TRH neurons are hypophysiotropic (17), were selected for electron-microscopic examination from all three animals used for the ultrastructural study. The numbers of immunoreactive axon terminals forming either symmetric or asymmetric synapses on the perikarya or dendrites of TRH neurons were determined. The synapses were categorized as symmetric or asymmetric synapses according to the criteria of Gray (18). These morphological characteristics were confirmed on neighboring ultrathin sections.
Results
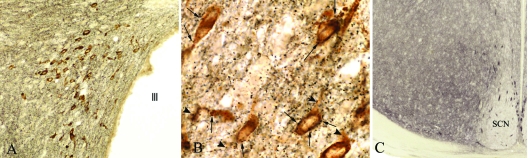
CB1 immunoreactivity appeared as fine, punctuate labeling in axons and axon terminals densely innervating all parvicellular subdivisions of the PVN (Fig. 1, A and B) but absent from some regions of the hypothalamus, like the suprachiasmatic nucleus (Fig. 1C), establishing specificity of the reaction. In the PVN, CB1-IR axon terminals marked by the black, silver/gold-intensified reaction product were closely apposed to the brown, DAB-immunolabeled cell bodies and proximal dendrites of all TRH neurons. In most instances, TRH neurons were surrounded by numerous CB1-IR boutons (Fig. 1B).
Figure 1.
A, Low-power photomicrograph illustrates the distribution of CB1-IR axons (black) and TRH-containing neurons (brown) in the caudal level of the PVN; B, high-power magnification of CB1-IR axon varicosities contacting perikarya (arrows) and proximal dendrites (arrowheads) of labeled TRH neurons in the medial parvicellular subdivision of the PVN; C, low-power micrograph illustrates the absence of CB1-IR axons in the suprachiasmatic nucleus.
Ultrastructural analysis of double-labeled preparations demonstrated that CB1-IR axons frequently established synaptic associations with hypophysiotropic TRH neurons (Figs. 2–4). The most intense CB1 signal was generally observed in the preterminal portion of the CB1-IR axons. The terminal portion of CB1-IR axons contained only a relatively weaker signal primarily in the region farthest from the synapse (Figs. 2–4). Tracing the juxtaposed CB1-IR terminals and TRH-IR neurons through a series of ultrathin sections, both symmetric (Fig. 2) and asymmetric (Figs. 3 and 4) synaptic specializations were observed on both the perikarya and dendrites of TRH-containing neurons. Analysis of 21 synapses between TRH neurons and CB1-IR terminals revealed 13 asymmetric and eight symmetric synaptic associations in the PVN.
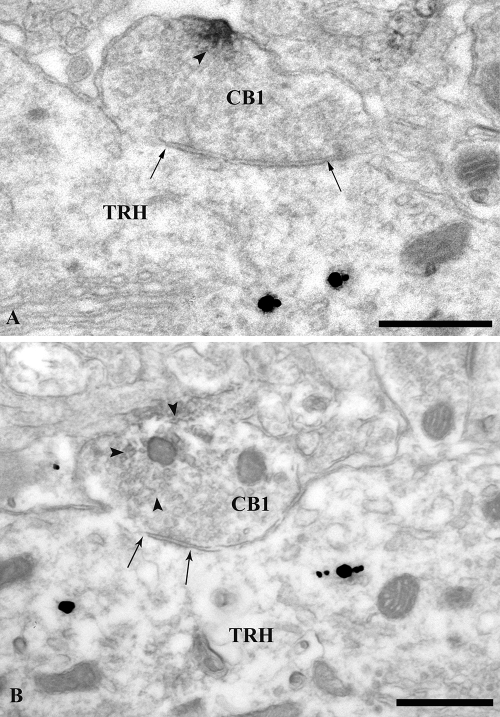
Figure 2.
Electron micrographs showing symmetric-type synaptic specializations between CB1-containing axon terminals and TRH-containing perikarya in the PVN. The TRH-IR perikarya (TRH) are identified with highly electron-dense silver-coated gold label, whereas the CB1-IR terminals are recognized by the presence of the medium electron-dense Ni-DAB (arrowheads). Arrows point to the synapses between the CB1- and TRH-IR structures. Scale bars, 500 nm.
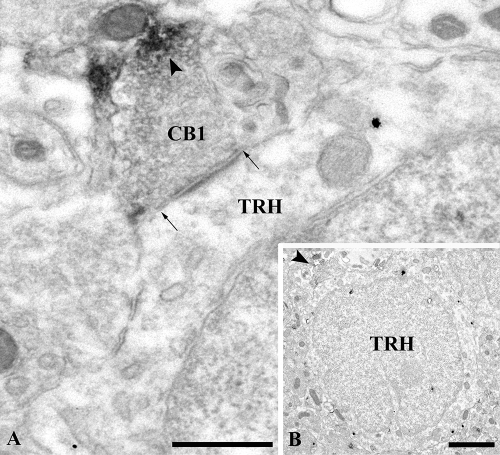
Figure 3.
Electron micrographs of an asymmetric-type axosomatic synapse between a CB1-IR axon terminal labeled with the moderately electron-dense Ni-DAB precipitate (arrowhead), and a TRH-IR perikaryon (TRH) labeled with highly electron-dense silver-gold particles. A, High-magnification image of the asymmetric synapse. Arrows point to the synaptic specialization. B, Low-magnification image of the innervated TRH neuron. Arrowhead points to the CB1-IR terminal. Scale bars, 500 nm (A) and 2 μm (B).
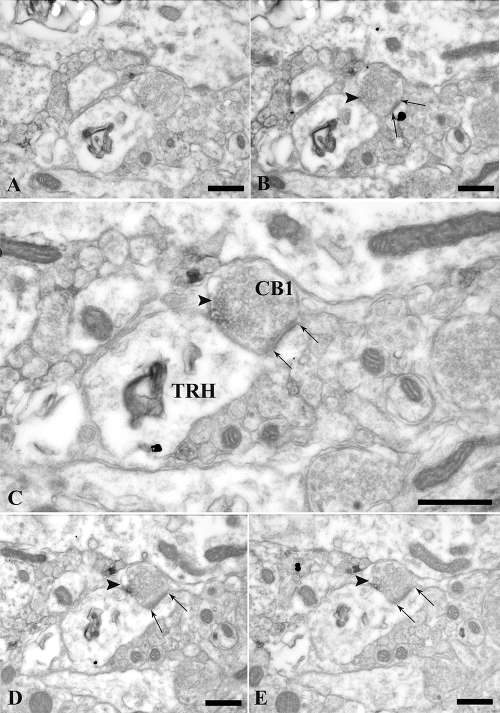
Figure 4.
Electron micrographs of series of ultrathin sections showing an asymmetric-type synaptic contact (arrows) between a TRH-containing dendritic spine and a CB1-containing axon terminal. The TRH-IR dendrite (*) and dendritic spine are labeled with the highly electron-dense silver particle, whereas the CB1-IR terminal (arrowheads) is recognized by the presence of the medium electron-dense Ni-DAB. Arrows point to the asymmetric synapse formed between the CB1-IR terminal and the TRH-IR dendritic spine. Scale bar, 500 nm (A–E).
Discussion
After discovery of the endogenous cannabinoid system and its role in the regulation of energy homeostasis, the number of research communications describing the role of cannabinoids in the regulation of hypothalamic functions exploded. CB1-immunoreactivity is discretely localized to several hypothalamic nuclei but absent from the suprachiasmatic and lateral mammillary nuclei (10). Despite the increasing number of functional research data, little is known about the hypothalamic neuronal groups innervated by CB1-containing axons.
In the present study, we demonstrate that CB1-containing axon terminals densely innervate the hypophysiotropic TRH neurons in the PVN. In addition, we show at the ultrastructural level that the CB1-immunoreactivity is present in both excitatory and inhibitory terminals forming synapses on TRH neurons.
The observed presynaptic location of CB1 is in agreement with the retrograde signaling role of endocannabinoids released from the postsynaptic structures to influence presynaptic functions (5). This concept is supported by the presence of endocannabinoid-synthesizing enzymes associated with the surface of the postsynaptic cells near the synapses (4). Upon activation of the postsynaptic cells, the synthesized endocannabinoids are released; they reach the presynaptic terminal by diffusion and bind to CB1 (4,5). By inhibiting the N- and P/Q-type voltage-activated Ca2+ channels, the receptor then suppresses the neurotransmitter release from the terminal (4). There are at least two ways by which the endocannabinoid retrograde signaling system can be activated. First, an increased firing of presynaptic axons results in a Ca2+ efflux into the postsynaptic neuron leading to endocannabinoid release and concomitant activation of CB1 on the presynaptic terminal resulting in inhibition of transmitter release (4,5). This is the mechanism of the depolarization-induced suppression of inhibition (DSI) and excitation (DSE) (4,5). The DSI and DSE are very well known phenomena in the hippocampus and play important role in learning (5). DSI has also been described in the hypothalamus (19). The second mode of activation of the endocannabinoid signaling is via peripheral hormones (12,20). Ghrelin and glucocorticoids increase endocannabinoid synthesis within the hypothalamus, whereas leptin inhibits it (12,19,20). Data from our group have demonstrated that a direct effect of ghrelin on parvicellular neurons of the PVN inhibits the glutamatergic input of these cells (20). Because this effect of ghrelin can be blocked by inhibition of the endocannabinoid synthesis and also by pharmacological antagonism of CB1 (20), we have proposed that the effect of ghrelin on the excitatory input of parvicellular neurons is mediated by the endocannabinoid retrograde signaling system. Di et al. (12) have observed that acute effects of glucocorticoids on the excitatory input of parvicellular neurons in the PVN are also mediated by endocannabinoid retrograde signaling. In contrast, a direct effect of leptin inhibits the endocannabinoid synthesis in lateral hypothalamic neurons and, therefore, inhibits the DSI of these cells (19).
Although exogenous administration of the plant cannabinoid Δ9THC decreases serum TSH and thyroid hormone levels (11), and the endocannabinoids can inhibit the glutamatergic input of TRH neurons (12), it is currently unclear how and under which circumstances endocannabinoids regulate TRH neurons.
An interesting observation in the study is the electron microscopic demonstration that CB1 axons in contact with hypophysiotropic TRH neurons establish both symmetric and asymmetric type synapses. Because symmetric synapses are generally associated with inhibitory and asymmetric synapses with a stimulatory action (21) and the hypophysiotropic neurons are densely innervated by axons containing either γ-aminobutyric acid (GABA), the main inhibitory transmitter, or glutamate, the main excitatory transmitter, the above observation suggests that CB1 triggered regulatory mechanisms are capable of interacting with both GABAergic and glutamatergic inputs of TRH neurons. This is not unusual for CB1, because both GABAergic and glutamatergic axons contain CB1 in the hippocampus (22,23). Therefore, Δ9THC should suppress simultaneously a portion of both the inhibitory and excitatory input of the TRH neurons that contain CB1. The inhibitory effect of Δ9THC on the HPT axis is, therefore, the net effect of this substance on the excitatory and inhibitory inputs of TRH neurons. This would suggest that the CB1-containing excitatory input of TRH neurons is more active under basal conditions than the CB1-containing inhibitory input of these cells.
In contrast to the exogenous treatment with cannabinoids, the release of endogenous cannabinoids may separately influence the two types of inputs on the same cell. Because fasting increases the hypothalamic level of endocannabinoids (7) and ghrelin, which has an increased level during fasting (24), inhibits the glutamatergic input of parvicellular neurons in the PVN via the endocannabinoid system (20), we hypothesize that inhibition of glutamatergic input by endocannabinoids may contribute to the fasting-induced inhibition of hypophysiotropic TRH neurons. Interestingly, however, the orexigenic neuropeptide Y (NPY)/agouti-related protein and the anorexigenic α-melanocyte-stimulating hormone/cocaine-and amphetamine-regulated transcript neurons of the arcuate nucleus that mediate the effects of fasting and leptin administration on hypophysiotropic TRH neurons (1) do not express CB1 (8). Because NPY can inhibit glutamate release from nerve terminals in the cortex through the inhibition of N- and P/Q-type Ca2+ channels (25), the same channel that is inhibited by the CB1 (4), the endocannabinoid signaling may be involved in the mediation of the effect of NPY on the glutamate release. Therefore, we hypothesize that the increased release of NPY in fasted animals may result in endocannabinoid release from the hypophysiotropic TRH neurons, leading to suppression of glutamatergic input of these neurons via CB1. This way, NPY may block the excitatory input of TRH neurons, which in turn may originate from leptin-insensitive neuronal groups located outside the arcuate nucleus. This putative mechanism may contribute to the fasting-induced inhibition of the HPT axis.
Because inflammation also increases the hypothalamic level of anandamide (26), the endocannabinoid system may also be involved in the inflammation-induced inhibition of the hypophysiotropic TRH neurons.
In summary, we conclude that the hypophysiotropic TRH neurons are densely innervated by CB1-containing axons. The presence of CB1 in both inhibitory and excitatory axons innervating the TRH neurons suggests that the TRH neurons can regulate their own excitatory and inhibitory inputs via the retrograde endocannabinoid signaling system.
Footnotes
This work was supported by grants from the Sixth EU Research Framework Programme (contract LSHM-CT-2003-503041), and National Institutes of Health (DK37021 and TW007834). The study was supported in part by Gedeon Richter Ltd. (Hungary).
Disclosure Statement: L.D., G.W., I.K., R.M.L., M.W., Z.L., and C.F. have nothing to declare.
First Published Online September 25, 2008
Abbreviations: CB1, Type 1 cannabinoid receptor; DSI, depolarization-induced suppression of inhibition; GABA, γ-aminobutyric acid; HPT, hypothalamic-pituitary-thyroid; IR, immunoreactive; NPY, neuropeptide Y; PB, phosphate buffer; PVN, hypothalamic paraventricular nucleus; Δ9THC, Δ9-tetrahydrocannabinol.
References
- Fekete C, Lechan RM 2007 Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Front Neuroendocrinol 28:97–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JE 2003 The thermogenic effect of thyroid hormone and its clinical implications. Ann Intern Med 139:205–213 [PubMed] [Google Scholar]
- Lechan RM, Fekete C 2006 The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153:209–235 [DOI] [PubMed] [Google Scholar]
- Piomelli D 2003 The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884 [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D 2003 Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066 [DOI] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R 2006 The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27:73–100 [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V 2002 Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol 136:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U 2003 The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G 2001 Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410:822–825 [DOI] [PubMed] [Google Scholar]
- Wittmann G, Deli L, Kallo I, Hrabovszky E, Watanabe M, Liposits Z, Fekete C 2007 Distribution of type 1 cannabinoid receptor (CB1)-immunoreactive axons in the mouse hypothalamus. J Comp Neurol 503:270–279 [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Farber NE, Hagen TC, Bloom AS 1984 The effects of Δ9-tetrahydrocannabinol on serum thyrotropin levels in the rat. Pharmacol Biochem Behav 20:547–550 [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG 2003 Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23:4850–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M 2004 Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci 19:2682–2692 [DOI] [PubMed] [Google Scholar]
- Liposits Z, Setalo G, Flerko B 1984 Application of the silver-gold intensified 3,3′-diaminobenzidine chromogen to the light and electron microscopic detection of the luteinizing hormone-releasing hormone system of the rat brain. Neuroscience 13:513–525 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Jackson IM 1982 Immunohistochemical localization of thyrotropin-releasing hormone in the rat hypothalamus and pituitary. Endocrinology 111:55–65 [DOI] [PubMed] [Google Scholar]
- Makara JK, Katona I, Nyiri G, Nemeth B, Ledent C, Watanabe M, de Vente J, Freund TF, Hajos N 2007 Involvement of nitric oxide in depolarization-induced suppression of inhibition in hippocampal pyramidal cells during activation of cholinergic receptors. J Neurosci 27:10211–10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Mihaly E, Luo LG, Kelly J, Clausen JT, Mao Q, Rand WM, Moss LG, Kuhar M, Emerson CH, Jackson IM, Lechan RM 2000 Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J Neurosci 20:9224–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG 1959 Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat 93:420–433 [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Chen YJ, Chua Jr SC, Talmage DA, Role LW 2005 Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron 48:1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Ajodha S, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M 2008 The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. Plos One e1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H 1991 The fine structure of the nervous system. Neurons and their supporting cells. 3rd ed. Oxford, UK: Oxford University Press [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF 1999 Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF 2006 Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci 26:5628–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Wang SJ 2005 Activation of neuropeptide Y Y1 receptors inhibits glutamate release through reduction of voltage-dependent Ca2+ entry in the rat cerebral cortex nerve terminals: suppression of this inhibitory effect by the protein kinase C-dependent facilitatory pathway. Neuroscience 134:987–1000 [DOI] [PubMed] [Google Scholar]
- Rettori V, Fernandez-Solari J, Prestifilippo JP, Mohn C, De Laurentiis A, Bornstein SR, Ehrhart-Bornstein M, Elverdin JC, McCann SM 2007 Endocannabinoids in TNF-α and ethanol actions. Neuroimmunomodulation 14:188–192 [DOI] [PubMed] [Google Scholar]