Abstract
Hypothalamic nesfatin-1, derived from the nucleobindin2 (NUCB2) precursor, inhibits nocturnal food intake and body weight gain in rats. Nesfatin-1 is able to cross the blood-brain barrier, suggesting a peripheral source of nesfatin-1. Many centrally acting food intake regulatory neuropeptides are also produced in the periphery, especially in the gastrointestinal tract. Therefore, we investigated the gene expression of NUCB2 and distribution of nesfatin-1-immunoreactive cells in the stomach. Microarray mRNA expression profiles in purified small endocrine cells of the gastric mucosa substantiated by quantitative RT-PCR showed significantly higher NUCB2 mRNA expression compared with brain and heart. Western blot confirmed the expression of NUCB2 protein and its transport into a secretory soluble fraction of gastric mucosal endocrine cell homogenates. Immunohistochemical colabeling for nesfatin-1 and ghrelin, histidine decarboxylase, or somatostatin revealed two subtypes of nesfatin-1-positive endocrine cells. Cells in the midportion of the glands coexpressed nesfatin-1 and ghrelin, whereas few cells in the glandular base coexpressed nesfatin-1 and somatostatin or histidine decarboxylase. High-resolution three-dimensional volume imaging revealed two separate populations of intracytoplasmic vesicles in these cells, one containing nesfatin-1 and the other ghrelin immunoreactivity. Microarray rat genome expression data of NUCB2 in small gastric endocrine cells confirmed by quantitative RT-PCR showed significant down-regulation of NUCB2 after 24 h fasting. In summary, NUCB2 mRNA expression as well as protein content is present in a specific subset of gastric endocrine cells, most of which coexpress ghrelin. NUCB2 gene expression is significantly regulated by nutritional status, suggesting a regulatory role of peripheral nesfatin-1 in energy homeostasis.
Nesfatin-1/nucleobindin 2 is co-expressed in gastric ghrelin-containing X/A-like cells, suggesting the release of orexigenic and anorexigenic peptides from the same endocrine cells regulating food intake.
Nesfatin-1, a novel 82-amino-acid polypeptide, was identified in 2006 by Oh and colleagues (1) as the amino-terminal fragment of nucleobindin2 (NUCB2), a protein that is highly conserved in humans, mice, and rats. The protein contains a 24-amino-acid N-terminal signal peptide sequence followed by a 396-amino-acid sequence that is proteolytically processed by pro-hormone convertase to at least three peptides, nesfatin-1 (1–82), nesfatin-2 (85–163), and nesfatin-3 (166–396), of which only nesfatin-1 suppresses nocturnal food intake and body weight gain in freely fed rats when administered into the rat cerebral ventricle (1). Interestingly, nesfatin-3 contains two EF-hand S100-like calcium-binding motifs, possibly suggesting intracellular calcium signaling properties for this peptide. Immunostaining studies have shown that NUCB2/nesfatin-1-containing proteins were present in the rat arcuate nucleus and the paraventricular nucleus of the hypothalamus (PVN) (2,3), which are important brain nuclei involved in the regulation of food intake (4). Nesfatin-1 alters the membrane potential of different subpopulations of PVN neurons (5). Twenty-four hours fasting decreased the mRNA expression of NUCB2 in the PVN (1), and conversely, refeeding resulted in a significant increase of activated nesfatin-1-immunoreactive neurons in the PVN, assessed by Fos immunohistochemistry (3). These results suggest a physiological role of hypothalamic nesfatin-1 in the regulation of food intake. Although hypothalamic protein samples did not show mature nesfatin-1, rat cerebrospinal fluid contained nesfatin-1, indicating processing and secretion of the mature peptide (1). Nesfatin-1 crosses the blood-brain barrier in both directions in a nonsaturable way (6,7). This observation and the fact that various centrally active food regulatory neuropeptides are produced in the periphery (8) raises the question as to whether nesfatin-1 has a peripheral site of production.
The stomach is the first site of food contact, and it is not surprising that the oxyntic mucosa contains a variety of endocrine cells (9,10), which are predominantly located in the basal region of the epithelium (11). Although in the antrum the main endocrine cell types are gastrin-producing cells (G cells) and somatostatin cells (D cells), the oxyntic mucosa accommodates a variety of physiologically important transmitters involved not only in the regulation of acid secretion but also in energy homeostasis of the body (9,12,13,14). In the rat, these include enterochromaffin-like cells (∼70%) (15), which release histamine and thereby stimulate acid secretion in parietal cells (16), somatostatin-producing D cells (∼5%) (15,17), and ghrelin-producing X/A-like cells (∼20%) (18), which play key roles in appetite control and gastrointestinal motility (14). In addition, there is a minority of other cell types containing small endocrine granules, namely serotonin-containing enterochromaffin cells, P cells, and D1 cells (10,14,15,19,20,21).
Therefore, we investigated whether NUCB2, the precursor of the novel anorexigenic peptide nesfatin-1, is also peripherally produced in the rat gastric mucosa. NUCB2 mRNA expression was studied using comparative microarray gene expression analysis and quantitative RT-PCR (RT-qPCR) in the gastric oxyntic mucosa compared with the brain and another viscera, the heart. The NUCB2 protein was analyzed by Western blot in the gastric mucosa and other endocrine tissues, the pituitary gland and pancreas. NUCB2/nesfatin-1-immunoreactive cells were visualized by immunohistochemistry and characterized using immunofluorescent double labeling of mucosal sections with antibodies against NUCB2/nesfatin-1 together with anti-ghrelin, anti-somatostatin, or an antibody against the histamine-synthesizing enzyme histidine decarboxylase (HDC) and confocal microscopy analysis. The regulation of NUCB2 mRNA expression during fasting was investigated by microarray analysis and RT-qPCR.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Harlan, San Diego, CA) (body weight 280–350 g) were housed in groups of four animals per cage under conditions of controlled illumination (12-h light, 12-h dark cycle, lights on at 0600 h and off at 1800 h), humidity (60%), and temperature (22 ± 2 C). Animals were fed with a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water ad libitum. Fasting was achieved by housing rats in groups of four rats per cage with free access to water without food for 24 h starting at 0900 h. The rats fed ad libitum were also housed in groups of four animals per cage for the same time period. Tissues of ad libitum-fed and 24-h-fasted rats were harvested on the following day at 0900 h after euthanasia by CO2 anesthesia followed by cervical dislocation.
Animal care and experimental procedures followed institutional ethics guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committees at Veterans Affairs Greater Los Angeles Healthcare System (Animal Protocol No. 04032-01).
Immunohistochemical and fluorescent staining and microscopy
Three ad libitum-fed rats were euthanized by CO2 anesthesia followed by cervical dislocation. Esophagus, stomach, small intestine, colon, pancreas, liver, spleen, heart, gastrocnemius muscle, lung, kidney, adrenal gland, testis, visceral fat, sc fat, and pituitary gland were quickly removed and ex situ fixed in Bouin’s fixative for 2 h. The tissues were rinsed in 50% ethanol three times to remove fixative residues and processed after standard procedures until paraffin block embedding. Five-micrometer sections were cut using a microtome, deparaffinized, and rehydrated with graded xylene-alcohol series, and washed with PBS three times for 15 min before immunostaining. Endogenous peroxidase was inactivated by 0.3% hydrogen peroxide in PBS for 30 min, and nonspecific binding was reduced by pretreatment with 3% normal goat serum. Sections were incubated overnight at 4 C in antirat nesfatin-1 (1–82) polyclonal antibody at a titer of 1:2000 (Phoenix Pharmaceuticals, Burlingame, CA) in 0.3% Triton X-100 in PBS and then in biotinylated goat antirabbit IgG (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA) in 0.3% Triton X-100 in PBS for 1 h at room temperature followed by incubation with avidin-biotin-peroxidase complex in 0.3% Triton X-100 in PBS (ABC; Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride hydrate and hydrogen peroxide (Sigma Chemical Co., St. Louis, MO) for 10 min and frequently checked on the light microscope. Each step of incubation was followed by three 5-min washes in PBS. Sections were mounted on Fisher SuperFrost Plus slides, air dried for 24 h, and completely dehydrated through a gradient of ethanol and xylene. Slides were studied using light microscopy.
The same protocol was applied for immunostaining after preabsorption of the anti-nesfatin-1 antibody. Fifty micrograms of rat nesfatin-1 (Phoenix Pharmaceuticals) were incubated with 1 ml rabbit antirat nesfatin-1 (1:2000) in 0.3% Triton X-100 in PBS for 2 h at room temperature followed by 22 h at 4 C. The solution was centrifuged for 15 min at 16,000 × g, and the supernatant was used for staining as described above.
For immunofluorescent double labeling, the gastric corpus was sectioned. After pretreatment with normal goat serum as described above, sections were incubated overnight at 4 C in anti-nesfatin-1 (1–82) antibody (1:500) together with either guinea pig anti-HDC (1:1000; Eurodiagnostics, Malmö, Sweden), mouse anti-ghrelin serum D4–7.1 (1:2000; Eli Lilly Research Laboratories, Indianapolis, IN) or mouse anti-somatostatin (1:500, SST CURE S6; UCLA Digestive Diseases Research Center, Los Angeles, CA) antibodies in 0.3% Triton X-100 in PBS. Tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat antirabbit IgG (1:2000; Jackson ImmunoResearch) together with either fluorescein isothiocyanate (FITC)-labeled antimouse (1:500; Jackson ImmunoResearch) or FITC-labeled anti-guinea-pig (1:100; Jackson ImmunoResearch) antibodies in 0.3% Triton X-100 in PBS were added for 2 h at room temperature. Each step of incubation was followed by three 5-min washes in PBS. Counterstaining was performed using 4′,6-diamidino-2-phenylindole (Sigma) 1:1000 for 6 min. The slides were mounted with antifade mounting medium (Vector Laboratories) and visualized by confocal microscopy (LSM 510; Zeiss, Oberkochen, Germany).
Cell separation from rat stomach
Cell suspensions of gastric mucosal cells, gastric small endocrine cells, and purified parietal cells were prepared as previously described applying a combined method of pronase digestion of oxyntic mucosa followed by Nycodenz gradient density centrifugation before counterflow elutriation and fluorescence-activated cell sorting (22,23,24).
SDS-PAGE and Western blot analysis
Soluble and membrane protein fractions of gastric corpus mucosa, pancreas, and pituitary gland were prepared from three freely fed rats. Animals were euthanized by CO2 anesthesia followed by cervical dislocation at 0900 h. Pancreas and pituitary gland were removed and kept on ice. Stomachs were immediately opened and rinsed, and the corpus mucosa was scraped off. All tissues were kept on ice during tight Dounce homogenization (Wheaton, Millville, NY) with PBS supplemented with one tablet of protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Crude protein fractions were obtained by centrifugation of the homogenates in the Sorvall centrifuge at 12,000 × g for 20 min to remove cell debris and nuclei. Soluble protein was separated from the membrane fraction by ultracentrifugation for 90 min at 100,000 × g. The supernatant was used directly as the soluble protein fraction. All centrifugation steps were executed at 4 C. Final protein concentrations for stomach mucosa, pancreas, and pituitary gland samples were determined using a BCA protein assay according to the manufacturer (Pierce Biotechnology, Rockford, IL).
Gastric mucosal small endocrine cells were isolated as described above. Cells were counted (Cellometer Disposable Cell Counting Chamber; Nexcelom Bioscience Lawrence, MA) and 400,000 cells incubated in lysis buffer [0.15 m NaCl, 1% Triton X-100, 4 mm EGTA in 15 mm Tris base (pH 8.0)] supplemented with protease inhibitor cocktail (Roche Applied Science) for 30 min on ice with gentle agitation. Thereafter, cell suspensions were centrifuged for 10 min at 18,000 × g at 4 C. The supernatant was used as the soluble cell protein fraction, whereas the pellet was resuspended in PBS supplemented with protease inhibitor cocktail (Roche Applied Science).
Gel samples were prepared by mixing protein samples with gel sample buffer [4% SDS, 0.05% bromphenol blue (wt/vol), 20% glycerol, 1% mercaptoethanol (vol/vol) in 0.1 Tris buffer, pH 6.8]. Samples were incubated at 60 C for 15 min before gel electrophoresis. Soluble and membrane protein obtained from 400,000 cells, 20 μg of stomach, pancreas, and pituitary soluble and membrane protein per lane, and 50 ng of synthetic nesfatin-1 peptide (Phoenix Pharmaceuticals) were loaded on a 4–12% SDS-polyacrylamide gel (NuPage; Invitrogen, Carlsbad, CA) and run in 2-(N-morpholino)ethanesulfonic acid buffer. After SDS-PAGE, proteins were transferred by electrophoresis to nitrocellulose membranes (BioPlot-NC; Costar, Cambridge, MA) for 6 h at 4 C. Membranes were washed in distilled water and stained in Ponceau-S in 3% trichloroacetic acid solution, and an image was taken. Membranes were washed twice with Tris-buffered saline (TBS; 10 mm Tris, 150 mm NaCl, and 0.05% Tween, vol/vol) and incubated in TBS containing 5% (wt/vol) nonfat milk (Carnation, Nestlé, Glendale, CA). After 30 min, the membranes were incubated in the primary antibody solution (polyclonal antibody nesfatin-1; Phoenix Pharmaceuticals) diluted 1:1000 in TBS. After 1 h, membranes were washed three times with TBS and incubated with the secondary antibody solution (antirabbit IgG conjugated to alkaline phosphatase; Promega, Madison, WI) diluted 1:2000 in TBS. After 1 h, membranes were washed three times before color development in alkaline phosphatase buffer [100 mm Tris, 100 mm NaCl, and 5 mm MgCl2 (pH 9.5)] containing 0.3% nitroblue tetrazolium solution (vol/vol) and 0.15% 5-bromo-4-chloro-3-indolyl-l-phosphate solution (vol/vol) according to the manufacturer’s instructions (Promega) for 5–10 min.
RT-qPCR and comparative gene expression microarray analysis
Total RNA from gastric mucosal fundus homogenates, purified parietal cells, and whole brain and heart from ad libitum-fed rats as well as RNA from gastric small endocrine cells of ad libitum-fed and 24-h-fasted rats was isolated. Rats were euthanized by CO2 anesthesia followed by cervical dislocation at 0900 h. Organs were quickly removed, and RNA was generated and integrity determined as described previously (24). One microgram total RNA was added to the RT reaction using Omniscript (QIAGEN, Valencia, CA) reverse transcriptase and an oligo (deoxythymidine) 12–18 primer (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Two microliters of RT product were then added to a real-time qPCR together with the Dynamo SYBR Green qPCR Kit (Finnzymes, Espoo, Finland) according to the manufacturer’s protocol. Primers used were rat NUCB2 (sense CCA TCC AAG CAC GGT ACT GTT TTC and antisense CCA GTG TCT TGA AGG GCA TCC) (GenBank accession no. NM 021663) together with rat vesicular monoamine transporter 2 (VMAT2) (sense CCA GCT CCT CAC TAA CCC ATT CAT and antisense TCA GCA AGG TCG TTA GAG GTG TCC) (NM 013031) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reference primers (sense GGG TGA TGC TGG TGC TGA GTA TGT and antisense CAG TGG ATG CAG GGA TGA TGT TCT) (NM 017008). Annealing temperature was 58 C.
Real-time qPCR was performed in six-well strips using a DNA Engine Opticon 2 U (MJ Research, Watertown, MA). Each amplification was followed by a melting curve resulting in only one peak for each amplicon indicative of amplification of only one product. This was confirmed by agarose gel electrophoresis of the RT-PCR products. The cycle of threshold C(T) was determined as the fluorescent signal (binding of SYBR green to double-stranded cDNA) of 1 sd over background. The efficiency of each primer pair was also measured by cloning each PCR product into pCR-4-TOPO cloning vector (Invitrogen) and amplification with known amounts of cDNA starting material (10 pg to 10 ng). The slope of the linear curve of C(T) vs. cDNA starting material was used to calculate the efficiency of the primer pair [efficiency = 10(−1/slope)]. Target primer pair amplifications of NUCB2 were compared with the reference primer pair amplifications (GAPDH) in the same experiment for each RT product tested. All reactions were carried out in duplicate, and four separate amplifications for each primer pair were performed. The relative expression ratio of the target gene compared with the reference gene GAPDH was calculated using the formula (25):
 |
Each transcriptome analysis was performed using subtractive hybridization on 44K whole rat genome expression oligonucleotide microarrays (Agilent Technologies, Santa Clara, CA) as described before (24). The microarray data were deposited for public access to the Gene Expression Omnibus NCBI database with the series number GSE6204 (purified gastric endocrine cells and gastric mucosa from 24-h-fasted rats compared with ad libitum-fed rats) and GSE3518 (normal purified gastric endocrine and parietal cells). In addition, microarray data from heart tissue were also included in the analysis. All microarray conditions were similar, and all data sets were analyzed using Genespring Pro 7.3 (Agilent) as a statistical analysis package and microarray data warehouse. At least three independent RNA-labeling procedures resulted in three independent channel intensities (including one dye swap) that were compared across the tissue samples by normalizing overall expression of housekeeping genes of the gastric mucosal samples.
Statistical analysis
Data are expressed as mean ± sd and were analyzed by one-way ANOVA. Differences between groups were evaluated by all pairwise multiple comparison procedures (Tukey post hoc test). In the study investigating the VMAT2 and NUCB2 mRNA expression after fasting differences between groups were evaluated by Student’s t test, P < 0.05 was considered significant.
Results
NUCB2 expression in the stomach
NUCB2 mRNA detected by RT-qPCR was highly expressed in the gastric oxyntic mucosa. The expression was at least 10-fold higher compared with total RNA extracts from rat brain shown as relative ratio compared with brain tissue in Fig. 1 (mean ± sd for gastric mucosa, 16.38 ± 0.85, P < 0.001; heart tissue, 1.40 ± 0.84).
Figure 1.
NUCB2 mRNA expression in the stomach of ad libitum-fed rats. The precursor of nesfatin-1, NUCB2, was significantly more highly expressed in the gastric oxyntic mucosa than in brain and heart. Data are expressed as mean ± sd. *, P < 0.001 vs. brain and P < 0.001 vs. heart, respectively.
Further analysis of microarray mRNA expression data sets of gastric mucosa, 99% purified parietal cells, and 90% purified small endocrine cells of the oxyntic mucosa showed significantly higher expression in the purified endocrine cell fraction compared with gastric mucosa (normalized intensities, mean ± sd for gastric mucosa 9073.55 ± 3702.32 vs. endocrine cells 47547.00 ± 8116.85, P < 0.001). NUCB2 expression was absent in gastric parietal cells (1825.33 ± 614.56, P < 0.001) (Fig. 2).
Figure 2.
Quantitative comparison of NUCB2 gene expression using whole-rat genome microarray expression analysis in diverse stomach cell type samples. NUCB2 expression was absent in purified gastric parietal cells but significantly increased in the purified small endocrine cell fraction compared with the whole stomach. Data are expressed as mean ± sd. *, P < 0.001 vs. whole stomach and P = 0.001 vs. parietal cells.
Western blot for nesfatin-1 in the gastric mucosa, pancreas, and pituitary gland
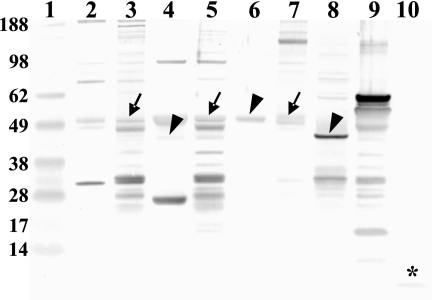
A Western blot of soluble and membrane proteins of gastric mucosa, pancreas, and pituitary gland with rat anti-nesfatin-1 antibody showed full-length NUCB2 protein (∼50 kDa) in all membrane protein fractions (Fig. 3). The soluble fraction of gastric mucosal endocrine cells and pancreatic tissue showed a reduction of the molecular mass of approximately 3 kDa (Fig. 3). In contrast, the soluble fraction of the pituitary gland showed full-length peptide of the same size as the membrane fraction (Fig. 3). Lane 10 shows the ability of the Western blot system used to detect 50 ng synthetic nesfatin-1 peptide (9.7 kDa) (Fig. 3). This band was not detected in any of the tissue samples tested.
Figure 3.
Western blot for nesfatin-1 in the gastric mucosa, pancreas, pituitary gland, and gastric oxyntic gland endocrine cells. Using rat anti-nesfatin-1 antibody, membrane fractions of gastric mucosa (lane 3), pancreas (lane 5), and pituitary gland (lane 7) showed full-length NUCB2 protein (∼50 kDa, arrow). The soluble fraction of pancreatic tissue (lane 4) and gastric mucosal endocrine cells (lane 8) shows a reduction of the molecular mass of approximately 3 kDa (∼47 kDa, arrowhead), whereas the soluble fraction of the pituitary gland (lane 6) shows no further processing of the full-length protein (∼50 kDa, arrowhead). Lane 9 is loaded with a resuspended pellet of nuclei, mitochondria, and crude membranes after removal of soluble proteins from gastric endocrine cells. Lane 10 was loaded with 50 ng synthetic nesfatin-1 peptide to illustrate the ability of the blot to detect the 9.7-kDa band (star). Lane 1 was loaded with a standard molecular weight marker; gastric mucosal soluble protein fraction was loaded in lane 2.
Nesfatin-1-immunoreactive cells in stomach, pancreas, and pituitary gland
Immunohistochemical staining showed nesfatin-1-immunoreactive cells in the gastric oxyntic mucosa (Fig. 4, A and B). These cells resembled an endocrine phenotype and were distributed particularly in the lower third and midportion of the oxyntic gland (Fig. 4, A and B). The staining was exclusively detected in a cytoplasmic vesicular compartment (Fig. 4, A and B). The antrum showed very few nesfatin-1-immunoreactive cells and nesfatin-1-immunopositive cells were absent in the esophagus, small intestine, and colon (data not shown). The pancreas showed strong cytoplasmic vesicular staining in islet endocrine cells (Fig. 4, C and D), whereas the anterior pituitary gland showed predominantly nuclear staining in a subset of endocrine cells (Fig. 4, E and F). Nesfatin-1-immunoreactive cells were also found in the rat testis in the interstitium next to the seminiferous tubules (data not shown). No nesfatin-1-immunopositive cells could be detected in liver, kidney, spleen, sc and visceral adipose tissue, adrenal gland, heart, gastrocnemius muscle, and lung (data not shown).
Figure 4.
Nesfatin-1-immunoreactive cells in the stomach, pancreas, and pituitary gland. Nesfatin-1-immunoreactive cells were detected in the oxyntic mucosa of the gastric corpus. Most of the immunopositive cells were located in the lower third and middle portion of the oxyntic glands (A, 100-fold magnification). The cytoplasmic vesicular staining as well as the endocrine appearance of these cells is shown in the inset with higher magnification (B, 400-fold magnification, arrows). The pancreas showed strong cytoplasmic vesicular staining in a subset of islet endocrine cells (C, 100-fold magnification). The inset shows higher magnification of this islet (D, 400-fold magnification). The pituitary gland showed predominantly nuclear staining in a subset of the endocrine cells (E, 100-fold magnification). The higher magnification highlights the nuclear staining (F, 400-fold magnification, arrows). Scale bar in A, 100 μm (A, C, and E); scale bar in B, 50 μm (B, D, and F).
After preabsorption of the antibody with rat nesfatin-1 or omission of anti-nesfatin-1 antibody, no immunostaining could be detected in all tissues studied (data not shown).
Colocalization of nesfatin-1 immunoreactivity with ghrelin, somatostatin, or HDC in gastric oxyntic mucosa using confocal microscopy
Similarly to the immunohistochemical analysis, fluorescent immunoreactivity of nesfatin-1 was detected in endocrine cells of the mid and basal portion of the gastric glands of the oxyntic mucosa (Fig. 5, A, C, and E). Most nesfatin-1 immunoreactivity (53%) of the midportion in the oxyntic mucosa colocalized with ghrelin-immunoreactive endocrine cells (Table 1 and Fig. 5, A and B). The majority of ghrelin-positive cells (86%) were also positive for nesfatin-1 (Table 1). High-resolution images of a representative single cell revealed separate pools of nesfatin-1- and ghrelin-immunoreactive cytoplasmic vesicles (Fig. 6).
Figure 5.
Confocal microscopy of doubly labeled oxyntic mucosal sections with antibodies against nesfatin-1, ghrelin, somatostatin, or HDC. The majority of nesfatin-1-immunoreactive cells (red) is located in the middle part of the gastric oxyntic glands colocalizing with ghrelin-containing X/A cells (green, A). The inset at higher magnification clearly illustrates colocalization of nesfatin-1 with the majority of ghrelin immunoreactive cells (B). Somatostatin-containing cells (green, C) were located mostly in the lower third of the gastric corpus mucosa. The inset (D) in higher magnification shows that only a few cells coexpress nesfatin-1 and somatostatin (arrow). Similar results were seen for HDC-positive cells (green, E), again located mostly in the lower third of the oxyntic mucosa. The inset (F) in higher magnification shows that only a few cells coexpress nesfatin-1 and HDC (arrow).
Table 1.
Percentage of nesfatin-1-immunoreactive cells in the gastric oxyntic mucosa colabeling with other endocrine cell markers and vice versa
| Modality of examination | Markers
|
||
|---|---|---|---|
| Ghrelin | Somatostatin | HDC | |
| % Nesfatin-1-immunoreactive cells colabeling with other markers | 53 | 15 | 21 |
| % Positive cells for other markers colabeling with nesfatin-1 | 86 | 25 | 14 |
Figure 6.
High-resolution confocal microscopy of a single cell coexpressing ghrelin and nesfatin-1. Nesfatin-1 (red) and ghrelin (green) were located in cytoplasmic vesicles. The image clearly shows nesfatin-1 and ghrelin immunoreactivity in separate vesicles within the same cell. Confocal microscopy was performed using a ×100 objective and digital zoom, eight images in z-axis (stack size 24 × 24 × 7 μm) with dual laser excitation at 488 and 543 nm. Images were collected in two optical channels and photomultiplier tubes using FITC and tetramethylrhodamine isothiocyanate (TRITC) filter sets, deconvoluted, and reconstructed into a three-dimensional image. Scale bar, 1 μm.
A small percentage of basally located endocrine cells showed coexpression of nesfatin-1 immunoreactivity with somatostatin or HDC (Table 1 and Fig. 5, C–F). Immunostaining without primary antibody or antibody preabsorption with purified nesfatin-1 peptide did not show any positive cells (data not shown).
Regulation of NUCB2 expression by fasting
Food deprivation for 24 h resulted in a significant down-regulation of NUCB2 and VMAT2 mRNA in a suspension of purified small endocrine cells of the rat gastric oxyntic mucosa as shown by comparative microarray whole-rat genome expression analysis (fold reduction, mean ± sd, fasted vs. fed ad libitum: NUCB2 1.67 ± 0.14, P < 0.02, and VMAT2 2.43 ± 0.73, P < 0.01; Fig. 7), whereas GAPDH did not change (1.02 ± 0.55, P > 0.05; Fig. 7). This was confirmed by RT-qPCR in a small endocrine cell-enriched fraction of the rat gastric corpus mucosa (fasted vs. fed ad libitum: NUCB2 3.78 ± 0.92, P < 0.01, and VMAT2 4.51 ± 0.91, P < 0.02; Fig. 7) with no change in GAPDH as housekeeping gene (0.90 ± 0.43, P > 0.05; Fig. 7).
Figure 7.
Regulation of NUCB2 expression during fasting and ad libitum feeding conditions in rats. Fasting for 24 h resulted in a significant down-regulation of NUCB2 and VMAT2 in a small endocrine cell-enriched fraction of the rat gastric corpus mucosa, whereas GAPDH did not change. Data are expressed as fold reduction of the mean of mRNA expression ratios ± sd. *, P < 0.01, VMAT2 fasted vs. fed ad libitum, P < 0.01 NUCB2 fasted vs. fed ad libitum using RT-qPCR, P = 0.002 VMAT2 fasted vs. ad libitum fed, and P = 0.014 NUCB2 fasted vs. ad libitum fed using comparative microarray whole-rat genome expression analysis.
Discussion
The present study shows that NUCB2 expression is limited to specific subsets of gastric oxyntic mucosal endocrine cells as shown by colocalization of immunoreactivity of nesfatin-1 with ghrelin, HDC, or somatostatin. The data clearly indicate that NUCB2 is highly expressed in two pools of endocrine cells in the oxyntic gastric glands. The majority of immunoreactive cells in the midportion of the glands coexpress ghrelin and represent most likely X/A cells. A minority of cells at the base of the glands coexpress either somatostatin or HDC.
Nesfatin-1 consists of 82 amino acids and has a predicted molecular mass of 9.7 kDa. However, this mature peptide has been detected only in cerebrospinal fluid and is not present in protein extracts from the hypothalamus (1). Likewise, the Western blots of all tissues and cells studied here failed to show a band at 9.7 kDa despite the fact that 50 ng synthetic nesfatin-1 peptide could be detected. One might speculate that the gastric and pancreatic protein is further processed upon secretion into the circulation generating the mature 9.7-kDa molecule. However, further studies including HPLC of stomach, pancreas, and serum samples are needed to address this question. It has been shown that full-length NUCB2 protein injected intracerebroventricularly does also exert anorexigenic effects (1), and therefore one might speculate that peripheral NUCB2 is the biologically active compound.
Immunohistochemical analysis of gastric and pancreatic islet endocrine cells showed strong vesicular cytoplasmic staining in contrast to almost exclusive nuclear staining of pituitary endocrine cells. In line with these findings, the soluble protein fraction of gastric mucosal endocrine cells and pancreatic tissue showed a reduction of size of the immunoreactive band of approximately 3 kDa. In contrast, the soluble protein fraction of the pituitary gland showed no further processing of the 50-kDa full-length immunoreactive band of the membrane fraction. This indicates that gastric mucosal and pancreatic islet endocrine cells release full-length protein into a secretory vesicular compartment of the cell by cleavage of a 3-kDa signal sequence.
Although the majority of immunoreactive soluble protein of gastric endocrine cells does not show any further processing, most of the pancreatic immunoreactive soluble protein appeared to be further processed to yield a 25-kDa band, most likely encompassing nesfatin-1 and -2. The biological significance of this peptide remains to be elucidated.
In the membrane fractions of all tissues studied as well as in the soluble protein fraction of gastric mucosal homogenates and endocrine cells, we detected a nonspecific breakdown product of NUCB2 at 30 kDa. This product correlates with a cleavage of the protein at a conserved caspase site (DGLD) (26).
We show that NUCB2 mRNA is about 10 times more expressed in the gastric mucosa compared with brain and heart tissues. After 24 h fasting, NUCB2 mRNA was markedly down-regulated as observed by comparative gene expression microarray analysis of purified oxyntic mucosal small endocrine cells and confirmed by RT-qPCR on gastric mucosal tissue. This pattern was qualitatively and quantitatively similar to a decrease in mRNA expression for VMAT2, the protein responsible for histamine accumulation into the secretory vesicle of enterochromaffin-like cells (27,28), as described previously (24). The initial report by Oh and colleagues (1) described a significant down-regulation of NUCB2 mRNA expression in the PVN after 24 h fasting in rats. Our findings expand this report to the gastric mucosa, thereby possibly adding another food regulatory peptide to the brain-gut axis.
It has been shown that fasting increases mRNA expression of the food intake-stimulating hormone ghrelin in the gastric fundus (29,30) as well as of its receptor, the GH secretagogue receptor, in the hypothalamus (30). Furthermore, peptide content of the X/A-like cells was decreased and plasma ghrelin levels elevated after fasting (29), indicating increased production and secretion of ghrelin. These findings corroborate the role of ghrelin as physiological orexigenic modulator of food intake. In contrast, in the present study, we showed a significant down-regulation of NUCB2 mRNA supporting the assumption of NUCB2/nesfatin-1 playing a role in energy homoeostasis not only in the brain but also in the stomach. We also demonstrated that ghrelin and nesfatin-1 immunoreactivity resides in different vesicles in the X/A-like endocrine cells of the oxyntic mucosa. This indicates that ghrelin and NUCB2/nesfatin-1 could be released separately under different metabolic conditions transmitted by separate secondary intracellular messaging systems suggesting a negative feedback loop in the peripheral regulation of food intake within the same cell.
In summary, nesfatin-1 immunoreactivity was selectively found in stomach, pancreas, pituitary gland, and testis, whereas all other tissues did not show nesfatin-1 immunostaining. The gastric mucosa expresses high amounts of NUCB2 compared with other viscera and the brain. We found that the majority of gastric oxyntic ghrelin-producing X/A-like cells coexpress NUCB2/nesfatin-1. The expression of NUCB2 mRNA in gastric endocrine cells is significantly down-regulated by 24 h fasting in the rat, suggesting a regulatory anorexigenic role of peripheral NUCB2/nesfatin-1 in energy homeostasis.
Footnotes
Disclosure Statement: A.S., M.G., I.Y., L.W., D.W., Y.T., G.S., and N.W.G.L. have nothing to disclose. T.C. is recently employed at Eli Lilly and Co.
This work was supported by German Research Foundation Grants STE 1765/1-1 (A.S.) and GO 1718/1-1 (M.G.), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 33061 (Y.T.), Veterans Affairs Research Career Scientist Award, Department of Veterans Affairs Merit Award (Y.T.), Center Grant DK-41301 (Animal Core, Y.T.), NIDDK 058333 (G.S.), and NIDDK 053642 (G.S.).
First Published Online September 25, 2008
Abbreviations: C(T), Cycle of threshold; FITC, fluorescein isothiocyanate; HDC, histidine decarboxylase; NUCB2, nucleobindin2; PVN, paraventricular nucleus of the hypothalamus; RT-qPCR, quantitative RT-PCR; TBS, Tris-buffered saline; VMAT2, vesicular monoamine transporter 2.
References
- Oh IS, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M 2006 Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443:709–712 [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ 2007 Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148:5088–5094 [DOI] [PubMed] [Google Scholar]
- Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T 2008 Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149:1295–1301 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB 2005 Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493:63–71 [DOI] [PubMed] [Google Scholar]
- Price CJ, Hoyda TD, Samson WK, Ferguson AV 2008 Nesfatin-1 influences the excitability of paraventricular nucleus neurones. J Neuroendocrinol 20:245–250 [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Kastin AJ 2007 Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides 28:2223–2228 [DOI] [PubMed] [Google Scholar]
- Price TO, Samson WK, Niehoff ML, Banks WA 2007 Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides 28:2372–2381 [DOI] [PubMed] [Google Scholar]
- Wren AM, Bloom SR 2007 Gut hormones and appetite control. Gastroenterology 132:2116–2130 [DOI] [PubMed] [Google Scholar]
- Capella C, Solcia E, Vassallo G 1969 Identification of six types of endocrine cells in the gastrointestinal mucosa of the rabbit. Arch Histol Jpn 30:479–495 [DOI] [PubMed] [Google Scholar]
- Forssmann WG, Orci L, Pictet R, Renold AE, Rouiller C 1969 The endocrine cells in the epithelium of the gastrointestinal mucosa of the rat. An electron microscope study. J Cell Biol 40:692–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adda T, Bertele A, Pilato FP, Bordi C 1989 Quantitative electron microscopy of endocrine cells in oxyntic mucosa of normal human stomach. Cell Tissue Res 255:41–48 [DOI] [PubMed] [Google Scholar]
- Sachs G, Zeng N, Prinz C 1997 Physiology of isolated gastric endocrine cells. Annu Rev Physiol 59:243–256 [DOI] [PubMed] [Google Scholar]
- Solcia E, Rindi G, Buffa R, Fiocca R, Capella C 2000 Gastric endocrine cells: types, function and growth. Regul Pept 93:31–35 [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K 2005 Ghrelin: structure and function. Physiol Rev 85:495–522 [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao CM, Lindstrom E, Hakanson R 1999 Rat stomach ECL cells up-date of biology and physiology. Gen Pharmacol 32:413–422 [DOI] [PubMed] [Google Scholar]
- Prinz C, Kajimura M, Scott DR, Mercier F, Helander HF, Sachs G 1993 Histamine secretion from rat enterochromaffinlike cells. Gastroenterology 105:449–461 [DOI] [PubMed] [Google Scholar]
- Alumets J, Ekelund M, El Munshid HA, Hakanson R, Loren I, Sundler F 1979 Topography of somatostatin cells in the stomach of the rat: possible functional significance. Cell Tissue Res 202:177–188 [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M 2000 Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261 [DOI] [PubMed] [Google Scholar]
- Solcia E, Capella C, Vassallo G, Buffa R 1975 Endocrine cells of the gastric mucosa. Int Rev Cytol 42:223–286 [DOI] [PubMed] [Google Scholar]
- Grube D, Forssmann WG 1979 Morphology and function of the entero-endocrine cells. Horm Metab Res 11:589–606 [DOI] [PubMed] [Google Scholar]
- Yu PL, Fujimura M, Hayashi N, Nakamura T, Fujimiya M 2001 Mechanisms in regulating the release of serotonin from the perfused rat stomach. Am J Physiol Gastrointest Liver Physiol 280:G1099–G1105 [DOI] [PubMed] [Google Scholar]
- Lambrecht NW, Yakubov I, Scott D, Sachs G 2005 Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiol Genomics 21:81–91 [DOI] [PubMed] [Google Scholar]
- Lambrecht NW, Yakubov I, Zer C, Sachs G 2006 Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion. Physiol Genomics 25:153–165 [DOI] [PubMed] [Google Scholar]
- Lambrecht NW, Yakubov I, Sachs G 2007 Fasting-induced changes in ECL cell gene expression. Physiol Genomics 31:183–192 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia CA, Cotten SW, Duan J, Liu R 2008 Modulation of nucleobindin-1 and nucleobindin-2 by caspases. FEBS Lett 582:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter D, Jimenez J, Liu Y, Kim J, Edwards RH 1994 The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J Biol Chem 269:7231–7237 [PubMed] [Google Scholar]
- De Giorgio R, Su D, Peter D, Edwards RH, Brecha NC, Sternini C 1996 Vesicular monoamine transporter 2 expression in enteric neurons and enterochromaffin-like cells of the rat. Neurosci Lett 217:77–80 [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S 2001 Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun 281:1220–1225 [DOI] [PubMed] [Google Scholar]
- Kim MS, Yoon CY, Park KH, Shin CS, Park KS, Kim SY, Cho BY, Lee HK 2003 Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport 14:1317–1320 [DOI] [PubMed] [Google Scholar]