Abstract
Activation of β2-adrenergic receptors inhibits osteoblastic bone formation and enhances osteoclastic bone resorption. Whether β-blockers inhibit ovariectomy-induced bone loss and decrease fracture risk remains controversial. To further explore the role of β-adrenergic signaling in skeletal acquisition and response to estrogen deficiency, we evaluated mice lacking the three known β-adrenergic receptors (β-less). Body weight, percent fat, and bone mineral density were significantly higher in male β-less than wild-type (WT) mice, more so with increasing age. Consistent with their greater fat mass, serum leptin was significantly higher in β-less than WT mice. Mid-femoral cross-sectional area and cortical thickness were significantly higher in adult β-less than WT mice, as were femoral biomechanical properties (+28 to +49%, P < 0.01). Young male β-less had higher vertebral (1.3-fold) and distal femoral (3.5-fold) trabecular bone volume than WT (P < 0.001 for both) and lower osteoclast surface. With aging, these differences lessened, with histological evidence of increased osteoclast surface and decreased bone formation rate at the distal femur in β-less vs. WT mice. Serum tartrate-resistance alkaline phosphatase-5B was elevated in β-less compared with WT mice from 8–16 wk of age (P < 0.01). Ovariectomy inhibited bone mass gain and decreased trabecular bone volume/total volume similarly in β-less and WT mice. Altogether, these data indicate that absence of β-adrenergic signaling results in obesity and increased cortical bone mass in males but does not prevent deleterious effects of estrogen deficiency on trabecular bone microarchitecture. Our findings also suggest direct positive effects of weight and/or leptin on bone turnover and cortical bone structure, independent of adrenergic signaling.
Mice lacking ß-adrenergic receptors have increased body weight, bone mineral density, and bone turnover versus controls, but are not protected from bone loss due to deficiency of estrogens..
Evidence indicates that β-adrenergic signaling plays a key role in the regulation of bone remodeling. Sympathetic nerve fibers are present in bone and bone marrow (1,2,3,4), and β-adrenergic receptors (β-AR) have been identified in osteoblastic cells (5,6,7,8,9). In vivo studies indicate that bone metabolism may be influenced both through indirect activation of β-adrenergic signaling via hypothalamic-derived neural pathways and through direct modulation of β-adrenergic activity by pharmacological intervention (8,9,10,11,12).
Mice devoid of leptin (ob/ob) or the signaling form of its receptor (db/db) have, despite reduced gonadal function, markedly increased body weight, fat, and vertebral trabecular bone volume (BV) but exhibit decreased cortical bone mass and thickness (13,14). The inhibitory effects of leptin on trabecular bone have been attributed to hypothalamic activation of sympathetic nervous system (SNS) activity that is ultimately mediated via β2-AR on osteoblasts, reducing osteoblast proliferation and bone formation, and stimulating osteoclastic activity by increasing RANKL expression (8,9,13). The initial findings in rodents have been confirmed in adult sheep, where intracerebroventricular infusion of leptin markedly reduces bone formation (15).
Direct modulation of adrenergic activity by administration of β-blockers or β-agonists also influences bone metabolism in vivo. For example, young mice treated with the nonspecific β-blocker propranolol have enhanced vertebral trabecular BV (8). Furthermore, bone loss induced by estrogen deficiency is reportedly attenuated in rodents treated with propranolol (8,10,16). Yet, reports differ as to whether treatment with propranolol inhibits the bone loss associated with skeletal unloading (17,18). In contrast, pharmacological activation of β-adrenergic signaling inhibits skeletal acquisition (8,12) and reduces bone mass and bone strength in adult animals (11). Altogether, the skeletal effects of pharmacological interventions that alter adrenergic signaling are challenging to interpret, because there is growing evidence that the bone-sparing effects of blocking β-adrenergic signaling are dose dependent with decreased benefits at higher doses (16).
Elefteriou and colleagues (9) reported that mice deficient in β2-AR [β2-AR knockout (KO)] have normal body weight but increased trabecular BV at 6 months of age. They also showed that during growth β2-AR KO mice are resistant to ovariectomy (OVX)-induced increases in bone resorption and decreases in trabecular BV, thus concluding that integrity of sympathetic signaling is necessary for the increase in bone resorption caused by gonadal failure (9). A potential confounding aspect to their study is the possibility of compensatory activity by other isoforms of the β-AR, which although not clearly established in bone, has been established in other tissues (19,20). Thus, we and others have suggested that β2-AR might not be the sole β-AR involved in the control of bone mass and remodeling (21,22).
Data supporting an effect of β-blocker use on skeletal integrity in humans are inconclusive, with studies reporting positive, negative, and no associations between β-blocker use and fracture risk or bone mineral density (BMD) (23,24,25,26,27,28,29). A randomized placebo-controlled trial in postmenopausal women showed that propranolol treatment led to significant decreases in serum osteocalcin, urinary free deoxypyridinoline, and serum albumin, with no effect on serum procollagen type I N-terminal peptide or total alkaline phosphatase activity, suggesting that in normal postmenopausal women β-adrenergic blockade decreases bone turnover (30).
To further examine the role of β-adrenergic signaling in the skeleton, we evaluated bone acquisition and the response to OVX in mice that lack the three known β-ARs (β-less). We hypothesized that relative to wild-type (WT) mice, β-less mice would have increased trabecular BV and would be resistant to the deleterious effects of estrogen deficiency. More precisely, if β2-AR is the main and/or only β-AR subtype involved in bone mass regulation, then β-less mice should present skeletal phenotypes similar to β2-AR-null mice.
Materials and Methods
Overall approach
We evaluated the skeletal phenotype and response to estrogen deficiency in mice lacking the three known β-AR (β-less). β-less and WT mice were derived on a mixed genetic background, are viable and fertile, develop mild obesity with increasing age on normal rodent chow, and develop marked obesity on a high-fat diet due to absence of diet-induced thermogenesis (31). In particular, as described previously by Bachman et al. (31), β-less mice were created by crossing the β1,2-AR KO with a genetic background of FVB, C57BL/6J, DBA/2, and 129/SvJ (32) to β3-AR KO with a genetic background of FVB (19). The first breeding produced mice that were heterozygous for disruption of all three β-AR. These were subsequently bred to select two lines each of mice that were either homozygous null for β1-, β2-, and β3-AR genes (β-less) or homozygous WT for all three β-ARs. We used one of these lines, and mice were subsequently bred as homozygous β-less or homozygous WT. Mice used in these experiments were approximately six to eight generations after the initial derivation of the WT and β-less lines. Thus, WT controls were derived from the same mixed background but were not littermate controls due to challenges of breeding a triple knockout from heterozygous parents. To evaluate the skeletal phenotype of these mice, we assessed body composition, BMD, serum leptin, and markers of bone turnover in vivo and trabecular and cortical bone morphology and femoral biomechanics ex vivo in male mice at 6 and 16 wk of age. To test whether β-less mice are resistant to deleterious effects of estrogen deficiency, we performed ovariectomy (OVX) or sham-OVX in 8-wk-old β-less and WT mice. In all experiments, mice were maintained under standard nonbarrier conditions and had access to mouse chow (Harlan Teklad 5542, 2.5% Ca, 1.2% Pi) and water ad libitum. Calcein (15 mg/kg) was injected sc 7 and 2 d before death. The protocol and procedures were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center (Boston, MA).
BMD and bone morphology
We evaluated body composition and total body, spine, and femoral BMD (grams per square centimeter) in vivo using peripheral dual-energy x-ray absorptiometry (pDXA, PIXImus; GE-Lunar, Madison, WI) (33,34,35,36,37). We assessed trabecular and cortical bone architecture using microcomputed tomography (μCT40; Scanco Medical AG, Basserdorf, Switzerland), employing a 12-μm isotropic voxel size. Specifically, trabecular bone architecture was evaluated at the fifth lumbar vertebra and distal femoral metaphysis, whereas cortical bone morphology was evaluated at the femoral midshaft, as previously described (36,37).
For all μCT evaluations, we used a nominal isotropic voxel size of 12 μm. Images were subjected to Gaussian filtration and segmented using an adaptive-iterative algorithm applied to each specimen (38). Morphometric parameters, including BV fraction [BV/total volume (TV), percent], trabecular number (TbN, per millimeter), trabecular thickness (TbTh, micrometers), trabecular separation (TbSp, micrometers), structure model index (SMI), and connectivity density (per cubic millimeter) were computed without assumptions regarding the underlying bone architecture (39,40). At the femoral midshaft, 50 transverse CT slices were obtained and used to compute the total cross-sectional area (TA, square millimeters), cortical bone area (BA, square millimeters), medullary area (MA, square millimeters), cortical thickness (CortTh, μm), and bone area fraction (BA/TA, percent). We also used the CT images to measure the anteroposterior and mediolateral diameters, the area moments of inertia about the anteroposterior and mediolateral axes (Iap and Iml, millimeter4), and the polar moment of inertia (J, millimeter4).
Femoral biomechanics
Femoral biomechanical properties were assessed by three-point bending in 16-wk-old male β-less and WT mice, as previously described (41,42). Load was applied at a constant rate (0.05 mm/sec) until failure. We measured failure load (newtons), bending stiffness (newtons per millimeter), and work-to-failure (newton-millimeters) from the load-displacement curve and computed the apparent elastic modulus (megapascal) and ultimate strength (gigapascal) using the relevant midfemoral cross-sectional geometry measured from μCT (43).
Bone histomorphometry
Femurs were dehydrated in graded ethanol and embedded in methylmethacrylate. Five and 8-μm-thick sagittal sections were cut with a Leica Corp. Polycut E microtome (Leica Corp. Microsystems AG, Glattbrugg, Switzerland) and stained with modified Goldner’s trichrome (5-μm sections) for assessment of static histomorphometric variables or left unstained (8-μm sections) for assessment of calcein fluorescence and dynamic indices of bone formation. Histomorphometric measurements were performed on the secondary spongiosa of the distal femoral metaphysis (beginning 690 μm proximal to the growth plate and extending 344 μm proximally) using a Leica Corp. Q image analyzer at ×40 magnification. All parameters were calculated and expressed according to standard formulas and nomenclatures (44).
Serum measurements
Serum leptin was measured as previously described (31). Serum IGF-I was assessed by RIA (Alpco, Windham, NH), as previously described (45). Serum osteocalcin was measured by RIA with a goat antimouse osteocalcin antibody and donkey antigoat secondary antibody (Biomedical Technologies Inc., Stoughton, MA). Serum tartrate-resistance alkaline phosphatase-5b (TRACP5b) was measured according to the manufacturer’s instructions (SBA Sciences, Turku, Finland).
Data analysis
Standard descriptive statistics were computed and data checked for normality. We used ANOVA and unpaired t tests to test for significant differences between β-less and WT mice. All tests were two-tailed, and differences were considered statistically significant at P < 0.05. Data are presented as mean ± sem, unless otherwise noted.
Results
Body composition and skeletal phenotype of male β-less mice
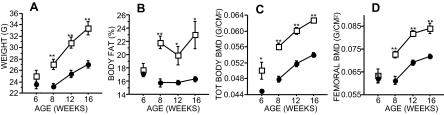
Whereas femoral length did not differ, male β-less mice had a higher body weight, lean mass, fat mass, body fat percentage (and fat mass), total body BMD, and femoral BMD than WT mice from 8–16 wk of age (Fig. 1). Consistent with their higher fat mass, male β-less mice had 2-fold higher serum leptin concentrations (β-less 4.95 ± 1.55 ng/ml vs. WT 1.18 ± 0.16 ng/ml, P < 0.01). At 6 wk of age, at both the vertebral body and distal femur, β-less had significantly higher trabecular BV (BV/TV, +33% at vertebral body and +77% at distal femur), thickness, and number along with decreased TbSp compared with WT (Table 1 and Figs. 2 and 3). However, by 16 wk of age, there were no significant differences in trabecular BV/TV at either site, although TbTh remained significantly higher in β-less compared with WT (Table 1). Trabecular BV/TV at distal femur declined 50% between 6 and 16 wk of age in β-less compared with less than 20% in WT.
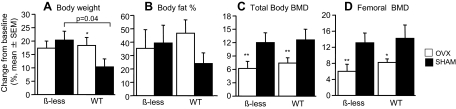
Figure 1.
Longitudinal in vivo assessment of body composition and BMD in male WT (○) and β-less (□) mice (n = 8–9 per group). A, Body weight (grams); B, body fat (percent); C, total body BMD (grams per square centimeter); D, femoral BMD (grams per square centimeter). *, P < 0.05; **, P < 0.001 β-less vs. WT. Error bars represent sem.
Table 1.
Skeletal characteristics of male β -less mice at 6 and 16 wk of age
| 6 wk
|
16 wk
|
|||
|---|---|---|---|---|
| WT (n = 8) | β-less (n = 7) | WT (n = 9) | β-less (n = 8) | |
| Vertebral trabecular | ||||
| BV/TV (%) | 18.5 ± 0.6 | 24.4 ± 1.0c | 23.0 ± 0.7 | 25.1 ± 0.9 |
| TbTh (μm) | 47.8 ± 0.3 | 53.9 ± 1.9b | 54.2 ± 0.8 | 64.1 ± 1.4c |
| TbN (mm−1) | 4.72 ± 0.13 | 5.21 ± 0.21 | 4.44 ± 0.13 | 4.18 ± 0.13 |
| TbSp (μm) | 217 ± 7 | 197 ± 9 | 232 ± 8 | 247 ± 8 |
| Distal femur | ||||
| BV/TV (%) | 16.2 ± 1.2 | 28.7 ± 1.2c | 13.4 ± 1.2 | 14.4 ± 1.3 |
| TbTh (μm) | 48.4 ± 0.6 | 57.5 ± 1.6c | 51.2 ± 0.9 | 57.4 ± 1.3b |
| TbN (mm−1) | 5.76 ± 0.21 | 7.22 ± 0.11c | 4.93 ± 0.17 | 4.55 ± 0.19 |
| TbSp (μm) | 174 ± 8 | 132 ± 3c | 204 ± 8 | 223 ± 10 |
| Femoral midshaft | ||||
| TA (mm2) | 1.54 ± 0.06 | 1.77 ± 0.10 | 1.56 ± 0.04 | 1.86 ± 0.07c |
| BA (mm2) | 0.70 ± 0.03 | 0.79 ± 0.05 | 0.75 ± 0.03 | 0.94 ± 0.04b |
| MA (mm2) | 0.85 ± 0.03 | 0.98 ± 0.05a | 0.81 ± 0.02 | 0.93 ± 0.03b |
| BA/TA (%) | 45.0 ± 0.5 | 44.6 ± 0.8 | 48.0 ± 1.2 | 50.2 ± 1.1 |
| Cort Th (μm) | 180 ± 4 | 188 ± 6a | 194 ± 7 | 223 ± 7a |
| Imax (mm4) | 0.17 ± 0.01 | 0.22 ± 0.01a | 0.18 ± 0.01 | 0.29 ± 0.02c |
| Imin (mm4) | 0.09 ± 0.01 | 0.13 ± 0.01a | 0.10 ± 0.01 | 0.15 ± 0.01 |
| J (mm4) | 0.26 ± 0.02 | 0.35 ± 0.03a | 0.29 ± 0.02 | 0.44 ± 0.03c |
| Femoral biomechanics | ||||
| Peak load (N) | NA | NA | 17.8 ± 0.6 | 26.5 ± 1.0c |
| Stiffness (N/mm) | NA | NA | 105 ± 7 | 135 ± 7b |
| Work-to-failure (N-mm) | NA | NA | 6.6 ± 0.5 | 10.5 ± 2.7b |
Results are mean ± sem. Cort Th, Cortical thickness; Imax, maximum moment of inertia; Imin, minimum moment of inertia; J, polar moment of inertia; N, newtons; NA, not available.
P < 0.05 vs. WT.
P < 0.01 vs. WT.
P < 0.001 vs. WT.
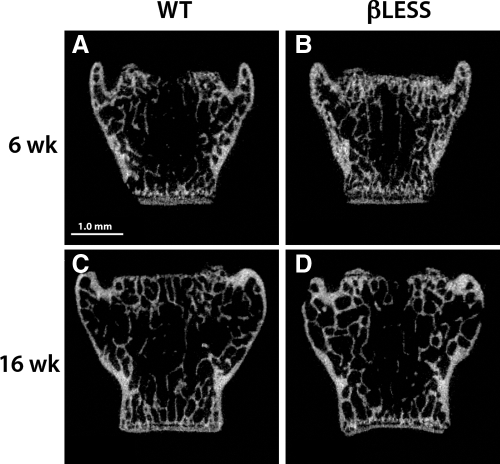
Figure 2.
Two-dimensional μCT-derived images of the fifth lumbar vertebrae in male 6-wk (A) and 16-wk (B) WT and 6-wk (C) and 16-wk (D) β-less mice. Scale bar, 1 mm.
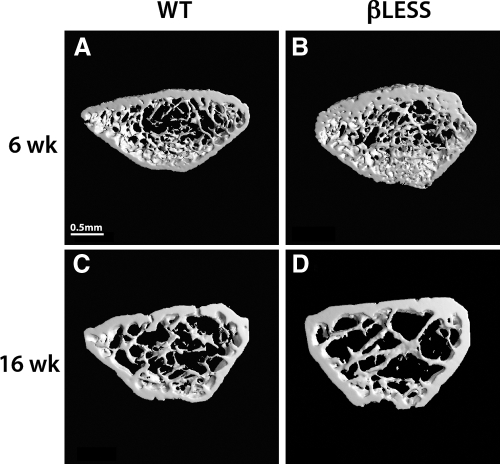
Figure 3.
Three-dimensional μCT-derived images of the distal femoral metaphysis in male 6-wk (A) and 16-wk (B) WT and 6-wk (C) and 16-wk (D) β-less mice.
At the femoral midshaft, total cross-sectional area (0.05 < P < 0.10) and area moments of inertia (P < 0.05) were greater in 6- and 16-wk-old β-less compared with WT mice (Table 1 and Fig. 4). Cortical TbSp and cortical thickness were increased in 16-wk-old β-less mice, although the ratio of cortical bone area to TA (i.e. BA/TA) did not differ at either age (Table 1). Biomechanical tests showed that femora from 16-wk-old β-less mice had increased peak load, stiffness, and work-to-failure (P < 0.01 for all). However, the estimated elastic modulus and ultimate strength did not differ (data not shown), indicating that the increased structural properties in β-less mice were due to their larger femoral size.
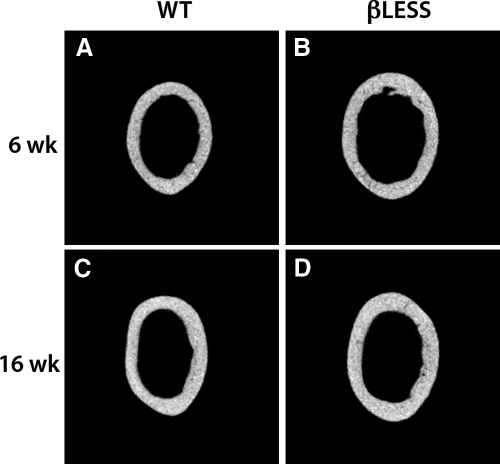
Figure 4.
Two-dimensional μCT images of the midfemoral cross-section in male 6-wk (A) and 16-wk (B) WT and 6-wk (C) and 16-wk (D) β-less mice.
Bone histomorphometry and serum measurements in β-less and WT mice
Histomorphometric analysis of the distal femoral metaphysis showed that at 6 wk of age, β-less mice had decreased osteoclast surface and number compared with WT, with no difference in bone formation indices (Table 2). In contrast, by 16 wk of age, β-less mice had slightly, although not significantly, higher osteoclast number and significantly reduced bone formation rate compared with WT (Table 2). Consistent with the age-related increase in osteoclast surface in β-less mice (as opposed to the decrease in WT), serum markers of bone turnover were higher in β-less compared with WT mice from 8–16 wk of age. Specifically, at 8 wk of age, TRACP5b (β-less 4.25 ± 0.27 U/liter vs. WT 2.61 ± 0.50 U/liter, P = 0.01) and osteocalcin (β-less 267 ± 28 ng/ml vs. WT 150 ± 27 ng/ml, P = 0.02) were 62 and 78% higher, respectively, in β-less than WT mice. At 16 wk of age, serum TRACP5b remained significantly higher in β-less mice, whereas osteocalcin was no longer increased compared with WT (data not shown).
Table 2.
Quantitative histomorphometry of the secondary spongiosa of the distal femur in male WT and β -less mice
| 6 wk
|
16 wk
|
|||
|---|---|---|---|---|
| WT | β -less | WT | β -less | |
| OcS/BS (%) | 9.9 ± 1.2 | 5.6 ± 0.4a | 3.5 ± 1.2 | 6.3 ± 0.5 |
| NOc/BA (mm−1) | 1076 ± 151 | 585 ± 95a | 500 ± 166 | 808 ± 66 |
| MS/BS (%) | 26.3 ± 2.8 | 32.4 ± 5.9 | 37.4 ± 5.3 | 21.6 ± 4.1 |
| MAR (μm/d) | 1.29 ± 0.29 | 1.08 ± 0.10 | 1.09 ± 0.06 | 0.73 ± 0.12 |
| BFR (μm3/μm2 · d) | 0.36 ± 0.11 | 0.35 ± 0.08 | 0.41 ± 0.06 | 0.15 ± 0.03a |
Results are mean ± sem; n = 4 per group. BFR, Bone formation rate, surface referent; BS, bone surface; MAR, mineral apposition rate; MS, mineralizing surface; NOc/BA, osteoclast number per bone area; OcS, osteoclast surface.
P < 0.05 vs. WT.
Skeletal response to estrogen deficiency in growing β-less and WT mice
To test whether lack of adrenergic signaling affects the skeletal response to estrogen deficiency, we performed OVX or sham surgery in 8-wk-old WT and β-less mice (n = 9–12 per group). In contrast to male β-less mice, at baseline, female β-less mice weighed slightly less than WT (β-less 19.1 ± 0.4 g vs. WT 21.5 ± 0.4 g, P < 0.001) and had similar total body, spine, and femur BMD as WT. Like the males, however, female β-less had significantly higher body fat percentage (β-less 22.2 ± 0.6% vs. WT 16.7 ± 0.4%, P < 0.001) and total body fat mass than WT.
Over the 8-wk study, body weight, fat mass, total body BMD, and femoral shaft BMD increased significantly compared with baseline in all groups (0. 0001 < P < 0.04, Fig. 5). In sham-operated mice, weight gain was greater in β-less than WT mice (P = 0.04). As expected, in WT mice, body weight and fat mass, expressed either as an absolute or percentage change, increased more in OVX than sham-operated mice (Fig. 5). For example, body weight increased 3.9 ± 0.6 g (vs. baseline) in WT-OVX vs. 2.1 ± 0.6 g in WT-sham (P = 0.048 vs. WT-OVX), and fat mass increased 1.7 ± 0.4 g in WT-OVX compared with 0.7 ± 0.2 g in WT-sham (P = 0.04 vs. WT-OVX). In contrast, OVX did not lead to further increases in body weight and fat mass in β-less mice. In both WT and β-less mice, OVX significantly reduced the gain in total body BMD and femoral BMD, expressed either in absolute units or percent increase (Fig. 5). Specifically, total body BMD increased 7.3 and 6.2% in WT-OVX and β-less OVX, respectively, compared with 12.7 and 11.0% in WT-SHAM and β-less sham (P < 0.005, OVX vs. sham). Gains in femoral BMD were similarly inhibited by estrogen deficiency, because femoral shaft BMD increased 8.1 and 5.9% in WT-OVX and β-less OVX mice, respectively, compared with 14.3 and 13.1% in WT-sham and β-less sham groups (P < 0.005, OVX vs. sham-OVX).
Figure 5.
Percent increase (mean ± se) from baseline in body weight (A), percent body fat (B), total body BMD (C), and femoral BMD (D) in β-less OVX (white bars, n = 10), β-less sham-OVX (black bars, n = 9), WT OVX (white bars, n = 11), and WT sham-OVX (black bars, n = 12) mice. *, P = 0.07; **, P < 0.05.
In sham groups, trabecular BV/TV at the spine and distal femur did not differ between β-less and WT females (Table 3), similar to males of the same age (i.e. 16 wk, Table 1). The β-less females tended to have smaller midfemoral cross-sectional area than WT, consistent with their lower body weight.
Table 3.
Effect of OVX on trabecular and cortical bone morphology in growing β -less and WT mice assessed by μCT
| WT
|
β -less
|
P value
|
||||
|---|---|---|---|---|---|---|
| Sham (n = 12) | OVX (n = 11) | Sham (n = 9) | OVX (n = 10) | OVX | Genotype | |
| Vertebral body | ||||||
| BV/TV (%) | 23.3 ± 1.4 | 18.8 ± 1.3b | 25.2 ± 1.4 | 20.6 ± 1.8c | 0.0003 | NS |
| TbTh (μm) | 52.7 ± 1.0 | 50.2 ± 0.8b | 53.3 ± 0.7 | 51.7 ± 1.1 | 0.04 | NS |
| TbN (mm−1) | 4.30 ± 0.19 | 3.67 ± 0.18b | 4.16 ± 0.13 | 3.65 ± 0.20c | 0.001 | NS |
| TbSp (μm) | 248 ± 13 | 291 ± 13b | 255 ± 9 | 294 ± 15b | 0.001 | NS |
| Connectivity density (mm−3) | 206 ± 12 | 154 ± 9c | 218 ± 16 | 165 ± 14b | 0.0003 | NS |
| SMI | 0.63 ± 0.15 | 1.00 ± 0.05b | 0.28 ± 0.18 | 0.66 ± 0.11a | 0.007 | 0.01 |
| Distal femur | ||||||
| BV/TV (%) | 11.2 ± 1.3 | 9.7 ± 0.5 | 9.2 ± 0.9 | 6.9 ± 0.7a | 0.05 | 0.018 |
| TbTh (μm) | 49.3 ± 0.8 | 48.2 ± 0.6 | 51.1 ± 0.8 | 50.9 ± 1.2 | NS | 0.01 |
| TbN (mm−1) | 3.47 ± 0.17 | 3.35 ± 0.09 | 3.19 ± 0.15 | 2.80 ± 0.14a | 0.08 | 0.006 |
| TbSp (μm) | 295 ± 18 | 301 ± 9 | 320 ± 17 | 366 ± 20a | NS | 0.009 |
| Connectivity density (mm−3) | 87.4 ± 11.7 | 80.6 ± 8.0 | 68.2 ± 10.0 | 43.5 ± 6.9a | NS | 0.007 |
| SMI | 2.43 ± 0.19 | 2.56 ± 0.07 | 2.77 ± 0.12 | 3.05 ± 0.12 | NS | 0.004 |
| Femoral midshaft | ||||||
| TA (mm2) | 1.50 ± 0.04 | 1.55 ± 0.03 | 1.27 ± 0.05 | 1.34 ± 0.04 | NS | <0.001 |
| BA (mm2) | 0.73 ± 0.02 | 0.70 ± 0.02 | 0.68 ± 0.02 | 0.70 ± 0.02 | NS | 0.04 |
| MA (mm2) | 0.77 ± 0.02 | 0.83 ± 0.02 | 0.59 ± 0.03 | 0.64 ± 0.03 | 0.06 | <0.001 |
| BA/TA (%) | 48.5 ± 0.7 | 47.0 ± 0.6 | 54.0 ± 1.0 | 52.3 ± 1.3 | 0.09 | <0.001 |
| Cort Th (μm) | 191 ± 4 | 189 ± 3 | 202 ± 3 | 200 ± 4 | NS | 0.003 |
Results are mean ± sem. Measurements were performed 8 wk after OVX or sham-OVX surgery (i.e. at 16 wk of age). Cort Th, Cortical thickness; NS, not significant.
a–c OVX vs. sham within genotype:
0.05 < P < 0.10;
P < 0.05;
P < 0.01.
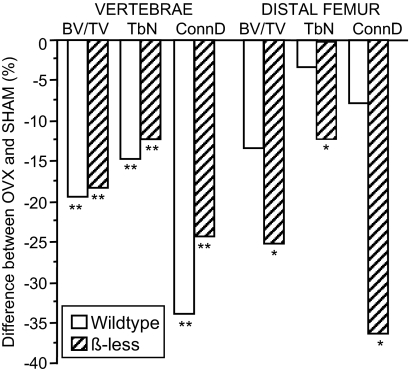
In response to OVX, trabecular architecture was significantly deteriorated in both WT and β-less compared with sham controls (Table 3 and Fig. 6). Specifically, compared with sham-operated mice, vertebral trabecular BV/TV, number and connectivity density were lower, whereas TbSp and SMI were higher in both WT and β-less OVX (0.01 < P < 0.06). At the vertebrae, the magnitude of difference between OVX and sham was similar in WT and β-less (Fig. 6). A similar pattern was seen at the distal femur, although the magnitude of difference between OVX and sham tended to be greater in β-less mice. There were no significant effects of OVX on midfemoral cortical parameters, although MA tended to be higher and TbSp fraction tended to be lower in OVX compared with sham in both β-less and WT (Table 3). Serum markers of bone turnover (osteocalcin and TRACP5b) were significantly higher in β-less than WT at baseline (P < 0.05), declined by approximately 50% from 8–16 wk of age irrespective of genotype or treatment (P < 0.01) and remained significantly elevated in β-less compared with WT at 16 wk of age (P < 0.01).
Figure 6.
Difference (in percent) in trabecular BV/TV, TbN, and connectivity density (ConnD) between OVX and sham-OVX at the vertebrae and distal femur in wild-type and β-less mice (n = 9–12 per group). *, 0.05 < P < 0.10; **, P < 0.05 for sham vs. OVX.
Discussion
In this study, we assessed body composition and skeletal characteristics in mice deficient in the three known β-AR (β-less) during normal growth and aging and in response to OVX. We confirmed previous findings of the role of β-adrenergic signaling in bone mass acquisition and maintenance but not in estrogen deficiency. We found that 1) male β-less have an early increase in total body BMD, trabecular BV, and cortical bone parameters and exhibit further increases in total body BMD and cortical bone as they become obese with age. In contrast, β-less mice exhibit an exaggerated loss of trabecular bone with age, consistent with an increase in osteoclast surface and bone turnover relative to WT. We also found that 2) growing female β-less and WT mice have similar skeletal deterioration after OVX, but β-less mice are protected from OVX-induced weight gain.
The β-less mice represent an interesting model in which to explore interactions between central and peripheral regulation of bone mass and microarchitecture. The higher body fat in β-less leads to increased circulating leptin. Generally, this increased circulating leptin would be predicted to influence bone mass via hypothalamic-SNS pathways; however, given the lack of β-AR, the increased leptin levels can exert their effects on bone only via peripheral pathways. Whereas the central (hypothalamic) actions of leptin increase sympathetic signaling, activate osteoblast β-AR, and decrease trabecular bone mass, circulating leptin has been shown to have direct anabolic effects on osteoblasts, thereby increasing periosteal bone formation (46). Thus, we initially hypothesized that β-less mice would have increased trabecular and cortical bone mass relative to WT mice and would be resistant to the deleterious effects of estrogen deficiency.
Our results partly support the hypothesis that trabecular bone mass will be increased in the absence of the negative effects of β-adrenergic signaling on bone remodeling, because both vertebral and femoral trabecular bone mass and microarchitecture were enhanced in young (i.e. 6-wk-old) β-less mice. This appears to be due to reduced osteoclast number, perhaps coupled with a trend toward increased mineralizing surface as measured by histomorphometry. These observations are concordant with the trabecular bone phenotype of β2-AR KO mice (9) and the proposed mechanism of action of β2-AR signaling (i.e. stimulation of RANKL and inhibition of osteoblastic bone formation). However, β-less mice did not have an increased bone formation rate and actually had a significantly lower bone formation rate at 16 wk of age, providing further evidence to support the tenet that β1- and/or β3-adrenergic signaling may be necessary to stimulate bone formation in the absence of β2 -AR (21,22).
At 16 wk of age, there was no difference in trabecular BV between WT and β-less mice, suggesting that the normal age-related changes in trabecular bone were enhanced in the absence of β-AR, particularly in the distal femoral metaphysis, perhaps due to increased bone turnover, as reflected by their higher osteocalcin and TRACP5b levels, and a trend for higher osteoclast surface. The mechanisms for the later increase in bone turnover are unknown but demonstrate clearly that absence of adrenergic signaling is not sufficient to prevent bone loss.
As hypothesized, β-less males had increased cortical bone mass, cross-sectional geometry, and biomechanical properties at the midfemoral diaphysis. These increases in diaphyseal cortical bone properties may be due to the higher body mass and/or increased peripheral leptin activity in β-less compared with WT. Note that increased fat mass in β-less mice was not deleterious to cortical bone properties. One might speculate that the higher weight and circulating leptin levels stimulate periosteal bone formation and could in turn trigger local adaptive mechanisms to inhibit trabecular bone formation or enhance trabecular bone resorption to remove cancellous bone that may be excessive in the presence of increased cortical bone properties (46).
As expected, ovariectomized WT mice had greater gains in weight and body fat than sham-operated controls. In contrast, β-less mice had no additional weight or fat gain in response to OVX compared with sham-operated controls. The mechanisms underlying this observation are unknown, but other studies have established that body weight regulation is altered in β-less mice (31). Furthermore, our observation is consistent with a previous study in which mice with germline deletion of hypothalamic neuropeptide Y2 (NPY2) receptors were protected from fat mass gain after OVX (47). Thus, at least two hypothalamic pathways are implicated in OVX-induced increases in body mass, and both likely involve changes in leptin secretion. Estrogen deficiency is associated with decreased circulating leptin and central leptin insensitivity (48,49), but increased leptin transgene expression in the hypothalamus blocks OVX-induced hyperphagia and weight gain (50). Interruption of this mechanism in β-less and NPY2 KO mice may offer some protection from OVX-induced fat gain.
With regard to OVX-induced bone loss, we found that β-less and WT exhibited similar declines in trabecular bone after OVX and that the magnitude actually tended to be greater in β-less compared with WT mice, consistent with the age-related trabecular bone loss in male β-less mice. Although OVX had no apparent effect on serum markers of bone turnover, likely due to the relatively late sampling time, they were higher in β-less than WT both before and after OVX and declined by approximately 50% from 8–16 wk in all groups. These results are somewhat unexpected, because prior studies reported OVX-induced bone loss was inhibited in mice lacking β2-AR and in rodents treated with β-blockers (8,10,16). One would therefore expect similar protection from OVX-induced bone loss in β-less mice. However, a comparison of the results of the current study to the phenotypes of the β2-AR and β1,2-AR KO suggests that different effects of β2-AR and β1-AR in bone may explain some of the differences in results (22).
Specifically, in contrast to β-less mice, the β2-AR KO has lower body weight and fat than WT, yet at older ages, the β2-AR KO has higher vertebral trabecular BV/TV, bone formation rate, and osteoblast number as well as decreased osteoclastic activity (9). In comparison, mice null for both the β1- and β2-AR (β1,2-AR KO) have lower body weight but similar fat mass to WT and do not have a high bone mass phenotype. Relative to WT, β1,2-AR KO mice have similar vertebral trabecular BV, and lower midfemoral cortical thickness and cross-sectional geometric properties (51). These differences support the possibility of opposing effects of the β1- and β2-AR on bone and may also explain conflicting data on the effects of β-blockers on bone (21). At low doses, propranolol stimulates bone formation and inhibits resorption, but this effect disappears at higher doses (52). It has been proposed that propranolol affects the β2-AR at lower doses, suppressing bone resorption, but acts through the β1-AR at higher doses, reducing its protective effects (21,46). Furthermore, although β3-AR is not expressed in bone, but rather primarily in adipose tissue, it may have an indirect role in mediating skeletal response to estrogen deficiency by regulation of fat mass and peripheral aromatization of estrogen.
Use of the β-less model has both strengths and limitations. On the one hand, with the ablation of all three AR, there is no possibility of compensatory up- or down-regulation of other isoforms of the β-AR, as is observed in models where a single β-AR is deleted (19,20). Yet, unlike the β2-AR KO, which has similar body composition to WT, β-less can have increased body weight and fat compared with WT even on a normal chow diet. This makes the model more complex, because in addition to absence of adrenergic signaling, β-less have increased mechanical loading as well as altered endocrine factors that influence bone metabolism. Furthermore, derivation of the β-less on a mixed genetic background and the lack of littermate controls may confound interpretation of these results. However, initial studies found similar metabolic phenotypes in two lines of β-less mice derived on the mixed genetic background, leading the study authors to conclude that the effects of genetic disruption were more profound than the possible contribution of the mixed genetic background (31). Although having β-less on a pure genetic background would eliminate these concerns, attempts to do this have thus far proven unsuccessful (D. Kong, personal communication).
These limitations notwithstanding, our data extend the knowledge regarding effects of β-adrenergic signaling on the skeleton. Of interest, the phenotype of β-less mice appears more similar to that of mice lacking leptin (i.e. ob/ob mice, who are obese with low sympathetic tone) than any model of deficient adrenergic signaling, except that β-less have high leptin levels and do not exhibit gonadal deficiency and hypercorticism, both present in the ob/ob model. High leptin levels in the β-less mouse likely explain the observation that whereas ob/ob mice have markedly reduced cortical bone mass and thickness (14), β-less mice have increased cortical parameters, presumably due to leptin stimulation of cortical bone through peripheral pathways. The β-less model therefore emphasizes the non-SNS-mediated effects of leptin on bone mass regulation.
Following observations that bone remodeling is mediated in part by β-adrenergic signaling via the central nervous system (13) and that pharmacological inhibition of β-adrenergic signaling inhibits deleterious skeletal effects of estrogen deficiency in animal models (8), there has been keen interest in determining effects of β-blockers on BMD and fracture risk. Results from observational studies of β-blocker use and fracture risk have been inconsistent, showing positive, negative, and no effect on fracture risk, highlighting the challenges in interpreting these types of studies. Confounding factors include the underlying disease for which the β-blockers are prescribed, along with possible comorbidities and medications (53). Although a metaanalysis showed protective effects of β-blocker use (54), large cohort studies and prospective randomized studies have shown modest or no effects of β-blocker use on fracture risk (53). Because β-less mice were not protected from the deleterious effects of estrogen deficiency, our study indicates that some of the positive skeletal effects seen in clinical studies of β-blockers may be due to concomitant medications or other confounding factors.
In summary, our data indicate that global absence of β-adrenergic signaling results in obesity and increased trabecular BV in young and increased cortical bone mass in older males but does not prevent deleterious effects of estrogen deficiency on trabecular bone. Thus, our data do not support the use of β-blockers to prevent postmenopausal bone loss and fractures. Our findings also suggest a direct positive effect of weight and/or increased circulating leptin on bone turnover and cortical bone structure, independent of adrenergic signaling.
Footnotes
Funding for this project was provided by the Swiss National Science Foundation Grant 631-62937 (S.L.F., D.D.P.), National Institutes of Health Grant AR49265 (M.L.B.), and the American Heart Association (H.D.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 18, 2008
Abbreviations: β-AR, β-Adrenergic receptor; BA, bone area; BMD, bone mineral density; BV, bone volume; Cort Th, cortical thickness; μCT, microcomputed tomography; KO, knockout; MA, medullary area; OVX, ovariectomy; SMI, structure model index; SNS, sympathetic nervous system; TA, total cross-sectional area; TbN, trabecular number; TbSp, trabecular spacing; TbTh, trabecular thickness; TRACP5b, tartrate-resistance alkaline phosphatase-5B; TV, total volume; WT, wild type.
References
- Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL 1986 Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 232:868–871 [DOI] [PubMed] [Google Scholar]
- Hill EL, Elde R 1991 Distribution of CGRP-, VIP-, DβH-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res 264:469–480 [DOI] [PubMed] [Google Scholar]
- Burt-Pichat B, Lafage-Proust MH, Duboeuf F, Laroche N, Itzstein C, Vico L, Delmas PD, Chenu C 2005 Dramatic decrease of innervation density in bone after ovariectomy. Endocrinology 146:503–510 [DOI] [PubMed] [Google Scholar]
- Denes A, Boldogkoi Z, Uhereczky G, Hornyak A, Rusvai M, Palkovits M, Kovacs KJ 2005 Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience 134:947–963 [DOI] [PubMed] [Google Scholar]
- Moore RE, Smith 2nd CK, Bailey CS, Voelkel EF, Tashjian Jr AH 1993 Characterization of β-adrenergic receptors on rat and human osteoblast-like cells and demonstration that β-receptor agonists can stimulate bone resorption in organ culture. Bone Miner 23:301–315 [DOI] [PubMed] [Google Scholar]
- Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T 1997 Expression of mRNAs for neuropeptide receptors and β-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett 233:125–128 [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Muller K, Richener H, Bilbe G 1998 Formoterol and isoproterenol induce c-fos gene expression in osteoblast-like cells by activating β2-adrenergic receptors. Bone 22:471–478 [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G 2002 Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G 2005 Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Bouxsein ML, Rizzoli R, Ferrari SL 2006 Combined treatment with a β-blocker and intermittent PTH improves bone mass and microarchitecture in ovariectomized mice. Bone 39:260–267 [DOI] [PubMed] [Google Scholar]
- Bonnet N, Benhamou CL, Brunet-Imbault B, Arlettaz A, Horcajada MN, Richard O, Vico L, Collomp K, Courteix D 2005 Severe bone alterations under β2 agonist treatments: bone mass, microarchitecture and strength analyses in female rats. Bone 37:622–633 [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Muzzin P, Bouxsein ML, Rizzoli R, Ferrari SL 2005 Mice null for β1β2-adrenergic receptors have low bone mass and architecture and are resistant to isoproterenol-induced inhibition of bone growth. Bone 36(Suppl):S130 [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G 2000 Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Newton D, Xie D, Isales C 2004 Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34:376–383 [DOI] [PubMed] [Google Scholar]
- Pogoda P, Egermann M, Schnell JC, Priemel M, Schilling AF, Alini M, Schinke T, Rueger JM, Schneider E, Clarke I, Amling M 2006 Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res 21:1591–1599 [DOI] [PubMed] [Google Scholar]
- Bonnet N, Beaupied H, Vico L, Dolleans E, Laroche N, Courteix D, Benhamou CL 2007 Combined effects of exercise and propranolol on bone tissue in ovariectomized rats. J Bone Miner Res 22:578–588 [DOI] [PubMed] [Google Scholar]
- Kondo H, Nifuji A, Takeda S, Ezura Y, Rittling SR, Denhardt DT, Nakashima K, Karsenty G, Noda M 2005 Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J Biol Chem 280:30192–30200 [DOI] [PubMed] [Google Scholar]
- Marenzana M, De Souza RL, Chenu C 2007 Blockade of β-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone 41:206–215 [DOI] [PubMed] [Google Scholar]
- Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB 1995 Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem 270:29483–29492 [DOI] [PubMed] [Google Scholar]
- Collins S, Surwit RS 2001 The β-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res 56:309–328 [DOI] [PubMed] [Google Scholar]
- Bonnet N, Pierroz D, Ferrari S 2008 Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact 8:94–104 [PubMed] [Google Scholar]
- Takeda S, Karsenty G 2008 Molecular bases of the sympathetic regulation of bone mass. Bone 42:837–840 [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC 2004 β-Adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res 19:19–24 [DOI] [PubMed] [Google Scholar]
- Schlienger RG, Kraenzlin ME, Jick SS, Meier CR 2004 Use of β-blockers and risk of fractures. JAMA 292:1326–1332 [DOI] [PubMed] [Google Scholar]
- Rejnmark L, Vestergaard P, Kassem M, Christoffersen BR, Kolthoff N, Brixen K, Mosekilde L 2004 Fracture risk in perimenopausal women treated with β-blockers. Calcif Tissue Int 75:365–372 [DOI] [PubMed] [Google Scholar]
- Reid IR, Gamble GD, Grey AB, Black DM, Ensrud KE, Browner WS, Bauer DC 2005 β-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J Bone Miner Res 20:613–618 [DOI] [PubMed] [Google Scholar]
- Levasseur R, Dargent-Molina P, Sabatier JP, Marcelli C, Breart G 2005 β-Blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l’Osteoporose prospective study. J Am Geriatr Soc 53:550–552 [DOI] [PubMed] [Google Scholar]
- Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, Benhamou CL 2007 Protective effect of β-blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 40:1209–1216 [DOI] [PubMed] [Google Scholar]
- Meisinger C, Heier M, Lang O, Doring A 2007 β-Blocker use and risk of fractures in men and women from the general population: the MONICA/KORA Augsburg cohort study. Osteoporos Int 18:1189–1195 [DOI] [PubMed] [Google Scholar]
- Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, Davidson JS, Grey AB 2005 Effects of a β-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 90:5212–5216 [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB 2002 βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297:843–845 [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK 1999 Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J Biol Chem 274:16701–16708 [DOI] [PubMed] [Google Scholar]
- Nagy TR, Clair AL 2000 Precision and accuracy of dual-energy x-ray absorptiometry for determining in vivo body composition of mice. Obes Res 8:392–398 [DOI] [PubMed] [Google Scholar]
- Nagy TR, Prince CW, Li J 2001 Validation of peripheral dual-energy x-ray absorptiometry for the measurement of bone mineral in intact and excised long bones of rats. J Bone Miner Res 16:1682–1687 [DOI] [PubMed] [Google Scholar]
- Iida-Klein A, Lu SS, Yokayama K, Dempster DW, Nieves J, Lindsay R 2003 Precision, accuracy, and reproducibility of dual x-ray absorptiometry measurements in mice in vivo. J Clin Densitom 6:25–33 [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Pierroz DD, Glatt V, Goddard DS, Cavat F, Rizzoli R, Ferrari SL 2005 β-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J Bone Miner Res 20:635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML 2005 Bone response to intermittent parathyroid hormone is altered in mice null for β-arrestin2. Endocrinology 146:1854–1862 [DOI] [PubMed] [Google Scholar]
- Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak-Novakovic G, Kaplan D 2005 Silk implants for the healing of critical size bone defects. Bone 37:688–698 [DOI] [PubMed] [Google Scholar]
- Hildebrand T, Rüegsegger P 1997 A new method for the model independent assessment of thickness in three-dimensional images. J Microsc 185:67–75 [Google Scholar]
- Hildebrand T, Rüesegger P 1997 Quantification of bone microarchitecture with the structure model index. Comp Meth Biomech Biomed Eng 1:5–23 [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Akkus OJ, Majeska RJ, Nadeau JH 2003 Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome 14:97–104 [DOI] [PubMed] [Google Scholar]
- Brodt MD, Ellis CB, Silva MJ 1999 Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res 14:2159–2166 [DOI] [PubMed] [Google Scholar]
- Turner CH, Burr DB 1993 Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608 [DOI] [PubMed] [Google Scholar]
- Parfitt A, Drezner M, Glorieux F, Kanis J, Recker R 1987 Bone histomorphometry: standardization of nomenclature, symbols and units. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, Horton LG, Delahunty KM, Beamer WG, Sipos J, Clemmons D, Nelson T, Bouxsein ML, Horowitz M 2004 Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35:1046–1058 [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Ferrari SL 2008 Leptin and the sympathetic connection of fat to bone. Osteoporos Int 19:905–912 [DOI] [PubMed] [Google Scholar]
- Allison SJ, Baldock P, Sainsbury A, Enriquez R, Lee NJ, Lin EJ, Klugman M, During M, Eisman JA, Li M, Pan LC, Herzog H, Gardiner EM 2006 Conditional deletion of hypothalamic Y2 receptors reverts gonadectomy-induced bone loss in adult mice. J Biol Chem 281:23436–23444 [DOI] [PubMed] [Google Scholar]
- Chu SC, Chou YC, Liu JY, Chen CH, Shyu JC, Chou FP 1999 Fluctuation of serum leptin level in rats after ovariectomy and the influence of estrogen supplement. Life Sci 64:2299–2306 [DOI] [PubMed] [Google Scholar]
- Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW 2001 Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord 25:1680–1688 [DOI] [PubMed] [Google Scholar]
- Torto R, Boghossian S, Dube MG, Kalra PS, Kalra SP 2006 Central leptin gene therapy blocks ovariectomy-induced adiposity. Obesity (Silver Spring) 14:1312–1319 [DOI] [PubMed] [Google Scholar]
- Pierroz D, Muzzin P, Glatt V, Bouxsein M, Rizzoli R, Ferrari SL 2004 β1β2-Adrenergic receptor KO mice have decreased total body and cortical bone mass despite increased trabecular number. J Bone Miner Res 19:S32 [Google Scholar]
- Bonnet N, Laroche N, Vico L, Dolleans E, Benhamou CL, Courteix D 2006 Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J Pharmacol Exp Ther 318:1118–1127 [DOI] [PubMed] [Google Scholar]
- Reid IR 2008 Effects of β-blockers on fracture risk. J Musculoskelet Neuronal Interact 8:105–110 [PubMed] [Google Scholar]
- Wiens M, Etminan M, Gill SS, Takkouche B 2006 Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med 260:350–362 [DOI] [PubMed] [Google Scholar]