Abstract
Corticotropin-releasing factor (CRF) overexpressing (OE) mice are a genetic model that exhibits features of chronic stress. We investigated whether the adaptive feeding response to a hypocaloric challenge induced by food deprivation is impaired under conditions of chronic CRF overproduction. Food intake response to a 16-h overnight fast and ip injection of gut hormones regulating food intake were compared in CRF-OE and wild type (WT) littermate mice along with brain Fos expression, circulating ghrelin levels, and gastric emptying of a nonnutrient meal. CRF-OE mice injected ip with saline showed a 47 and 44% reduction of 30-min and 4-h cumulative food intake response to an overnight fast, respectively, compared with WT. However, the 30-min food intake decrease induced by ip cholecystokinin (3 μg/kg) and increase by ghrelin (300 μg/kg) were similar in CRF-OE and WT mice. Overnight fasting increased the plasma total ghrelin to similar levels in CRF-OE and WT mice, although CRF-OE mice had a 2-fold reduction of nonfasting ghrelin levels. The number of Fos-immunoreactive cells induced by fasting in the arcuate nucleus was reduced by 5.9-fold in CRF-OE compared with WT mice whereas no significant changes were observed in other hypothalamic nuclei. In contrast, fasted CRF-OE mice displayed a 5.6-fold increase in Fos-immunoreactive cell number in the dorsal motor nucleus of the vagus nerve and a 34% increase in 20-min gastric emptying. These findings indicate that sustained overproduction of hypothalamic CRF in mice interferes with fasting-induced activation of arcuate nucleus neurons and the related hyperphagic response.
Sustained over-production of brain corticotrophin-releasing factor interferes with fasting-activated neuronal activation in the arcuate nucleus and results in reduction of food intake dissociated from gastric emptying.
Corticotropin-releasing factor (CRF) is a peptide that is released in the brain by stress to integrate the endocrine, behavior, autonomic, and visceral responses (1,2). CRF functions both as a neuroendocrine hormone and neurotransmitter via the hypothalamic-pituitary-adrenal (HPA) axis and hypothalamic and extrahypothalamic neuronal pathways (3). In particular, CRF acts in the brain to induce a rapid onset reduction of food intake and gastric emptying in rodents and plays a role in stress-related alterations of food intake and gastric motor function (2,4,5,6). CRF also modulates the actions of other neuropeptides and hormones that regulate food intake, such as neuropeptide Y (NPY) and leptin (7). For instance, blockade of CRF signaling in the brain by selective antagonists or CRF antibody enhances NPY-induced food intake in rats (8,9).
CRF overexpressing mice have been developed using the metallothionein-1 promoter, which closely reflects chronic amplification of brain CRF pathways (10,11). This transgenic model displays features of chronic stress as characterized by the sustained HPA hyperactivity, increased anxiogenic behavior, truncal obesity, and hypervisceral responsiveness to an acute stress (10,12,13,14). However, there has been insufficient exploration of the model in regard to feeding behavior (15,16).
Brain signals are modulated by feeding and fasting. In particular, fasting alters neuronal pathways and expression of neuropeptide genes in the hypothalamus to promote energy intake (16,17). The arcuate nucleus (Arc) in the hypothalamus is the key integrative center with close connection to the paraventricular nucleus (PVN) in which many CRF-producing neurons project to the HPA axis and autonomic centers (16,18). Fasting in mice induces a robust increase in neuronal activity as shown by immunohistochemical detection of an early gene product, c-Fos (19), and expression of orexigenic peptides, NPY, and Agouti gene-related protein in the Arc (20,21,22). Likewise, ip injection of ghrelin, a gut hormone that stimulates food intake and is released during fasting, activates NPY-synthesizing neurons (23). Whether CRF overexpression alters the hypothalamic neuronal response to food deprivation has not been investigated.
In the present study, we examined whether the compensatory feeding response to an acute metabolic stress induced by overnight fasting was impaired under conditions of chronic CRF overexpression using transgenic mice and investigated possible central and peripheral underlying mechanisms. In particular, to gain insight into brain pathways, we assessed the pattern of neuronal activation in response to an overnight fast in CRF-overexpressing (OE) mice and wild-type (WT) littermates as monitored by Fos immunohistochemistry in hypothalamic nuclei (Arc and PVN), and brainstem nuclei, nucleus of the solitary tract (NTS) and dorsal motor nucleus of the vagus nerve (DMV) involved in the regulation of the feeding response to a fast and gastric motor function (17,24). We also investigated whether the change of food intake in CRF-OE mice was related to alteration of the orexigenic ghrelin signaling known to be activated by a fast (25,26) or to increased responsiveness to the cholecystokinin (CCK) satiation effect (27). Lastly, gastric emptying that also contributes to influence food intake (28) was monitored in fasted CRF-OE compared with WT mice.
Materials and Methods
Animals
Experiments were conducted in adult (9–11 months old) male CRF-OE mice (body weight 31.0 ± 1.2 g) and their littermates (body weight 33.5 ± 1.1 g) (Oregon Health and Science University, Portland, OR) generated as previously described (10). The chimeric CRF transgene was composed of the rat genomic CRF gene replacing the 5′ regulatory region by a mouse metallothionein-1 promoter and microinjected into the male pronucleus of fertilized eggs (C57/B6 × SJL). Mice were group housed (three to four per cage) and maintained under controlled temperature (20–23 C) and lighting (0600–1800 h) conditions with ad libitum access to a standard laboratory rodent diet (Prolab RMH 2500, 5P14; LabDiet, St. Louis, MO) and tap water. All experiments were performed between 0900 and 1300 h to avoid confounding variables of diurnal rhythm influence on the hypothalamic pituitary axis under basal and stress conditions (29). Before testing, mice were housed singly and deprived of food but not water overnight for 16 h except otherwise stated. All procedures were approved by the Animal Research Committees at Veterans Affairs Greater Los Angeles Healthcare System (04012-06).
Peptides
Sulfated CCK-8 (CCK-8S; Bachem, Torrance, CA) and human octanylated ghrelin (gift from Dr. S. St. Pierre, University of Montréal, Montréal, Canada) were dissolved in saline as stock solution, aliquoted, and stored at −80 C. Immediately before the start of the experiments, ghrelin and CCK-8S were diluted in sterile saline (0.9% NaCl; Hospira, Inc., Lake Forest, IL) to a final concentration of 300 μg/kg body weight (BW) for ghrelin or 3 μg/kg BW for CCK-8S in a total volume of 4 ml/kg per injection. Ghrelin and CCK doses were chosen based on our previous dose-response studies whereby ip injection of ghrelin at 300 μg/kg (10 μg/mouse) was the minimal dose significantly increasing food intake in freely fed mice (23), and CCK at 3 μg/kg was the subthreshold to inhibit feeding in fasted mice (30).
Measurement of food intake and gastric emptying
Overnight fasted CRF-OE and WT littermate mice were injected ip with saline or CCK (3 μg/kg). In separate groups, nonfasted CRF-OE and WT littermate mice were injected ip with saline or ghrelin (300 μg/kg). Thereafter, preweighed chow was made available to the animals for the subsequent 4 h. Food intake was determined by measuring the difference between the preweighed standard chow and the weight of chow and spillage at the end of the first 30 min or 1, 2, and 4 h of food exposure and expressed as gram per 25 g BW. Gastric emptying of a nonnutrient viscous meal was determined in overnight fasted CRF-OE and WT littermate mice by the phenol red method as previously described (31).
Measurement of plasma ghrelin levels
Nonfasted and overnight fasted CRF-OE and WT littermate mice were deeply anesthetized with sodium pentobarbital (Nembutal, 100 mg/kg; Abbott Laboratories, Chicago, IL). Blood was withdrawn by cardiac puncture and collected in ice-cooled tubes containing EDTA (7.5%, 10 μl/0.5 ml blood; Sigma, St. Louis, MO) and aprotinin (0.6 Trypsin Inhibitory Unit per 0.5 ml blood; ICN Pharmaceuticals, Costa Mesa, CA). Plasma ghrelin was measured using a commercial RIA kit for rat/mouse ghrelin (Phoenix Peptides, Belmont, CA) that detects total ghrelin.
Immunohistochemistry for Fos in hypothalamic and brainstem nuclei
At the end of the 16-h fasting period, CRF-OE and WT littermate mice were deeply anesthetized with an ip injection of pentobarbital. Thereafter, transcardial perfusion was performed as described before (19) with 40–50 ml of 4% paraformaldehyde and 14% saturated picric acid in 0.1 m phosphate buffer (pH 7.2). Brains were removed, overnight postfixed in the same fixative, and then rinsed and cryoprotected in 10% sucrose for 24 h, snap frozen in dry ice-cooled 2-methylbutane and sectioned at 25 μm in a cryostat. Brain sections were collected in three sets. One set of free floating sections was pretreated with 0.3% hydrogen peroxide and 3% normal goat serum, and incubated overnight at 4 C in rabbit anti-c-Fos serum at 1:10,000 (Ab-5, catalog no. PC38; Oncogene, Cambridge, MA) and then in biotinylated goat antirabbit IgG at 1:1,000 (Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature followed by avidin-biotin-peroxidase complex (Vector, Vermont, CA) for 1 h at room temperature. Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride hydrate and hydrogen peroxide (Sigma) for 7–10 min. Each step of incubation was followed by a 3 × 15 min rinsing in PBS. Sections were mounted on SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA), air dried for 24 h, and completely dehydrated through a gradient of ethanol and cleared in xylenes before coverslipping.
Fos-immunoreactive (ir) cells were counted unilaterally in six to eight sections per brain nucleus (Arc, PVN, NTS, and DMV) by an investigator blinded to the genotype of mice. Only cell nuclei with clear brown immunostaining were counted in the Arc (−1.46 to −1.94 mm from the bregma), PVN (−0.58 to −0.94 mm from bregma), NTS, and DMV at the level of the area postrema according to the mouse brain atlas of Franklin and Paxinos (32). Then the average number of Fos-ir cells per section for each animal was calculated in each brain nucleus.
Data and statistical analysis
Data are expressed as mean ± sem and were analyzed by one-way ANOVA. Differences between groups were evaluated by all pairwise multiple comparison procedures (Tukey post hoc test). Two-way ANOVA was used to compare differences between genotypes. P < 0.05 was considered significant.
Results
Food intake in overnight fasted CRF-OE and WT littermate mice injected ip with vehicle or CCK-8S
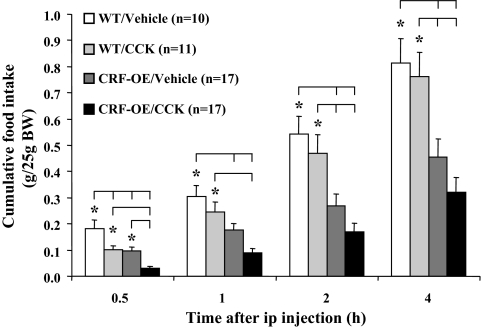
The cumulative food intake response to an overnight fast was significantly lower in CRF-OE than WT mice injected ip with saline as monitored at 30 min and 1, 2, and 4 h period of refeeding (e.g. 4 h cumulative food intake, grams per 25 g BW: 0.46 ± 0.07 vs. 0.81 ± 0.09, P = 0.01; Fig. 1). Two-way ANOVA revealed a significant difference between genotypes in cumulative food intake at all time points (e.g. 4 h: F = 27.8, P < 0.001). Time course indicated that food intake was significantly inhibited in the first 30 min and 1–2 h refeeding periods in CRF-OE compared with WT mice (grams per 25 g BW: 0–30 min: 0.10 ± 0.02 vs. 0.18 ± 0.03, P = 0.007; 1–2 h: 0.09 ± 0.02 vs. 0.24 ± 0.03, P = 0.003). The peripheral injection of CCK-8S (3 μg/kg) significantly decreased food intake only in the first 30 min compared with vehicle in both WT (grams per 25 g BW: 0.10 ± 0.01 vs. 0.18 ± 0.03, P = 0.027; Fig. 1) and CRF-OE mice (0.03 ± 0.01 vs. 0.10 ± 0.02, P = 0.021; Fig. 1) and no longer thereafter when assessed as noncumulatively (data not shown) and cumulatively (Fig. 1). Saline-treated CRF-OE mice even exhibited a lower 4-h cumulative food intake than WT mice injected with CCK-8S (0.46 ± 0.07 vs. 0.76 ± 0.09, P = 0.026; Fig. 1).
Figure 1.
CRF-OE mice had a reduced food intake response to an overnight fast compared with WT mice, whereas the CCK satiation effect was not altered. After an overnight fast, CRF-OE and WT littermate mice were injected ip with saline or CCK-8S (3 μg/kg BW) and cumulative food intake (expressed as grams per 25 g BW) was measured at 30 min and 1, 2, and 4 h. Data are expressed as mean ± sem; the numbers of mice per group are indicated in parentheses. *, P < 0.05.
Food intake in freely fed CRF-OE and WT littermate mice and in response to ip ghrelin
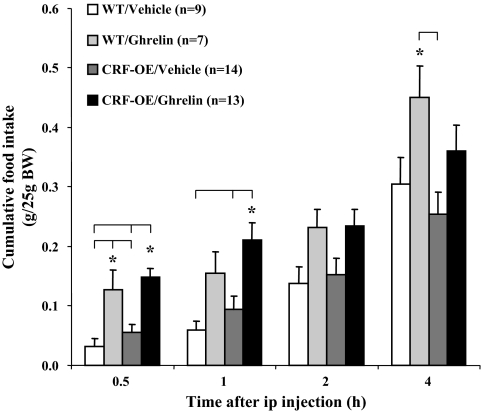
Nonfasted CRF-OE mice had similar food intake as the WT mice when monitored either hourly or during the 4-h cumulative period after the ip vehicle injection during the light phase (Fig. 2 and data not shown). Ghrelin (300 μg/kg ip) significantly increased food intake compared with vehicle similarly in both WT (grams per 25 g BW: 0.13 ± 0.03 vs. 0.03 ± 0.01, P = 0.01) and CRF-OE mice (0.15 ± 0.02 vs. 0.06 ± 0.01, P < 0.001) during the first 30 min after ip injection (Fig. 2). The ghrelin effect was no longer significant after the first 30-min period (data not shown). However, the cumulative food intake for 1 h was still significantly increased in ghrelin-administered CRF-OE mice compared with vehicle (0.21 ± 0.03 vs. 0.09 ± 0.02, P = 0.006), whereas not reaching significance in WT mice (0.16 ± 0.04 vs. 0.06 ± 0.02, P = 0.27; Fig. 2). Two hours after ghrelin injection, neither CRF-OE nor WT mice showed a significantly different cumulative food intake compared with their vehicle-treated controls (Fig. 2).
Figure 2.
Freely fed CRF-OE mice had a similar food intake response to ip ghrelin as WT mice. Freely fed CRF-OE and WT littermate mice were injected ip with saline or ghrelin (300 μg/kg BW) and cumulative food intake (expressed as grams per 25 g BW) was measured at 30 min and 1, 2, and 4 h. Data are expressed as mean ± sem; the numbers of mice per group are indicated in parentheses. *, P < 0.05.
Plasma ghrelin levels in ad libitum-fed and fasted CRF-OE compared with WT littermate mice
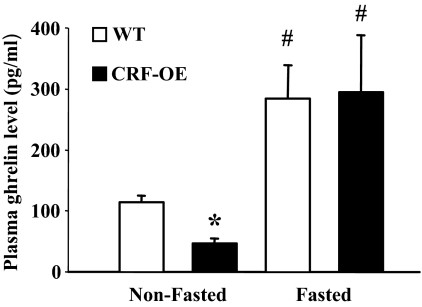
CRF-OE mice displayed significantly lower plasma levels of total ghrelin than WT mice under ad libitum feeding conditions (picograms per milliliter: 37.5 ± 3.3 vs. 113.9 ± 11.2, P < 0.05; Fig. 3). However, overnight fasting resulted in an elevation of plasma levels of total ghrelin and values were similar in CRF-OE and WT mice (picograms per milliliter: 294.8 ± 93.5 vs. 284.1 ± 55.3, P > 0.05; Fig. 3). The fasting levels of total plasma ghrelin were significantly different from those in the same nonfasted genotype (P < 0.05; Fig. 3).
Figure 3.
Plasma levels of total ghrelin in nonfasted and overnight fasted CRF-OE and WT littermate mice. Data are expressed as mean ± sem of n = 4 and 6 (nonfasted) or n = 3 and 4 (fasted) CRF-OE and WT mice. *, P < 0.05 vs. WT nonfasted; #, P < 0.05 vs. the nonfasted same genotype.
Gastric emptying in CRF-OE and WT littermate mice after overnight fasting
CRF-OE mice showed a significant increase in 20-min gastric emptying of a nonnutrient viscous solution compared with WT mice (83.2 ± 0.8 vs. 48.9 ± 3.9%, n = 4 and 6, respectively; P < 0.05).
Effect of overnight fasting on Fos expression in hypothalamic and brainstem nuclei in CRF-OE and WT littermate mice
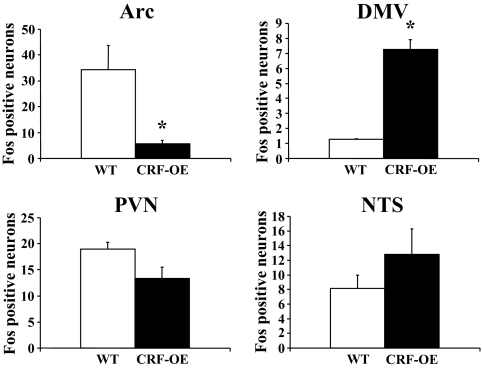
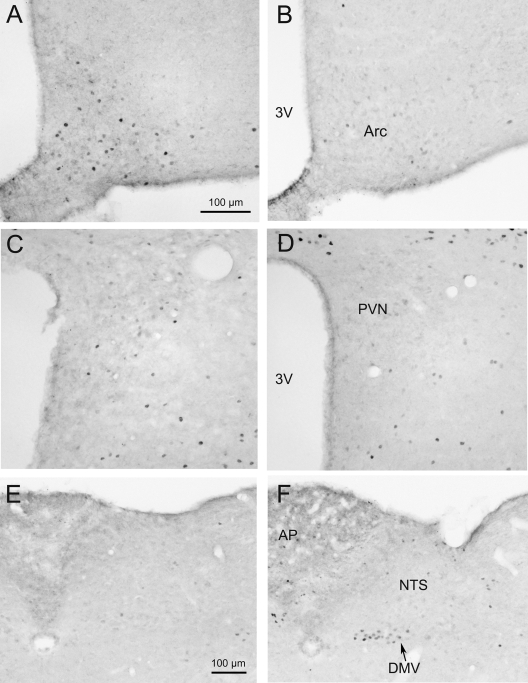
CRF-OE mice exhibited an 83% reduction in the number of Fos-ir neurons in the Arc compared with WT mice (5.8 ± 1.3 vs. 34.4 ± 9.3, P = 0.04) whereas no significant differences could be detected in the PVN (13.4 ± 2.1 vs. 19.0 ± 1.3, P = 0.09) and the NTS (12.8 ± 3.5 vs. 8.2 ± 1.8, P = 0.305) (Figs. 4 and 5). Overnight fasted CRF-OE mice had a significantly increased number of Fos-ir neurons in the DMV (7.3 ± 0.7 vs. 1.3 ± 0.05, P = 0.001; Figs. 4 and 5). Other brain areas that were investigated including the bed nucleus of the stria terminalis, amygdala, dorsomedial and ventromedial hypothalamic nuclei, locus coeruleus, and area postrema did not show obvious differences between CRF-OE and WT mice (data not shown).
Figure 4.
The number of Fos-ir neurons in hypothalamic and brain stem nuclei in overnight-fasted CRF-OE and WT mice (n = 3 and 4). Each bar represents the mean ± sem of the number of Fos-ir cells/section counted unilaterally in the Arc, PVN, DMV, and NTS. *, P < 0.05 vs. WT mice.
Figure 5.
Microphotographs of Fos immunoreactivity in the Arc, PVN, and dorsal-vagal complex (DMV and NTS) of overnight fasted CRF-OE (right panels) and WT (left panels) mice. Overnight fasted CRF-OE mice showed much less Fos-ir neurons in the Arc (B) compared with WT mice (A). No difference was detectable in the PVN (C and D) and NTS (E and F). Overnight fasting resulted in a significantly increased number of Fos-ir neurons in the DMV of CRF-OE mice (F) compared with WT mice (E). The scale bar in photo A represents the magnification for photos A–D and the one in E for E and F. 3V, Third cerebral ventricle; AP, area postrema.
Discussion
In the present study, we showed that the CRF-OE mice displayed a 47 and 44% reduction of the 30-min and 4-h cumulative food intake, respectively, in response to an overnight fast compared with WT littermates. The inhibition is specific to the refeeding after a state of energy deprivation because CRF-OE mice fed ad libitum did not show a significant change in food intake during the 4-h testing period. To our knowledge, this is the first report that revealed an impaired adaptive response to a metabolic stress in CRF-OE mice.
Mechanisms through which the refeeding response after a fast is compromised in CRF-OE mice are likely to involve alterations in the activation of NPYergic neurons in the Arc. Compelling evidence indicates that NPYergic neurons in the Arc projecting to the PVN are critical pathways integrating the feeding response to food deprivation (16,17,33). In particular, the Arc neuronal activity correlates with the nutritional state in lean mice as shown by the occurrence of Fos expression induced by fasting that is reversed by re-feeding and glucose treatment (34,35). In the present study, the Arc of fasted WT littermate mice contains a high number of Fos-ir neurons located in the ventromedial part of the nucleus consistent with the localization of NPY neurons as previously reported (21,23). In another study, we found that in overnight fasted male C57BL/6 mice (6–8 wk old), about 94% Fos-ir neurons in the Arc were expressing NPY mRNA assessed by dual labeling with immunohistochemistry and in situ hybridization (Wang L and Y Taché, unpublished data). By contrast, in fasted CRF-OE, Fos expression in the Arc was reduced by 83% compared with WT littermate mice. Taken together, the data suggest that under fasting conditions, CRF-OE mice have reduced activation of orexigenic neurons in the Arc, which may account for the reduced drive in food intake after an overnight food deprivation.
The Arc is a receptive hypothalamic nucleus for numerous feeding-related peripheral signals (16,36). We and others previously reported that ghrelin injected peripherally in lean freely fed mice or rats stimulated food intake and activated NPY neurons in the Arc as shown by the induction of Fos-immunoreactivity in the Arc with more than 90% of them expressing NPY (23,37,38,39). However, it is unlikely that the underlying mechanism of reduced activation of Arc neurons after a fast be related to an altered ghrelin signaling in CRF-OE mice. In the present study, fasting induced a rise in plasma levels of ghrelin reaching values similar in WT and CRF-OE mice, even though CRF-OE mice had 3-fold lower nonfasting ghrelin levels. In addition, freely fed CRF-OE and WT mice were capable of producing a similar orexigenic response to a ghrelin dose that we previously found to be the lowest dose inducing a significant increase in food intake in fed mice, indicating that the ghrelin-signaling pathway remains functional. Collectively, these data indicate that under fasting conditions, the dampened activation of neurons in the Arc translates into reduced food intake occurring in presence of fasting-induced rise of ghrelin release and functional growth hormone secretagogue receptor densely expressed in the Arc and known to mediate ghrelin orexigenic action (40,41). Our study also extends a recent report that endogenous ghrelin may not play a prominent role in the activation of Arc neurons during food deprivation as shown by the persistence of Fos expression induced by a 14-h fast in mice treated with a ghrelin antagonist (42). Of note, the lower plasma ghrelin levels in CRF-OE than WT mice under freely fed conditions is in line with the low plasma ghrelin levels reported in humans under chronic conditions of hypercortisolism of endogenous (Cushing’s syndrome) or exogenous (30 mg prednisolone for 5 d) origin (43). However, the reduced ghrelin levels in normally fed CRF-OE mice were not associated with changes in day time feeding compared with WT mice, which was also observed in genetically ghrelin-deficient mice (44).
A possible mechanism leading to impairment of fasting-induced arcuate neuronal activation in CRF-OE mice may be linked with the heightened constitutive expression of brain CRF-signaling pathways that override the fasting-stimulated NPY signals in the Arc. Consistent with this view, the Arc that is not a major site of CRF gene or peptide expression displayed a robust signal for CRF mRNA in CRF-OE mice in addition to the PVN as shown by in situ hybridization (10). Furthermore, it is well documented that hypothalamic CRF inhibits food intake and NPY orexigenic effects in the PVN independently from the HPA axis (4,8,9). There is also evidence that hypothalamic CRF suppresses NPY gene expression, and conversely, food deprivation decreases CRF mRNA to promote NPY orexigenic action (45,46). Hence, it is conceivable that in CRF transgenic mice, the overexpression of brain CRF combined with the additional stress of overnight single housing and fasting may abrogate the reduction of CRF expression and physiological compensatory stimulation of orexigenic NPY pathways, which play a key role in eliciting the refeeding response to a fast. Other studies showed that CRF-OE mice exhibit a CRF receptor-mediated anxiogenic behavior that is unrelated to the elevated activation of the HPA axis (12,47) likewise, existing evidence does not support a primary involvement of HPA axis in the decreased refeeding behavioral response to a fast in CRF-OE mice. CRF-OE mice did not respond to acute stressors with a further elevation in corticosterone levels, whereas WT mice reached the levels of CRF-OE mice (10,11,48). In addition, high glucocorticoid levels have not been associated with a reduction of food intake in experimental animals (49,50), and central treatment with dexamethasone even induced hyperphagia in rats (51).
The NTS receives peripheral satiety signals and sends inputs to other autonomic centers including the Arc and DMV to regulate feeding behavior (52). In the present study, we demonstrated in CRF-OE mice compared with their littermates that Fos expression was not modified in the NTS but increased in the DMV and that gastric emptying was increased by 34%. Acceleration of gastric emptying in fasted CRF-OE mice is likely to be centrally mediated and related to increased vagal cholinergic outflow stimulating gastric propulsive motility (53,54). This is supported by the 4.6-fold increase in Fos expression in the DMV in CRF-OE mice, whereas few Fos signal was observed in WT mice. In previous studies, we established that Fos induction in the DMV by cold stress is associated with a vagus-dependent stimulation of gastric secretory and motor functions in rats (55,56). Chronic restraint stress has been recently reported to accelerate gastric emptying, whereas acute restraint has the opposite effect in rats (57). Taken together, our data indicate that the NTS and DMV may not be involved in the decreased feeding response to a fast in CRF-OE and the enhanced signals in the DMV stimulate the gastric emptying.
Lastly, CRF-OE and WT mice had a similar reduction in food intake in response to ip CCK during the 30-min refeeding period after an overnight fast regardless of CRF-OE mice having ingested significantly less food than WT mice. It supports that CRF-OE mice maintain an early satiety in response to CCK. Whether other metabolic cues that signal to NPY neurons in the Arc such as insulin, glucose, and leptin (33,45) may also contribute to dampen the feeding response to a fast in CRF-OE awaits further elucidation. In particular, there is evidence that enhanced plasma leptin correlates to a high degree with intraabdominal fat content in mice (58), and as initially reported (10), in our study CRF-OE mice displayed truncal obesity. We previously established a synergic interaction between CCK and leptin to induce early satiety signaling in fasted mice (30). However, whether leptin in fasted CRF-OE mice exerts a direct action on the arcuate NPY neurons expressing leptin receptors (59), which may contribute to the reduction of Fos expression in response to fasting, is an additional mechanism that needs to be investigated (60).
In summary, CRF-OE mice exposed to a metabolic stress showed a reduction of the refeeding response to a 16-h food deprivation compared with WT littermates. The dampened feeding response is not related to an impaired ghrelin signaling and is associated with the blockade of fasting-induced neuronal activity in the Arc. This may reflect a dysfunction of NPY networking response to a fast in the PVN-Arc pathway due to overexpression of CRF signaling in CRF transgenic mice. Another important finding is the uncoupling of brain pathways regulating food intake and gastric emptying in fasted CRF-OE mice. There was an increase in gastric emptying of a nonnutrient meal in fasted CRF-OE compared with WT littermate mice, whereas the feeding response to a fast was reduced. The acceleration of gastric transit is likely to be centrally mediated as evidenced by the increase in the number of activated DMV neurons that are well established to provide the vagal stimulatory input to propulsive gastric motor function (61).
Acknowledgments
We are grateful to Dr. S. St. Pierre for his generous supply of human ghrelin and Honghui Liang for her excellent technical support.
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 33061 (to Y.T.) and 68155 (to M.M.); Veterans Affairs Research Career Scientist Award, Department of Veterans Affairs Merit Award (to Y.T.); Center Grant DK-41301 (Animal Core; to Y.T.); and Grant R24 AT002681-01 (Animal core, Dr. M. Fanselow), German Research Foundation Grant STE 1765/1-1 (to A.S.), and Grant GO 1718/1-1 (to M.G.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 11, 2008
Abbreviations: Arc, Arcuate nucleus; BW, body weight; CCK, cholecystokinin; CCK-8S, sulfated CCK-8; CRF, corticotropin-releasing factor; DMV, dorsal motor nucleus of the vagus nerve; HPA, hypothalamic-pituitary-adrenal; ir, immunoreactive; NPY, neuropeptide Y; NTS, nucleus of the solitary tract; OE, overexpressing; PVN, paraventricular nucleus; WT, wild type.
References
- Bale TL, Vale WW 2004 CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557 [DOI] [PubMed] [Google Scholar]
- Taché Y, Bonaz B 2007 Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 117:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF 2004 Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311:427–440 [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Taché Y, Koob GF 2003 Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci 24:421–427 [DOI] [PubMed] [Google Scholar]
- Richard D, Lin Q, Timofeeva E 2002 The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol 440:189–197 [DOI] [PubMed] [Google Scholar]
- Miragaya JR, Harris RB 2008 Antagonism of corticotrophin-releasing factor receptors in the fourth ventricle modifies responses to mild but not restraint stress. Am J Physiol Regul Integr Comp Physiol 295:R404–R416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Zapanti E 2004 The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: the role of corticotropin releasing hormone. Nutr Neurosci 7:271–280 [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Heinrichs SC, Pich EM, Tilders FJ, Koob GF 1993 Functional impairment of hypothalamic corticotropin-releasing factor neurons with immunotargeted toxins enhances food intake induced by neuropeptide Y. Brain Res 618:76–82 [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF 1993 Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res 611:18–24 [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W 1992 Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology 130:3378–3386 [DOI] [PubMed] [Google Scholar]
- Coste SC, Murray SE, Stenzel-Poore MP 2001 Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides 22:733–741 [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW 1994 Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci 14:2579–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T 2002 Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci 15:2007–2015 [DOI] [PubMed] [Google Scholar]
- Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Taché Y 2007 Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol 292:R1429–R1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Bouwknecht JA, Hijzen TH, Van Der GJ, Ronken E, Verbeek JS, Veening JG, Dederen PJ, Korosi A, Schoolderman LF, Roubos EW, Olivier B 2002 Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. Eur J Neurosci 16:1751–1760 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Dallman MF, Woods SC 1995 Hypothalamic response to starvation: implications for the study of wasting disorders. Am J Physiol 269:R949–R957 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG 1980 The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194:555–570 [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Barrachina MD, Taché Y 1998 Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res 791:157–166 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV 1999 Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140:4551–4557 [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Ueyama E, Senba E 2004 Fasting-induced activation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus. J Neuroendocrinol 16:105–112 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Erickson JC, Baskin DG, Palmiter RD 1998 Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology 139:2629–2635 [DOI] [PubMed] [Google Scholar]
- Wang L, Saint-Pierre DH, Taché Y 2002 Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 325:47–51 [DOI] [PubMed] [Google Scholar]
- Taché Y, Yang H, Miampamba M, Martinez V, Yuan PQ 2006 Role of brainstem TRH/TRH-R1 receptors in the vagal gastric cholinergic response to various stimuli including sham-feeding. Auton Neurosci 125:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K 2001 Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86:4753–4758 [DOI] [PubMed] [Google Scholar]
- Smith GP, Gibbs J 1994 Satiating effect of cholecystokinin. Ann NY Acad Sci 713:236–241 [DOI] [PubMed] [Google Scholar]
- Cuomo R, Sarnelli G 2004 Food intake and gastrointestinal motility. A complex interplay. Nutr Metab Cardiovasc Dis 14:173–179 [DOI] [PubMed] [Google Scholar]
- Dallman MF 1993 Stress update Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab 4:62–69 [DOI] [PubMed] [Google Scholar]
- Barrachina MD, Martinez V, Wang L, Wei JY, Taché Y 1997 Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 94:10455–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey A, Wang L, Jamieson PM, Basa NR, Million M, Czimmer J, Vale W, Taché Y 2003 Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology 125:654–659 [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G 1997 The mouse brain in stereotaxic coordinates. San Diego: Academic Press, Inc. [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M 1993 Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 14:303–347 [DOI] [PubMed] [Google Scholar]
- Singru PS, Sanchez E, Fekete C, Lechan RM 2007 Importance of melanocortin signaling in refeeding-induced neuronal activation and satiety. Endocrinology 148:638–646 [DOI] [PubMed] [Google Scholar]
- Becskei C, Lutz TA, Riediger T 2008 Glucose reverses fasting-induced activation in the arcuate nucleus of mice. Neuroreport 19:105–109 [DOI] [PubMed] [Google Scholar]
- Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL 2002 The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes 51:3412–3419 [DOI] [PubMed] [Google Scholar]
- Hewson AK, Dickson SL 2000 Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol 12:1047–1049 [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M 2001 Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120:337–345 [DOI] [PubMed] [Google Scholar]
- Riediger T, Traebert M, Schmid HA, Scheel C, Lutz TA, Scharrer E 2003 Site-specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci Lett 341:151–155 [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG 2004 Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101:4679–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camina JP, Carreira MC, El Messari S, Llorens-Cortes C, Smith RG, Casanueva FF 2004 Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue receptor 1a. Endocrinology 145:930–940 [DOI] [PubMed] [Google Scholar]
- Becskei C, Bilik KU, Klussmann S, Jarosch F, Lutz TA, Riediger T 2008 The anti-ghrelin Spiegelmer NOX-B11–3 blocks ghrelin- but not fasting-induced neuronal activation in the hypothalamic arcuate nucleus. J Neuroendocrinol 20:85–92 [DOI] [PubMed] [Google Scholar]
- Otto B, Tschop M, Heldwein W, Pfeiffer AF, Diederich S 2004 Endogenous and exogenous glucocorticoids decrease plasma ghrelin in humans. Eur J Endocrinol 151:113–117 [DOI] [PubMed] [Google Scholar]
- Sun Y, Ahmed S, Smith RG 2003 Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 23:7973–7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V 1999 Starvation: early signals, sensors, and sequelae. Endocrinology 140:4015–4023 [DOI] [PubMed] [Google Scholar]
- Kaneda T, Makino S, Nishiyama M, Asaba K, Hashimoto K 2001 Differential neuropeptide responses to starvation with ageing. J Neuroendocrinol 13:1066–1075 [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Min H, Tamraz S, Carmouche M, Boehme SA, Vale WW 1997 Anti-sexual and anxiogenic behavioral consequences of corticotropin-releasing factor overexpression are centrally mediated. Psychoneuroendocrinology 22:215–224 [DOI] [PubMed] [Google Scholar]
- Groenink L, Dirks A, Verdouw PM, Schipholt M, Veening JG, Van Der GJ, Olivier B 2002 HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol Psychiatry 51:875–881 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen AG, Rutters F 2008 The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav 94:169–177 [DOI] [PubMed] [Google Scholar]
- Warwick BP, Romsos DR 1988 Energy balance in adrenalectomized ob/ob mice: effects of dietary starch and glucose. Am J Physiol 255:R141–R148 [DOI] [PubMed] [Google Scholar]
- Asensio C, Muzzin P, Rohner-Jeanrenaud F 2004 Role of glucocorticoids in the physiopathology of excessive fat deposition and insulin resistance. Int J Obes Relat Metab Disord 28(Suppl 4):S45–S52 [DOI] [PubMed] [Google Scholar]
- Valassi E, Scacchi M, Cavagnini F 2008 Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 18:158–168 [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL 1991 Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 260:R200–R207 [DOI] [PubMed] [Google Scholar]
- Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA 2007 A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol 292:R291–R307 [DOI] [PubMed] [Google Scholar]
- Bonaz B, Taché Y 1994 Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res 652:56–64 [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Taché Y 2001 Central TRH receptor 1 antisense blocks cold-induced gastric emptying but not brain c-Fos induction. Peptides 22:81–90 [DOI] [PubMed] [Google Scholar]
- Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K, Arakawa T 2008 Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci 82:862–868 [DOI] [PubMed] [Google Scholar]
- Ahren B, Mansson S, Gingerich RL, Havel PJ 1997 Regulation of plasma leptin in mice: influence of age, high-fat diet, and fasting. Am J Physiol 273:R113–R120 [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P 1996 Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol 8:733–735 [DOI] [PubMed] [Google Scholar]
- Fekete C, Singru PS, Sanchez E, Sarkar S, Christoffolete MA, Riberio RS, Rand WM, Emerson CH, Bianco AC, Lechan RM 2006 Differential effects of central leptin, insulin, or glucose administration during fasting on the hypothalamic-pituitary-thyroid axis and feeding-related neurons in the arcuate nucleus. Endocrinology 147:520–529 [DOI] [PubMed] [Google Scholar]
- Taché Y 2002 The parasympathetic nervous system in the pathophysiology of the gastrointestinal tract. In: Bolis CL, Licinio J, eds. Handbook of autonomic nervous system in health and diseases. New York: Marcel Dekker, Inc.; 463–503 [Google Scholar]