Abstract
Hashimoto thyroiditis can be partially reproduced in mice by immunization with thyroglobulin or, more recently, thyroperoxidase. This experimental autoimmune thyroiditis (EAT) model has been extensively characterized during early disease phases (up to d 35 after immunization). By extending the analysis of EAT to 100 d after immunization, we noted a remarkable regenerative capacity of the thyroid and the expression of Oct-4, suggesting in vivo the existence of adult thyroid stem cells. After an almost complete destruction of the follicular architecture, occurring between d 21 and 28, the thyroid was capable of restoring its follicles and reducing the mononuclear infiltration, so that by d 100 after immunization, it regained its normal morphology and function. During this regeneration process, thyrocytes expressed high levels of CD24. We therefore assessed the role of CD24 in thyroid regeneration by inducing EAT in mice lacking CD24. Regeneration was faster in the absence of CD24, likely a consequence of the effect of CD24 on the infiltrating lymphocytes. The study suggests that the EAT model can also be used as a tool to investigate adult thyroid stem cells.
The murine thyroid is capable of restoring its architecture after an insult that almost completely destroys it.
Hashimoto thyroiditis, one of the most prevalent autoimmune diseases (1,2), was first described in 1912 in four women who underwent thyroidectomy because of a goiter characterized pathologically by four key features: marked lymphocytic infiltration (often organized into true lymphoid follicles with germinal centers), destruction of thyroid follicles, Hürthle cell metaplasia of the thyrocytes, and interstitial fibrosis (3). Some of these features were reproduced in 1956 by Rose and Witebsky (4) who immunized rabbits with thyroid extracts and established the first model of experimental autoimmune thyroiditis (EAT).
Since those classic experiments, EAT has appeared in hundreds of publications from numerous laboratories using various animal species. EAT of the mouse, first reported in 1968 (5,6), has quickly become the premiere model due to the richness of immunological and genetic mouse tools. EAT is induced by injecting thyroglobulin mixed to an adjuvant (usually complete Freund’s adjuvant or lipopolysaccharide) into mice of susceptible strains (like those having the H-2k or H-2s major histocompatibility complex haplotype). Mice are then typically killed between d 21 and 28 after the first immunization and analyzed for the outcome of interest.
Like any other animal model, EAT is not a perfect replica of the human disease. For example, EAT is considered to regress spontaneously and to lack signs of hypothyroidism (7), whereas Hashimoto thyroiditis is chronic and often associated with clinical hypothyroidism. Only a few papers, however, have analyzed the evolution of EAT beyond 1 month after immunization (8). Okayasu et al. (8), for example, followed CBA mice for 18 months noting that EAT did not regress but rather persisted for a long time with only a moderate decrease in thyroid lymphocytic infiltration and no decline in antibodies. Even fewer papers have assessed thyroid function during EAT (9).
We designed the first part of the study to characterize the evolution of EAT up to d 100 after immunization. We noted that after a virtually complete destruction, the thyroid is capable of regeneration, suggesting the presence and activation of adult thyroid stem cells. Given that CD24 has long been hypothesized to be a marker of gastrointestinal stem cells (10), that it is now known to be required for homeostatic cell renewal (11), and that we found it highly represented in a serial analysis of a gene expression thyroid library (unpublished data), we designed the second part of the study to assess the role of CD24 during EAT.
CD24 is a small protein (27 amino acids), anchored to the plasma membrane via a glycosyl-phosphatidylinositol linker (12); it is heavily and differentially glycosylated, so that its molecular weight ranges from 28–68 kDa (13,14). CD24 is expressed on a variety of tissues and has been studied in the fields of leukocyte biology, oncology, and regenerative medicine.
CD24, also known in mice as heat-stable antigen for its resistance to heat-induced denaturation (15), is expressed strongly on lymphocytes and dendritic cells, and binds to P-selectin, a receptor expressed on activated endothelial cells. It has been involved in cell adhesion (16), lymphocyte proliferation for homeostatic purposes (17), and lymphocyte costimulation via CD28-independent pathways (18). For example, transgenic expression of CD24 on central nervous system astrocytes enhances development of experimental autoimmune encephalomyelitis (19). Similarly, the absence of CD24 on dendritic cells of recipient mice that have been rendered lymphopenic induces a massive proliferation of donor T cells that rapidly kill the recipient (20).
More recently, CD24 has been proposed as a putative oncogene in gastrointestinal and other malignancies (21). It is, in fact, expressed early during carcinogenesis and when blocked with monoclonal antibodies or small interfering RNA, it reduces tumor growth in xenograft mouse models (22). The role of CD24 in regeneration is less characterized, although one study has shown that CD24 is expressed on human regenerating muscles (23).
Materials and Methods
The study was structured in two phases. The first phase used 139 CBA/J mice [H-2k haplotype from The Jackson Laboratory (Bar Harbor, ME); stock no. 000656] to characterize the evolution of EAT. The second phase used 89 C57BL6-H2k congenic mice (from The Jackson Laboratory; stock number 001148) to assess the role of CD24 in the thyroid regeneration observed during EAT.
EAT induction in CBA/J mice
EAT was induced in 2-month-old, female CBA/J mice using mouse thyroglobulin as the immunogen and complete Freund’s as the adjuvant. Mouse thyroglobulin was prepared by gel filtration chromatography, dissolved in PBS at a concentration of 1.5 mg/ml, and emulsified 1:1 in complete Freund’s adjuvant. The adjuvant (from Sigma Chemical Co., St. Louis, MO) was supplemented with Mycobacterium tuberculosis (strain H37Ra, from BD Biosciences, Franklin Lakes, NJ) to a final concentration of 5 mg/ml.
Mice were injected sc on d 0 and 7 with 100 μl of the emulsion and therefore received with each injection 75 μg gel-purified mouse thyroglobulin and 250 μg heat-killed M. tuberculosis. Mice were then killed on d 10 (n = 19), d 14 (n = 25), d 21 (n = 14), d 28 (n = 15), d 35 (n = 23), d 42 (n = 8), d 70 (n = 7), or d 100 (n = 12) after the first immunization to analyze three main outcome measures: thyroid histopathology, serum total T4, and thyroglobulin antibodies. In addition, eight mice were killed on d 35 after immunization for thyroidal BrdU incorporation and Oct-4 expression, comparing results in eight d-0 mice. Mice were maintained in the pathogen-free animal facility of the Johns Hopkins University School of Medicine, in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Thyroid histopathology
Thyroid histopathology was evaluated for two parameters: the presence of hematopoietic cells infiltrating into the thyroid parenchyma (infiltration score) and the presence of regenerating thyroid follicles (regeneration score). The infiltration score was expressed on a continuous scale from 0–100 as percentage of the total thyroid area, as previously described (24). The regeneration score was expressed on a discrete scale from 0 (no regeneration) to 3 (marked regeneration) based on the presence of follicular buds, microfollicles, and signs of glandular remodeling.
Serum total T4 level
Thyroid function was estimated by measuring serum total T4 (GammaCoat [125I]T4; Diasorin, Stillwater, MN). For the CBA/J strain, the normal T4 range was determined in 30 nonimmunized 2-month-old females and found to be comprised between 2.77 (5th percentile) and 5.15 (95th percentile) μg/dl. For the C57BL6-H2k congenic strain, the normal T4 range was determined in 31 nonimmunized 2-month-old females and found to be comprised between 2.10 (5th percentile) and 5.91 (95th percentile) μg/dl.
Subclass-specific thyroglobulin antibodies
The production of thyroglobulin antibodies, an expected event occurring after immunization with thyroglobulin, was measured to monitor the strength and quality of B-cell responses and to evaluate their correlation with thyroid function. Sera were diluted in PBS (1:100 for d 7, 10, 70, and 100; 1:400 for d 14 and 42; 1:1600 for d 21, 28, and 35) and added in triplicate to Immulon2 ELISA plates (Thermo Fisher Scientific, Inc., Waltham, MA) coated with 100 ng/well mouse thyroglobulin. The assay was performed as described (25), using alkaline phosphatase-conjugated secondary antibodies to IgG1, IgG2a, or IgG2b (MP Biomedicals, Solon, OH). Results are expressed as arbitrary units per milliliter and then analyzed for a transformation that converted the antibodies into a more normally distributed variable (which turned out to be the square root transformation).
Thyroidal bromodeoxyuridine (BrdU) incorporation and proliferating cell nuclear antigen (PCNA) detection in thyroid
Thyroidal BrdU incorporation was measured on d 35 after immunization (the peak of regeneration activity) and in nonimmunized (d 0) controls. BrdU (1 mg/20 g body weight) was injected ip twice a day for 4 consecutive days. After euthanasia, thyroids were dissected and fixed for 2 h in ice-cold 80% methanol and overnight in 10% neutral buffered formalin. After processing and paraffin embedding, 5-μm sections were cut, deparaffinized in xylene (three times for 5 min), and then rehydrated (100% ethanol for 3 min twice, 90% ethanol for 1 min, 70% ethanol for 1 min, 30% ethanol for 1 min, washing buffer for 1 min three times). Thyroid sections underwent the following pretreatment steps: DNA denaturation [2 n HCl at 37 C for 30 min followed by rinsing with Tris-buffered saline supplemented with 0.01% Tween 20 (TBS-T) for 2 min three times], neutralization (0.1 m Na2B4O7, pH 8.5, at 37 C for 2 min followed by rinsing three times with TBS-T for 2 min three times), and enzymatic digestion [0.1% (wt/vol) trypsin at 37 C for 20 min followed by rinsing with TBS-T for 2 min three times]. After pretreatment, thyroid sections were stained using the BrdU labeling and detection kit II (Roche Applied Science, Indianapolis, IN).
Thyroidal expression of PCNA was assessed by immunohistochemistry using 5-μm paraffin-embedded thyroid sections. Sections were deparaffinized in xylene, rehydrated in a graded series of ethanol (100, 90, 70, and 30%). After steaming in Target Retrieval Solution (Dako, Carpinteria, CA), sections were treated with 3% hydrogen peroxidase for 30 min to quench endogenous peroxidase. After 1 h incubation in 5% goat serum to block nonspecific binding, sections were incubated overnight at 4 C in a humid chamber with a rat monoclonal antibody to PCNA (clone PC10; Santa Cruz Biotechnology, Inc., Santa Cruz, CA; diluted 1:500 in PBS/1% BSA). After a wash in PBS, sections were incubated for 1 h at room temperature in a humid chamber with a biotinylated goat antimouse Ig antibody (Jackson ImmunoResearch, West Grove, PA; diluted 1:1000 in PBS/1% BSA). The sections were then incubated for 30 min at room temperature in a humid chamber with peroxidase-conjugated streptavidin (Dako; diluted 1:500) developed with diaminobenzidine substrate (Sigma), and counterstained with Mayer’s hematoxylin (Polyscientific, Bay Shore, NY).
Oct-4 mRNA thyroidal expression
Oct-4, a stem cell marker described recently in adult human thyroids (26), was measured by RT-PCR to estimate the presence of stem cells within the thyroid, comparing d 35 after immunization to nonimmunized mice. Thyroid lobes were harvested, frozen in liquid nitrogen, mechanically homogenized, and then digested with deoxyribonuclease I (Invitrogen, Carlsbad, CA). mRNA was purified by oligo-(deoxythymidine)25 magnetic beads (Invitrogen) and reverse transcribed using Superscript II (Invitrogen). The resulting cDNA was amplified by PCR (40 cycles) using Finnzymes Phusion high-fidelity DNA polymerase (New England BioLabs, Inc., Ipswich, MA), and the following primers: 5′-GGC GTT CTC TTT GGA AAG GTG TTC-3′ (forward) and 5′-CTC GAA CCA CAT CCT TCT CT-3′ (reverse). The Oct-4 amplicon was confirmed by sequencing the 313-bp band purified from an agarose gel. Mouse β-actin was amplified (30 cycles) as quantitation control using forward primer 5′-ATG AAG ATC CTG ACC GAG CG-3′ and reverse primer 5′-TAC TTG CGC TCA GGA GGA GC-3′. Briefly, cDNA from each sample was diluted and then amplified for β-actin. The dilutions that gave similar β-actin intensities were used for Oct-4 amplification.
CD24 protein expression on thyroid cells
Thyroidal expression of CD24 was assessed by immunohistochemistry using 5-μm zinc-fixed, paraffin-embedded thyroid sections attached to SuperFrost plus slides (Thermo Fisher Scientific). Sections were deparaffinized in xylene, rehydrated in a graded series of ethanol (100, 90, 70, and 30%), and treated with 3% hydrogen peroxidase for 30 min to quench endogenous peroxidase. After 1 h incubation in 5% goat serum to block nonspecific binding, sections were incubated overnight at 4 C in a humid chamber with a rat monoclonal antibody to mouse CD24 (clone M1/69; BD Bioscience; diluted 1:100 in PBS/1% BSA). After a wash in PBS, sections were incubated for 1 h at room temperature in a humid chamber with a biotinylated goat antirat Ig antibody (Jackson ImmunoResearch; diluted 1:1000 in PBS/1% BSA). The sections were then incubated for 30 min at room temperature in a humid chamber with peroxidase-conjugated streptavidin (Dako; diluted 1:500), developed with diaminobenzidine substrate (Sigma), and counterstained with Mayer’s hematoxylin (Polyscientific).
Use of CD24 knockout mice in the EAT model
CD24 knockout mice, a gift of Dr. Yang Liu from Ohio State University, were originally made on the C57BL/6J (H-2b) background, known to be resistant to the induction of EAT (27). We therefore backcrossed them for six generations to C57BL/6J-H-2k (Jackson Laboratory; stock number 001148), a congenic strain that has the susceptible H-2k haplotype on the BL6 background. Mice were screened (supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http:// endo.endojournals.org) by PCR and flow cytometry on peripheral blood CD45-positive leukocytes for the expression of CD24 (phycoerythrin-conjugated anti-CD24 reagent by BioLegend, San Diego, CA) and the H-2 haplotype, using fluorescein isothiocyanate-conjugated anti-H-2b (BD Biosciences) and phycoerythrin-conjugated anti-H-2k (AbD Serotec, Raleigh, NC) reagents and immunohistochemistry as described above.
EAT was induced in 36 CD24-H2k knockout mice and 53 wild-type littermates, following the protocol described above for the CBA strain. Mice were killed to assess thyroid histopathology, serum total T4, and thyroglobulin antibodies, on the following days after the first immunization: d 7 (n = 3: two wild type, one knockout), d 12 (n = 9: five wild type, four knockout), d 14 (n = 23: 16 wild type, seven knockout), d 16 (n = 14: seven wild type, seven knockout), d 18 (n = 8: four wild type, four knockout), d 21 (n = 13: eight wild type, five knockout), d 35 (n = 11: seven wild type, four knockout), and d 42 (n = 8: four wild type, four knockout).
Statistical analysis
Relationships between the outcomes and time after immunization as well as comparison between genotypes were explored using multiple linear regression modeling as previously described (28). All analyses were performed using Stata statistical software, release 10 (from Stata Corp., College Station, TX).
Results
The murine thyroid is capable of regeneration
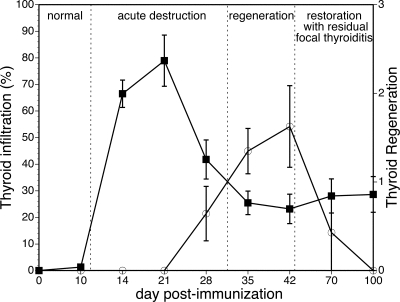
A detailed study of EAT beyond the canonical d 21–28 endpoints allowed us to identify a remarkable regeneration capacity of the murine thyroid gland. This observation led us to classify EAT into four broad stages: normal thyroid, acute destruction, regeneration, and restoration (Fig. 1).
Figure 1.
Thyroid regeneration and EAT progression in CBA/J mice. Thyroid infiltration (left y-axis, ▪) and regeneration (right y-axis, ○) are shown as mean ± se according to the day after thyroglobulin immunization.
Normal, baseline, stage (up to d 10)
A normal thyroid structure still prevailed up to d 10 after immunization. Round, uniform follicles were evenly distributed in a delicate interstitial stroma free of infiltrating cells (data not shown). However, three of 19 mice showed a sign of the beginning of infiltration (Fig. 2A).
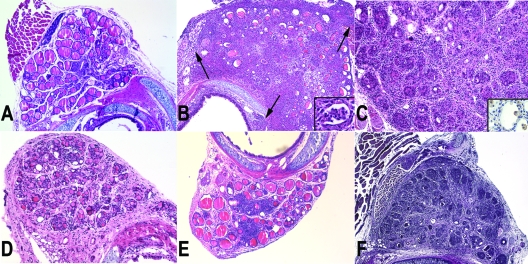
Figure 2.
A, Day 10 after immunization, hematoxylin and eosin (H&E) stain; magnification, ×20. Infiltration of mononuclear cells started around vessel (arrow). B, Day 14 after immunization, H&E stain; magnification, ×20. Note the massive infiltration of the thyroid with hematopoietic cells, extending also to the perithyroidal tissues (arrows), and the loss of thyroid follicles. The inset shows the presence of colloidal micro-abscesses. C, Day 35 after immunization, H&E stain; magnification, ×40. Note the appearance of compact, cellular, regenerating thyroid follicles. The higher magnification shows that at this stage, the infiltrate is mainly mononuclear in nature. The inset shows intrafollicular macrophages that stain positive for F4/80. D, Day 70 after immunization, H&E stain; magnification, ×20. The thyroid has largely restored its follicular architecture, and many small follicles are observed. E, Day 100 after immunization, H&E stain; magnification, ×20. The thyroid has largely restored its follicular architecture, although foci of mononuclear infiltration remain. F, Day 35 after immunization, van Gieson stain; magnification, ×20. The stain highlights the largely conserved connective tissue framework among which appear the budding thyroid follicles.
Acute destruction stage (d 14–28)
During this stage, the thyroid became intensely infiltrated by hematopoietic cells and lost most of its follicles (Fig. 2B). The infiltrate included both acute (neutrophils) and chronic (lymphocytes) components: neutrophils were mostly intrafollicular, often permeating the thyroid epithelium and forming colloidal micro-abscesses (Fig. 2B, inset), whereas lymphocytes populated the interstitium. In areas of severe follicular destruction, the inflammation extended into the surrounding perithyroidal soft tissues (Fig. 2B, arrows). The degree of follicular dropout and destruction varied according to the extent of the hematopoietic infiltration, ranging from patchy (around 30% of the total thyroid area on d 28) to near complete effacement of the thyroid architecture.
Regeneration stage (d 35–42)
The prevailing feature of this stage was the emergence of compact, cellular (colloid-less) follicles, suggestive of active thyrocyte regeneration (Fig. 2C). Follicles arose as rosettes within a connective tissue framework that remained conserved, even at the peak of glandular destruction (Fig. 2F). Inflammation, although less severe than that of the acute destruction phase, was still prominent during this stage and mostly lymphocytic (Fig. 2C). Intrafollicular macrophages (Fig. 2C, inset) replaced the neutrophils seen during the acute destruction phase. There was a mild increase in stromal fibrosis, without broad areas of collagen deposition.
Restoration stage (d 70–100)
During the restoration stage (d 70), most of the thyroid parenchyma returned to its normal architecture, but patchy chronic inflammation and intermittent follicle dropout persisted in small foci (Fig. 2D). Thyroidal colloids with normal size were observed, and thyroid architecture was improved compared with an acute destruction stage, although a modest lymphocytic infiltration still remained on d 100 after immunization (Fig. 2E). It is perhaps this late, rarely studied, phase of EAT that is the one more reminiscent of the human Hashimoto thyroiditis, in particular of the asymptomatic forms of focal thyroiditis found in autopsy and surgical specimens.
PCNA expression and BrdU incorporates into regenerating thyroid follicles
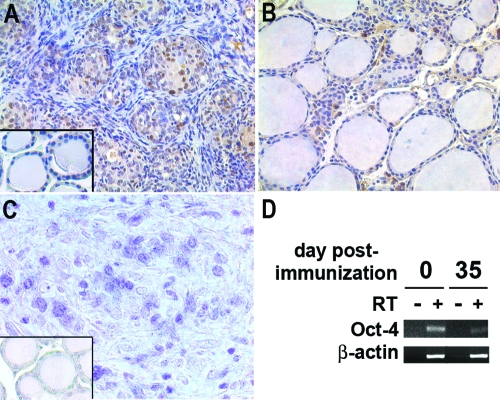
To better characterize the regenerating capacity of thyrocytes, PCNA expression was examined in thyroids on d 0, 35, and 100 after immunization. Thyrocytes in nonimmunized mice were largely negative (Fig. 3A, inset). PCNA-positive thyrocytes were found in the regenerating rosettes on d 35 after immunization (Fig. 3A), reflecting active thyrocyte proliferation. PCNA-positive thyrocytes were significantly decreased on d 100 after immunization (Fig. 3B), suggesting that the regeneration was not active in this period. Additionally, BrdU was injected daily for 4 consecutive days and then quantified in the thyroid gland to evaluate cell proliferation. Several BrdU-positive thyrocytes were found in the regenerating rosettes on d 35 after immunization, reflecting active thyrocyte proliferation (Fig. 3C). In contrast, thyrocytes were largely BrdU negative in nonimmunized controls (Fig. 3C, inset). The stem cell marker Oct-4 mRNA, although detectable both at baseline (d 0) and on d 35 after immunization, decreased significantly on d 35 (Fig. 3D), suggesting a commitment of the thyroid progenitors present at baseline to thyrocyte differentiation. Oct-4 expression is, in fact, known to decline once stem cells begin differentiation (29).
Figure 3.
PCNA expression, BrdU uptake, and Oct-4 expression during EAT of CBA/J mice. A, High level of PCNA expression is observed in thyroid on d 35 after immunization. Dark brown nuclei represent PCNA positivity. PCNA-positive cells with high grade locate in follicles arose as rosettes rather than a connective tissue framework. The inset shows for comparison PCNA-negative thyrocytes before immunization. B, PCNA-positive cells decrease in thyroid on d 100 after immunization. Most of thyrocytes (follicular cells) are PCNA negative. C, Regenerating thyrocytes are BrdU positive on d 35 after immunization. Dark blue nuclei represent BrdU positivity. The inset shows for comparison BrdU-negative thyrocytes before immunization. D, Oct-4 mRNA expression by semiquantitative PCR. Note the decrease in Oct-4 transcript on d 35 after immunization.
Thyroid function initially decreases, then increases, and finally normalizes during EAT
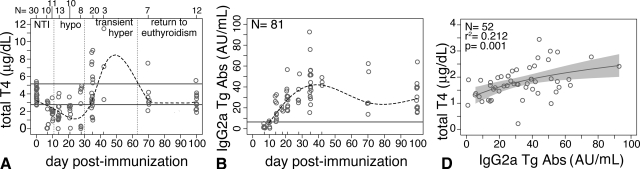
Thyroid function decreased rapidly during the first 10 d after the first immunization (Fig. 4A), a decrease known to be due to the nonthyroidal illness induced by the adjuvant (30). From d 10–30 after immunization, thyroid function then remained significantly lower than normal (Fig. 4A), likely a consequence of the marked loss and destruction of thyroid follicles.
Figure 4.
Serum T4 and thyroglobulin antibodies during EAT of CBA/J mice. A, Thyroid function changes repeatedly after immunization; it decreases initially due to the nonthyroidal illness (NT) effect, remains low during the destructive phase of EAT, then increases transiently, and finally normalizes after d 70. B, IgG2a thyroglobulin-specific antibodies. Thyroglobulin antibodies increase as expected after immunization and remain elevated up to 3 months thereafter. C, T4 levels positively and significantly correlates with thyroglobulin antibody levels.
Beginning on d 35, thyroid function started to return to normal levels, normalizing between d 70 and 100 in the majority of the mice (13 of 19, 68%, Fig. 4A). The regeneration stage, however, was notable for the presence of frank hyperthyroidism in nine of 23 (39%, Fig. 4A) mice. T4 levels did not correlate with the degree of regeneration (data not shown).
Thyroglobulin antibodies positively correlate with T4 during EAT
IgG2a thyroglobulin-specific antibodies became clearly elevated on d 14 after immunization, reached a peak between d 28 and 35, and then decreased gradually up to d 100, still remaining significantly higher than the preimmunization levels (Fig. 4B). The trend was similar for the G1 (data not shown) and G2b (data not shown) Ig subclasses, although at lower titers.
Interestingly, thyroglobulin antibodies positively correlated with T4, with the highest antibody levels seen with the most severe hyperthyroidism (P = 0.001 for IgG2a, Fig. 4C; P = 0.006 for IgG1; and P = 0.039 for IgG2b).
Lack of CD24 accelerates recovery from EAT
CD24 was constitutively expressed on normal thyrocytes (Fig. 5A) and persisted throughout the evolution of EAT. CD24 clearly identified the emergence of regenerating follicles on d 35 after immunization (Fig. 5B). We therefore assessed the role of CD24 in EAT and thyroid regeneration using CD24-H2k knockout mice.
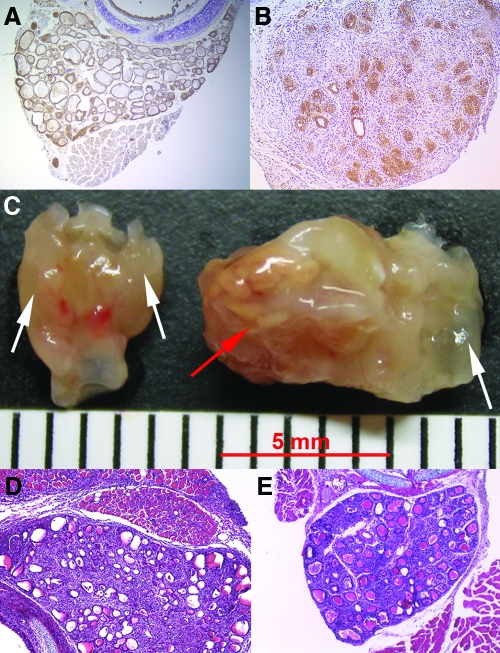
Figure 5.
CD24 expression on the thyroid and EAT development in CD24-H2k knockout mice and wild-type controls. A, CD24 is expressed in normal baseline thyroids and throughout the course of EAT. B, CD24 clearly identifies the regenerating thyrocytes on d 35 after immunization. C, Gross appearance of the thyroid gland in the two genotypes during the initiation of EAT. Note the more severe infiltration of the perithyroidal soft tissues in the CD24-deficient genotype. D, Day 14 after immunization in CD24-H2k knockout mice, hematoxylin and eosin (H&E) stain; magnification, ×20. Note the severe infiltration of the thyroid and the perithyroidal structures. E, Day 42 after immunization in wild-type littermates, H&E stain; magnification, ×20. The thyroid has recovered its normal architecture but still shows foci of infiltration, whereas it was completely normal in CD24-H2k knockout mice (not shown).
EAT kinetics developed earlier in the C57BL6-H2k strain than in the classical CBA strain, with frank thyroid lesions appearing on d 12 after immunization and recovery of thyroid morphology by d 35 (Fig. 6A).
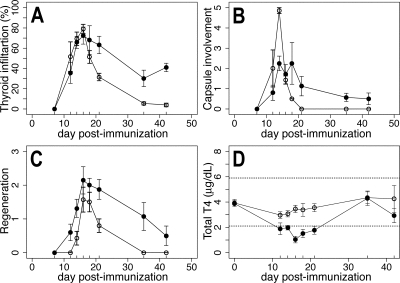
Figure 6.
Scores of EAT and thyroidal function in CD24-H2k knockout mice and wild-type controls. A, Infiltration scores. EAT develops similarly in CD24-H2k knockout mice (○) and wild-type controls (•) but ameliorates more rapidly in the absence of CD24. B, Capsular involvement scores. CD24-H2k knockout mice (○) feature a more severe infiltration of the perithyroidal capsule than wild-type littermates (•) during the initiation of EAT. C, Thyroid regeneration scores in CD24-H2k knockout (○) and wild-type littermates (•). D, Serum T4 levels in CD24-H2k knockout (○) and wild-type littermates (•).
EAT developed similarly in CD24-H2k knockout and wild-type controls up to d 16 after immunization (Fig. 6A), although it tended to be more severe in the absence of CD24. Particularly striking was the involvement of the thyroid capsule on d 14 after immunization (Fig. 6B). The thyroid capsule of CD24-H2k knockout mice was so massively infiltrated that a clean dissection of the gland from the surrounding anatomical structures, including trachea and sterno-thyroid muscles, was impossible (Fig. 5D). The mononuclear infiltration was limited to the thyroid region and not found in more distant structures, such as pancreas and quadriceps muscle (data not shown). After d 16, CD24 knockout mice showed a prompt recovery of the normal thyroid morphology so that by d 42 after immunization their thyroids appeared completely normal (Fig. 6A, ○), whereas wild-type controls still showed scattered foci of lymphocytic infiltration (Figs. 5E and 6A). These findings suggest that retention and survival of infiltrating cells in the thyroid are impaired in the absence of CD24, in keeping with its role in cell adhesion (as a ligand for P-selectin) and lymphocyte proliferation (21).
Regeneration occurred equally well in the thyroids of CD24 knockout mice and wild-type controls, indicating that expression of CD24 on thyrocytes is not required for their efficient regeneration, but it was accelerated in the absence of CD24 (Fig. 6C, ○), likely a consequence of the milder hematopoietic infiltration.
Total T4 was similar at baseline in CD24 knockout and wild-type controls, indicating that the expression of CD24 on thyrocytes is not required for thyroid hormonogenesis. T4 was also similar between the two genotypes on d 35 and 42 after immunization, a time when the thyroid architecture has been, to some degree, restored. Between d 14 and 21, however, T4 remained in the normal range in CD24 knockout mice (Fig. 6D, ○), whereas it decreased as expected in wild-type controls (Fig. 6D, •). The better thyroid function is in keeping with the more prompt and complete thyroid regeneration seen in CD24 knockout mice. Thyroglobulin antibodies developed similarly in CD24 knockout and wild-type controls (data not shown), indicating that CD24 is not required for autoantibody production.
Discussion
Our study mainly reports the ability of the murine thyroid gland to regenerate after an insult that almost completely destroyed its follicular architecture. This architecture was effaced between d 21 and 28 after immunization but emerged on d 35 with small regenerating follicles that expressed PCNA and incorporated BrdU, gradually restoring the normal morphology by d 70.
Thyroid regeneration could arise from duplication of the residual normal follicles, although very few were seen at the peak of destruction (between d 21 and 28) or from the activation of adult thyroid stem cells. We detected mRNA expression of Oct-4 (a well-established stem cell marker) in d 0 mouse thyroids, suggesting that the adult mouse thyroid does indeed contain stem cells. In addition, Oct-4 expression decreased on d 35 after immunization, in keeping with the notion that Oct-4 expression decreases when the stem cell begins to differentiate (29).
Stem cells residing in an adult organ (adult stem cells) are extremely attractive because they can in principle be used as an alternative to embryonic stem cells for tissue regeneration (31). Adult stem cells of the human thyroid gland were first postulated in 1992 (32), then suspected in 2003 by the demonstration of p63 expression in solid cell nests of the thyroid (33), and finally proven in 2006 by the Oct-4, Gata-4, HNF4α, and Pax8 expression in cultured cells derived from goiters (26). Three additional publications have identified adult thyroid stem cells, the first two using side population cells sorted by the Hoechst 33342 efflux technique, the third one with thyrospheres. In humans, Lan and colleagues (34) isolated stem cells from primary thyroid cultures of goitrous thyroids and grew them as monolayers or embedded in collagen. In response to TSH and serum, these cells differentiated into three-dimensional spheres that expressed thyroglobulin, Pax8, sodium iodide symporter, TSH receptor, and thyroperoxidase mRNA and showed TSH-dependent [125I]iodide uptake (34). In mice, Hoshi and colleagues (35) showed the presence of side population cells expressing stem cell markers and low levels of thyroid differentiation markers. Very recently, Fierabracci et al. (36) reported the isolation of CD34+ CD45− thyrospheres from surgical thyroid specimens, endowed with the ability to generate thyroid cells and also nonthyroidal cells. Our morphological findings and the Oct-4 mRNA suggest the presence of adult stem cells in the mouse thyroid that can initiate differentiation after a severe insult. Further studies are necessary to demonstrate the identity of the Oct-4-expressing cells, their isolation, and the more detailed contribution to the regeneration observed in EAT.
An additional contribution of our study is the novel observation that a transient hyperthyroidism occurs after thyroglobulin immunization (around d 35–42). The mechanism for this finding, which was not the initial focus of the paper, remains unknown. Considering that the hyperthyroidism was most severe in mice with the highest thyroglobulin antibody levels, it could be caused by the appearance of thyroid-stimulating antibodies, as seen in patients with Graves disease. Measurements of thyroid stimulating antibodies in EAT and adoptive transfer of d 35–42 sera will help elucidating whether EAT, long used as a model of Hashimoto thyroiditis, can also serve as a model of Graves hyperthyroidism. Another possible mechanism is that the small, regenerating thyroid follicular buds, with their limited colloid content, release T4 immediately upon synthesis, without storing it in the colloid. We did not find, however, a significant correlation between T4 levels and the magnitude of thyroid regeneration.
Finally, the study highlighted the role of CD24 in thyroid regeneration and EAT development. CD24, although expressed on regenerating thyrocytes, was not required for the efficient thyroid regeneration occurring after the nearly complete autoimmune destruction. On the contrary, CD24 significantly altered the course of EAT. CD24-deficient mice developed a more aggressive EAT during the initial phases but cleared the hematopoietic infiltrate more promptly and restored a normal thyroid morphology sooner. These results suggest that CD24 is required to maintain the pathogenic infiltrate within the target organ (37) and that therapies aimed to block CD24 may be beneficial for treating autoimmune diseases, as have been therapies that block TNF-α (38). Other groups have successfully exploited other molecules, such as IL-10, for the resolution of experimental thyroiditis (39,40,41).
In summary, we provide additional evidence of regeneration in the mouse thyroid and propose the use of the tried and true EAT model as a tool to investigate adult thyroid stem cells.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant DK55670 and DK080334 (to P.C.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 18, 2008
Abbreviations: BrdU, Bromodeoxyuridine; EAT, experimental autoimmune thyroiditis; PCNA, proliferating cell nuclear antigen; TBS-T, Tris-buffered saline supplemented with 0.01% Tween 20.
References
- Jacobson DL, Gange SJ, Rose NR, Graham NM 1997 Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84:223–243 [DOI] [PubMed] [Google Scholar]
- Cooper GS, Stroehla BC 2003 The epidemiology of autoimmune diseases. Autoimmun Rev 2:119–125 [DOI] [PubMed] [Google Scholar]
- Hashimoto H 1912 Zur Kenntniss der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa). Arch Klin Chirurg 97:219–248 [Google Scholar]
- Rose NR, Witebsky E 1956 Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol 76:417–421 [PubMed] [Google Scholar]
- Twarog FJ, Rose NR 1968 The production of thyroid autoantibodies in mice. J Immunol 101:242–250 [PubMed] [Google Scholar]
- Nakamura RM, Weigle WO 1968 Experimental thyroiditis in complement intact and deficient mice following injections of heterologous thyroglobulins without adjuvant. Proc Soc Exp Biol Med 129:412–416 [DOI] [PubMed] [Google Scholar]
- Wang SH, Bretz JD, Phelps E, Mezosi E, Arscott PL, Utsugi S, Baker Jr JR 2002 A unique combination of inflammatory cytokines enhances apoptosis of thyroid follicular cells and transforms nondestructive to destructive thyroiditis in experimental autoimmune thyroiditis. J Immunol 168:2470–2474 [DOI] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Kong YC 1989 Long-term observation and effect of age on induction of experimental autoimmune thyroiditis in susceptible and resistant mice. Clin Immunol Immunopathol 53:254–267 [DOI] [PubMed] [Google Scholar]
- Vladutiu AO, Kenney EM 1985 Thyroid function in mice with experimental autoimmune thyroiditis. Clin Exp Immunol 61:257–264 [PMC free article] [PubMed] [Google Scholar]
- Akashi T, Shirasawa T, Hirokawa K 1994 Gene expression of CD24 core polypeptide molecule in normal rat tissues and human tumor cell lines. Virchows Arch 425:399–406 [DOI] [PubMed] [Google Scholar]
- Nieoullon V, Belvindrah R, Rougon G, Chazal G 2007 Mouse CD24 is required for homeostatic cell renewal. Cell Tissue Res 329:457–467 [DOI] [PubMed] [Google Scholar]
- Kay R, Takei F, Humphries RK 1990 Expression cloning of a cDNA encoding M1/69-J11d heat-stable antigens. J Immunol 145:1952–1959 [PubMed] [Google Scholar]
- Alterman LA, Crispe IN, Kinnon C 1990 Characterization of the murine heat-stable antigen: an hematolymphoid differentiation antigen defined by the J11d, M1/69 and B2A2 antibodies. Eur J Immunol 20:1597–1602 [DOI] [PubMed] [Google Scholar]
- Rougon G, Alterman LA, Dennis K, Guo XJ, Kinnon C 1991 The murine heat-stable antigen: a differentiation antigen expressed in both the hematolymphoid and neural cell lineages. Eur J Immunol 21:1397–1402 [DOI] [PubMed] [Google Scholar]
- Springer T, Galfre G, Secher DS, Milstein C 1978 Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol 8:539–551 [DOI] [PubMed] [Google Scholar]
- Aigner S, Ruppert M, Hubbe M, Sammar M, Sthoeger Z, Butcher EC, Vestweber D, Altevogt P 1995 Heat stable antigen (mouse CD24) supports myeloid cell binding to endothelial and platelet P-selectin. Int Immunol 7:1557–1565 [DOI] [PubMed] [Google Scholar]
- Li O, Zheng P, Liu Y 2004 CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med 200:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbe M, Altevogt P 1994 Heat-stable antigen/CD24 on mouse T lymphocytes: evidence for a costimulatory function. Eur J Immunol 24:731–737 [DOI] [PubMed] [Google Scholar]
- Liu JQ, Carl Jr JW, Joshi PS, RayChaudhury A, Pu XA, Shi FD, Bai XF 2007 CD24 on the resident cells of the central nervous system enhances experimental autoimmune encephalomyelitis. J Immunol 178:6227–6235 [DOI] [PubMed] [Google Scholar]
- Li O, Chang X, Zhang H, Kocak E, Ding C, Zheng P, Liu Y 2006 Massive and destructive T cell response to homeostatic cue in CD24-deficient lymphopenic hosts. J Exp Med 203:1713–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen G, Sammar M, Altevogt P 2004 Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol 35:255–262 [DOI] [PubMed] [Google Scholar]
- Sagiv E, Starr A, Rozovski U, Khosravi R, Altevogt P, Wang T, Arber N 2008 Targeting CD24 for treatment of colorectal and pancreatic cancer by monoclonal antibodies or small interfering RNA. Cancer Res 68:2803–2812 [DOI] [PubMed] [Google Scholar]
- Figarella-Branger D, Moreau H, Pellissier JF, Bianco N, Rougon G 1993 CD24, a signal-transducing molecule expressed on human B lymphocytes, is a marker for human regenerating muscle. Acta Neuropathol 86:275–284 [DOI] [PubMed] [Google Scholar]
- Caturegli P, Rose NR, Kimura M, Kimura H, Tzou SC 2003 Studies on murine thyroiditis: new insights from organ flow cytometry. Thyroid 13:419–426 [DOI] [PubMed] [Google Scholar]
- Kimura H, Kimura M, Tzou SC, Chen YC, Suzuki K, Rose NR, Caturegli P 2005 Expression of class II major histocompatibility complex molecules on thyrocytes does not cause spontaneous thyroiditis but mildly increases its severity after immunization. Endocrinology 146:1154–1162 [DOI] [PubMed] [Google Scholar]
- Thomas T, Nowka K, Lan L, Derwahl M 2006 Expression of endoderm stem cell markers: evidence for the presence of adult stem cells in human thyroid glands. Thyroid 16:537–544 [DOI] [PubMed] [Google Scholar]
- Vladutiu AO, Rose NR 1971 Autoimmune murine thyroiditis relation to histocompatibility (H-2) type. Science 174:1137–1139 [DOI] [PubMed] [Google Scholar]
- Kimura H, Kimura M, Tzou SC, Chen YC, Suzuki K, Rose NR, Caturegli P 2005 Expression of class II major histocompatibility complex molecules on thyrocytes does not cause spontaneous thyroiditis but mildly increases its severity after immunization. Endocrinology 146:1154–1162 [DOI] [PubMed] [Google Scholar]
- Schoorlemmer J, Jonk L, Sanbing S, van Puijenbroek A, Feijen A, Kruijer W 1995 Regulation of Oct-4 gene expression during differentiation of EC cells. Mol Biol Rep 21:129–140 [DOI] [PubMed] [Google Scholar]
- Rocchi R, Kimura H, Tzou SC, Suzuki K, Rose NR, Pinchera A, Ladenson PW, Caturegli P 2007 Toll-like receptor-MyD88 and Fc receptor pathways of mast cells mediate the thyroid dysfunctions observed during nonthyroidal illness. Proc Natl Acad Sci USA 104:6019–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbling M, Estrov Z 2003 Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med 349:570–582 [DOI] [PubMed] [Google Scholar]
- Dumont JE, Lamy F, Roger P, Maenhaut C 1992 Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev 72:667–697 [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS, Preto A, Soares P, Ricardo S, Cameselle-Teijeiro J, Sobrinho-Simoes M 2003 p63 expression in solid cell nests of the thyroid: further evidence for a stem cell origin. Mod Pathol 16:43–48 [DOI] [PubMed] [Google Scholar]
- Lan L, Cui D, Nowka K, Derwahl M 2007 Stem cells derived from goiters in adults form spheres in response to intense growth stimulation and require thyrotropin for differentiation into thyrocytes. J Clin Endocrinol Metab 92:3681–3688 [DOI] [PubMed] [Google Scholar]
- Hoshi N, Kusakabe T, Taylor BJ, Kimura S 2007 Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology 148:4251–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierabracci A, Puglisi MA, Giuliani L, Mattarocci S, Gallinella-Muzi M 2008 Identification of an adult stem/progenitor cell-like population in the human thyroid. J Endocrinol 198:471–487 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zheng P 2007 CD24: a genetic checkpoint in T cell homeostasis and autoimmune diseases. Trends Immunol 28:315–320 [DOI] [PubMed] [Google Scholar]
- Chen K, Wei Y, Sharp GC, Braley-Mullen H 2007 Decreasing TNF-α results in less fibrosis and earlier resolution of granulomatous experimental autoimmune thyroiditis. J Leukoc Biol 81:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, DeMarco VG, Sharp GC, Braley-Mullen H 2007 Expression of transgenic FLIP on thyroid epithelial cells inhibits induction and promotes resolution of granulomatous experimental autoimmune thyroiditis in CBA/J mice. Endocrinology 148:5734–5745 [DOI] [PubMed] [Google Scholar]
- Fang Y, Wei Y, Demarco V, Chen K, Sharp GC, Braley-Mullen H 2007 Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis in DBA/1 mice. Am J Pathol 170:875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Sharp GC, Braley-Mullen H 2008 Interleukin-10 promotes resolution of granulomatous experimental autoimmune thyroiditis. Am J Pathol 172:1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.