Abstract
Stress-like elevations in plasma glucocorticoids suppress gonadotropin secretion and can disrupt ovarian cyclicity. In sheep, cortisol acts at the pituitary to reduce responsiveness to GnRH but does not affect GnRH pulse frequency in the absence of ovarian hormones. However, in ewes during the follicular phase of the estrous cycle, cortisol reduces LH pulse frequency. To test the hypothesis that cortisol reduces GnRH pulse frequency in the presence of ovarian steroids, the effect of cortisol on GnRH secretion was monitored directly in pituitary portal blood of follicular phase sheep in the presence and absence of a cortisol treatment that elevated plasma cortisol to a level observed during stress. An acute (6 h) cortisol increase in the midfollicular phase did not lower GnRH pulse frequency. However, a more prolonged (27 h) increase in cortisol beginning just before the decrease in progesterone reduced GnRH pulse frequency by 45% and delayed the preovulatory LH surge by 10 h. To determine whether the gonadal steroid milieu of the follicular phase enables cortisol to reduce GnRH pulse frequency, GnRH was monitored in ovariectomized ewes treated with estradiol and progesterone to create an artificial follicular phase. A sustained increment in plasma cortisol reduced GnRH pulse frequency by 70% in this artificial follicular phase, in contrast to the lack of an effect in untreated ovariectomized ewes as seen previously. Thus, a sustained stress-like level of cortisol suppresses GnRH pulse frequency in follicular phase ewes, and this appears to be dependent upon the presence of ovarian steroids.
A sustained stress-like level of cortisol reduces GnRH pulse frequency in the follicular phase and appears to require the presence of ovarian steroids.
Stress activates the hypothalamic-pituitary-adrenal axis, stimulating secretion of glucocorticoids such as cortisol, and this may reduce pulsatile LH secretion (1,2,3). Recent studies in ovariectomized sheep indicate that either psychosocial stress, or an increment in plasma cortisol comparable to that attained during psychosocial stress, acutely reduces LH pulse amplitude by suppressing pituitary responsiveness to GnRH (4,5,6,7,8). Importantly, this action of cortisol, which is expressed directly at the level of the pituitary gland via the type II glucocorticoid receptor (4,9), is necessary for the reduction in pituitary responsiveness to GnRH during psychosocial stress (5).
In contrast to strong evidence that cortisol inhibits reproductive neuroendocrine function at the level of the pituitary gland, a suppressive effect of cortisol on GnRH secretion has not been definitively identified. A hypothalamic effect has been inferred indirectly by the finding that the suppressive effect of long-term (months) exposure to cortisol on gonadotropin secretion in orchidectomized monkeys could not be accounted for by inhibition of pituitary responsiveness to GnRH (10). However, that finding does not definitively demonstrate a hypothalamic effect because GnRH secretion was not monitored. Direct monitoring of pulsatile GnRH secretion in pituitary portal blood has revealed that acute (6 h) exposure to a stress-like level of plasma cortisol did not inhibit episodic GnRH secretion in ovariectomized ewes devoid of ovarian steroids, although this treatment did reduce LH pulse amplitude (6). However, during the follicular phase of the ovarian cycle, cortisol did reduce LH pulse frequency in sheep and women (11,12). Although these collective findings suggest that cortisol can reduce GnRH pulse frequency, but ovarian steroids are required to sensitize the GnRH neurosecretory system to cortisol, this is not necessarily the case. Given that GnRH and LH pulses in the follicular phase are very low in amplitude (13,14) and that cortisol suppresses pituitary responsiveness to GnRH as described above, cortisol might lower amplitude of LH pulses to the point that they are no longer detectable. In that case the reduction in frequency of LH pulses would actually reflect a pituitary effect.
The initial goal of this study was to determine whether cortisol has a hypothalamic effect to reduce GnRH pulse frequency during the follicular phase of the estrous cycle. For this purpose we took advantage of the ability to monitor the GnRH secretory profile directly in serial samples of pituitary portal blood of ewes and conducted two experiments to determine whether either acute (6 h) or prolonged (27 or 31 h) exposure to cortisol reduces GnRH pulse frequency during the follicular phase. The second objective was to test the hypothesis that the gonadal steroid milieu of the follicular phase enables cortisol to reduce GnRH pulse frequency.
Materials and Methods
Animals and experimental procedures
Three experiments were conducted in the breeding season (October through December 2003, 2004, and 2006) using adult Suffolk ewes maintained under standard husbandry conditions at the Sheep Research Facility near Ann Arbor, MI. The ewes were fed hay and alfalfa pellets, and had free access to water and mineral licks. Expression of estrus, which begins on the day of the preovulatory LH surge (15), was determined using vasectomized rams. Surgical procedures were conducted under aseptic conditions using halothane nitrous oxide anesthesia. All procedures were approved by the Committee for the Use and Care of Animals at the University of Michigan.
The surgical preparation for installing the apparatus to collect pituitary portal blood has been described by Caraty et al. (16). After surgery, the ewes were housed in rooms in which photoperiod was controlled to simulate that of the outdoors. One to 4 d before sampling, ewes were moved to a collection room and individually penned adjacent to one or two companion sheep to avoid isolation stress. Each ewe was equipped with two indwelling jugular catheters, one for collecting peripheral blood and one for infusing heparinized saline (640 U/ml infused at a rate of 250 U/min) with or without added cortisol (Solu-Cortef, hydrocortisone sodium succinate, aqueous solution, 125 mg/ml; Pharmacia & Upjohn, Kalamazoo, MI). This dose was targeted to achieve a plasma cortisol concentration (∼80 ng/ml) within the range that we observe during psychosocial and immuno/inflammatory stress (5,17,18). For each of the three experiments, a pilot study was performed to check that the cortisol preparation achieved the desired plasma cortisol concentration, and the infusion rate was adjusted as necessary (rate specified in description of each experiment).
Sampling of pituitary portal and jugular blood was performed 2 wk after surgery on two to three sheep at a time using a remote, automated sampling procedure (16) in which investigators were in a room separate from the animals. This procedure permits collection of pituitary portal blood from fully conscious and nonsedated ewes that are not compromised physiologically, and appear to be nonstressed based on their behavior and a low basal plasma cortisol concentration (6). Blood was withdrawn continuously and dispensed into 5-min (experiment 3) or 6-min fractions (experiments 1 and 2) for analysis of LH and cortisol (jugular blood) and GnRH (portal blood). After sample collection, ewes were euthanized with a barbiturate overdose (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI), and the pituitary was inspected to confirm appropriate placement of the cut in the portal vasculature.
Synchronization of follicular phase endocrine events
The ovine estrous cycle is 16–17 d in length, consisting of a luteal phase of approximately 13–14 d and a follicular phase of approximately 3 d (19). To enable sampling pituitary portal blood at a precisely defined time of the follicular phase, the follicular phase was synchronized among randomly cycling ewes using two intravaginal progesterone-releasing devices [controlled internal drug release (CIDR); Dec NZ, Hamilton, New Zealand]. These devices maintain plasma progesterone concentrations (2–4 ng/ml) comparable to those of the midluteal phase (20). After 16 d treatment (adequate time for corpus luteum regression), CIDRs were removed, leading to a rapid decline in plasma progesterone levels (progesterone withdrawal) and initiation of follicular phase endocrine events culminating in generation of the preovulatory LH surge approximately 40–48 h after CIDR removal (20).
Experiment 1: effect of acute exposure to cortisol on GnRH and LH pulsatility in the follicular phase
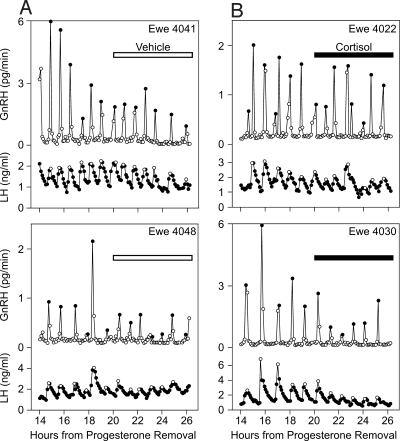
This experiment tested the response to a 6-h cortisol infusion beginning in the midfollicular phase. Surgery for placement of the portal blood collection apparatus was performed on d 2–4 after the synchronized estrus, and new CIDRs were inserted at that time to enable subsequent sampling of pituitary portal blood at the desired time within the follicular phase. Samples were obtained in the subsequent follicular phase, which was initiated by progesterone withdrawal (CIDR removal). Ewes were penned in the collection room 1 d before sampling. Pituitary portal and jugular blood were obtained from two groups of ewes at 6-min intervals for 12 h between 14 and 26 h after progesterone withdrawal, i.e. beginning approximately 34 h before the expected LH surge (Fig. 1A). Control ewes (n = 7) received an iv infusion of vehicle (heparin saline) throughout. Experimental ewes (n = 7) received vehicle for the first 6 h sampling, followed by cortisol (0.300 mg/kg · h, iv) for the last 6 h sampling. Plasma cortisol concentrations were monitored once an hour during pituitary portal blood collection, less frequently at other times.
Figure 1.
Design of experiments 1 (A), 2 (B), and 3 (C). Time is depicted as hours relative to progesterone withdrawal (removal of CIDRs), indicated by an arrow and -P. Horizontal bars indicate the period of cortisol or vehicle infusion. Estradiol (E) was added at 16 h in experiment 3. Time of frequent sampling for GnRH and LH pulse analysis and anticipated LH surge is indicated.
Experiment 2: effect of sustained elevation in plasma cortisol on GnRH and LH pulsatility in the follicular phase
This experiment tested the effect of a more prolonged exposure to cortisol beginning just before progesterone withdrawal. Surgery for placement of the portal blood collection apparatus was performed on d 5–6 after the detection of estrus, and samples were obtained in the subsequent follicular phase, initiated by progesterone withdrawal as described previously. Ewes were penned in the collection room 1 d before sampling. Beginning 1 h before progesterone withdrawal and continuing for 27 h, ewes received a constant iv infusion of either vehicle (heparin saline, n = 6) or cortisol (0.225 mg/kg · h; n = 6) (Fig. 1B). Pituitary portal and jugular blood were collected at 6-min intervals from 16–26 h after progesterone withdrawal (i.e. beginning ∼32 h before the expected LH surge). The period of jugular blood sampling was extended to monitor the preovulatory LH surge; samples were collected every 3 h between 30 and 84 h after progesterone withdrawal. Plasma cortisol concentrations were monitored hourly during pituitary portal blood collection.
Experiment 3: influence of cortisol on GnRH and LH pulsatility in the artificial follicular phase
The goal of this experiment was to determine whether cortisol reduces GnRH pulse frequency in ovariectomized ewes if they are treated with gonadal steroids to mimic the follicular phase of the cycle. This was based on our findings that cortisol did not suppress GnRH secretion in ovariectomized ewes devoid of gonadal steroids (6), but it did so in ewes during the follicular phase of the estrous cycle (experiment 2). This experiment made use of an artificial follicular phase model used extensively in our laboratory to study hormonal interactions of the follicular phase (21,22,23). To set up this model, ewes were bilaterally ovariectomized at surgery to install the apparatus for pituitary portal collection (d 3–5 after expression of estrus) and immediately thereafter treated with a hormone replacement regime that simulated plasma patterns and concentrations of both estradiol and progesterone of the natural estrous cycle. Estradiol was replaced using sc estradiol implants, and progesterone was replaced with CIDRs. The patterns and levels of circulating estradiol and progesterone, as well as the secretory profiles of GnRH and LH in the artificial follicular phase model, are well characterized (21,22,23,24). GnRH and LH pulse frequency increase markedly after progesterone withdrawal, and GnRH and LH surges typically begin approximately 20 h after the estradiol increase (i.e. ∼36 h after progesterone withdrawal).
Ewes were penned in the collection room 4 d before sampling. Pituitary portal and jugular blood were collected at 5-min intervals for 8 h, beginning 6 h after the estradiol increase (∼14 h before the LH surge, when GnRH pulse frequency would be high) (Fig. 1C). The experiment included control ewes (n = 11) receiving vehicle (heparinized saline, iv) and experimental ewes (n = 9) receiving a continuous infusion of cortisol (0.200 mg/kg · h, iv) from 1 h before progesterone withdrawal until the end of sampling (30 h). One animal from each group was excluded due to technical difficulties in sampling pituitary portal blood. The sustained cortisol treatment was used because experiments 1 and 2 showed that this was more effective in lowering GnRH pulse frequency than a 6-h treatment (see Results). Plasma cortisol concentrations were monitored once an hour during pituitary portal blood collection, less frequently at other times.
Hormone assays
GnRH was measured in duplicate methanol extracts of portal plasma (∼250 μl plasma extract per assay tube) using a previously described RIA (21,25). Intraassay and interassay coefficients of variation (CVs) were 10.4 and 15.3%, respectively, and assay sensitivity averaged 0.2 pg/tube (48 assays). LH concentrations were determined in duplicate aliquots (10–200 μl) of plasma using a modification (15) of a previously described RIA (26,27) and are expressed in terms of NIH-LH-S12. The mean intraassay and interassay CVs were 4.9 and 10.3%, respectively, and assay sensitivity averaged 0.7 ng/ml (29 assays). Total plasma cortisol concentrations were determined in duplicate 50-μl aliquots of unextracted plasma using the Coat-A-Count cortisol assay kit (Siemens Healthcare Diagnostics Inc., Los Angeles, CA), validated for use in sheep (28). Mean intraassay and interassay CVs were 7.4 and 6.7%, respectively, and assay sensitivity averaged 0.7 ng/ml (17 assays).
Data analysis
GnRH and LH pulses were identified using the Cluster pulse detection algorithm (29). As in our previous studies (6,8,28), cluster sizes for peaks and nadirs were defined as one and one for GnRH and two and two for LH, and the t statistic used to identify significant increase or decrease was 3.8 for GnRH and 2.6 for LH. GnRH in pituitary portal blood was calculated as a collection rate (pg/min) rather than concentration to minimize error due to contamination of portal samples with peripheral blood or cerebrospinal fluid (judged to be minimal), or due to changes in the rate of portal blood collection resulting from changes in the ewe’s posture. It should be noted that the procedure measures GnRH only during its initial pass through the portal system, not recirculated GnRH from the periphery, and that portal and jugular blood were collected continuously (i.e. integrated samples). GnRH pulse amplitude was defined as the total GnRH collected during a pulse, i.e. the sum of GnRH values in all samples during a pulse (generally no more than two samples) minus the nadir. LH pulse amplitude was defined as the difference between the peak concentration and its preceding nadir. Frequency of GnRH/LH pulses was defined as the number of pulses/sampling period. Before statistical analysis, plasma hormone concentrations were log transformed, and pulse frequencies were square-root transformed to normalize variability across a range of values, and a mean pulse amplitude was obtained in each ewe. Hormonal concentrations below assay sensitivity were assigned a value equal to assay sensitivity for the purpose of data analysis.
Experiment 1 was conducted according to a repeated measures design in which samples were obtained for 6 h before (period 1) and 6 h during (period 2) infusion of cortisol in experimental ewes and for comparable periods in vehicle-infused controls (Fig. 1A). Group differences in GnRH and LH pulse amplitude and frequency in experiment 1 were assessed by repeated measures ANOVA to identify treatment × period interactions (i.e. effects of cortisol). Experiments 2 and 3 each consisted of two groups: ewes infused with cortisol, and controls treated with vehicle throughout the entire sampling period (i.e. not a repeated measures design). Group differences in pulse parameters in experiments 2 and 3 were identified by the Student’s t test. Significance level was set at P < 0.05.
In experiment 2, the LH surge was defined as an increase in plasma LH concentration more than 3-fold above the presurge baseline for at least 6 h (LH surge not monitored in experiments 1 and 3). An unambiguous LH surge was evident in all ewes of experiment 2. The Student’s t test was used to compare the peak of the LH surge (maximal value) and the interval from withdrawal of progesterone to surge peak (latency) between vehicle- and cortisol-treated ewes.
Results
Plasma cortisol concentrations
Plasma cortisol concentrations for all experiments are presented in Fig. 2 and Table 1. During vehicle treatment, values remained at a stable basal level averaging less than 15 ng/ml, within the range of values we observe under nonstressed conditions (5,8). Mean plasma cortisol concentrations during cortisol infusion averaged 70–116 ng/ml in the three experiments. These values are within the range of maximal plasma cortisol concentrations that we observe during psychosocial stress (5) or infusion of endotoxin (17,18), a model of immune/inflammatory stress. The duration of the experimentally produced elevation in plasma cortisol was considerably longer than that that we have observed during psychosocial stress (Fig. 2A) but similar to that that we have observed during infusion of endotoxin in follicular phase ewes (17,18) (Fig. 2B).
Figure 2.
Mean ± sem plasma cortisol concentrations during infusion of cortisol in experiment (Exp) 1 (A) and experiments 2 and 3 (B; open and filled circles, respectively). Shaded areas depict the range of sem surrounding the mean previously observed during psychosocial stress (Ref. 5; A) or infusion of endotoxin in follicular phase ewes (Ref. 18; B). In experiment 2, cortisol was monitored only during the period of pituitary portal blood collection. Note, time axis in B is condensed relative to that in A.
Table 1.
Plasma cortisol in ewes infused with cortisol or vehicle
| Treatment | Cortisol (ng/ml) | |
|---|---|---|
| Experiment 1 | ||
| Vehicle, vehicle | 14.8 ± 1.3 | 12.3 ± 1.7 |
| Vehicle, cortisol | 10.7 ± 1.4 | 93.5 ± 8.2 |
| Experiment 2 | ||
| Vehicle | 13.9 ± 2.6 | |
| Cortisol | 116.3 ± 7.9 | |
| Experiment 3 | ||
| Vehicle | 13.5 ± 2.8 | |
| Cortisol | 69.6 ± 4.0 | |
Values are mean ± sem.
Experiment 1: effect of acute exposure to cortisol on GnRH and LH pulsatility in the follicular phase
Pulsatile GnRH and corresponding LH patterns of two representative cortisol- and vehicle-treated ewes are illustrated in Fig. 3; mean values for all ewes and results of the statistical analysis are presented in Table 2. In both groups, GnRH pulse frequencies were high, approximately one pulse per hour, which is typical of those normally seen during the follicular phase of the estrous cycle in ewes (13,22), and most GnRH pulses were associated with LH pulses (Fig. 3). Repeated measures ANOVA for GnRH or LH pulse frequency revealed no significant treatment or period effect and no treatment × period interaction. Thus, acute cortisol treatment did not alter pulse frequency.
Figure 3.
Profiles of GnRH in pituitary portal blood (top of each box) and LH in peripheral blood (bottom of each box) in two representative vehicle-treated ewes (A) and two ewes before and during administration of cortisol (B) in experiment 1. Peaks of pulses are depicted with contrasting open or filled circles. Horizontal open and solid bars depict vehicle and cortisol infusion, respectively.
Table 2.
Effects of acute cortisol on GnRH and LH pulses in natural follicular phase
| Treatment | Pulse frequency (pulses/6 h)
|
Pulse amplitudea
|
||
|---|---|---|---|---|
| 14–20 h | 20–26 h | 14–20 h | 20–26 h | |
| GnRH | ||||
| Vehicle, vehicle | 7.0 ± 0.3 | 6.7 ± 0.8 | 1.8 ± 0.4 | 1.2 ± 0.3b |
| Vehicle, cortisol | 6.3 ± 0.7 | 6.3 ± 1.2 | 1.9 ± 0.6 | 0.9 ± 0.2b |
| LH | ||||
| Vehicle, vehicle | 6.6 ± 0.4 | 6.1 ± 0.6 | 1.2 ± 0.2 | 0.9 ± 0.1c |
| Vehicle, cortisol | 6.1 ± 0.5 | 5.4 ± 0.5 | 1.5 ± 0.3 | 0.8 ± 0.1c,d |
Values are mean ± sem for seven control (vehicle, vehicle) and seven experimental (vehicle, cortisol) ewes.
Amplitude expressed as pg/min for GnRH and ng/ml for LH.
Period effect (14–20 vs. 20–26 h; P < 0.01) as determined by repeated measures ANOVA.
Period effect (14–20 vs. 20–26 h; P < 0.0001) as determined by repeated measures ANOVA.
Treatment × period interaction (P < 0.05) as determined by repeated measures ANOVA.
With respect to amplitude, repeated measures ANOVA identified a significant period effect for both GnRH and LH pulses. Amplitude was lower during the second sampling period (20–26 h) compared with the first (14–20 h). Furthermore, there was a significant treatment × period interaction for LH but not GnRH pulse amplitude (Table 2), indicating that the decrease in amplitude of LH (but not GnRH) pulses over time was more pronounced in ewes treated with cortisol (mean LH suppression, 21 and 44% in vehicle and cortisol-treated animals, respectively; P < 0.05).
Experiment 2: effect of sustained elevation in plasma cortisol on GnRH and LH pulsatility in the follicular phase
Examples of GnRH and corresponding LH patterns of three ewes treated with either vehicle or cortisol from the time of progesterone withdrawal are illustrated in Fig. 4; composite results are depicted in Table 3. Again, mean GnRH and LH pulse frequencies in vehicle-treated ewes were approximately one pulse per hour (Table 3), and most GnRH pulses were associated with LH pulses (Fig. 4). However, in this experiment sustained cortisol treatment significantly reduced frequency of both GnRH and LH pulses by 40–45% (Table 3 and Fig. 4B). Cortisol also increased the amplitude of GnRH pulses but, of interest, did not affect LH pulse amplitude. All ewes exhibited a preovulatory LH surge. Cortisol increased the latency to the LH surge peak by 10 h (Fig. 5A), but it did not affect LH surge amplitude (Fig. 5B).
Figure 4.
Profiles of GnRH in pituitary portal blood (top of each box) and LH in peripheral blood (bottom of each box) in three representative vehicle (A) and three cortisol-treated (B) ewes in experiment 2. Peaks of pulses are depicted with contrasting open or filled circles.
Table 3.
Effects of sustained cortisol on GnRH and LH pulses in natural follicular phase
| Treatment | Frequency (pulses/10 h) | Amplitudea |
|---|---|---|
| GnRH | ||
| Vehicle | 12.4 ± 2.3 | 1.05 ± 0.2 |
| Cortisol | 6.83 ± 1.0b | 1.84 ± 0.2b |
| LH | ||
| Vehicle | 9.6 ± 0.8 | 1.14 ± 0.2 |
| Cortisol | 6.0 ± 0.7c | 1.05 ± 0.2 |
Values are mean ± sem for five vehicle and six cortisol-treated ewes.
Amplitude expressed as pg/min for GnRH and ng/ml for LH.
Treatment effect (P < 0.05).
Treatment effect (P < 0.01).
Figure 5.
Mean ± sem latency to peak LH (A) and plasma LH concentration during the preovulatory LH surge (B) in all cortisol-treated and control ewes in experiment 2. Controls are depicted with open circles or bars, and the cortisol group is shown with filled circles or bars. B, LH is plotted normalized to the peak of the LH surge. Cortisol significantly delayed the time to the peak of the LH surge.
The GnRH pattern of one vehicle-treated control was atypical of the other five control ewes of this experiment, as well as the 14 follicular phase ewes of experiment 1 and all other ewes we have previously examined in the follicular phase (13,30), in that only one pulse was detected during the entire 10 h sampling. The pulsatile LH pattern in this ewe was also atypical relative to that that we observe in follicular phase ewes, being highly irregular and not clearly episodic. Data for this ewe were excluded from the statistical analysis. This ewe did have an LH surge that peaked 42 h after progesterone withdrawal.
Experiment 3: influence of cortisol on GnRH and LH pulsatility in the artificial follicular phase
Examples of pulsatile GnRH and LH patterns during the artificial follicular phase of three ewes treated with vehicle or cortisol from the time of progesterone withdrawal are depicted in Fig. 6, and mean values for all animals are summarized in Table 4. In vehicle-treated ewes, GnRH and LH pulse frequencies were approximately one pulse every 75–100 min (Table 4), and again most GnRH pulses were associated with LH pulses (Fig. 6). Sustained cortisol treatment had no significant effect on GnRH and LH pulse amplitude but reduced frequency of GnRH and LH pulses by about 70% (Table 4 and Fig. 6B). The reduction in frequency was often severe. Notably, two of the eight cortisol-treated ewes secreted no detectable GnRH or LH pulses (e.g. ewe 6010, Fig. 6B, top panel), and in three others, only one pulse was observed during the 8-h sampling period (e.g. ewe 6031, Fig. 6B, middle panel).
Figure 6.
Profiles of GnRH in pituitary portal blood (top of each box) and LH in peripheral blood (bottom of each box) in three representative vehicle (A) and three cortisol-treated (B) ewes in experiment 3. Peaks of pulses are depicted with contrasting open or filled circles. No pulses were detected in ewe 6010.
Table 4.
Effects of cortisol on GnRH and LH pulses in artificial follicular phase
| Treatment | Frequency (pulses/8 h) | Amplitudea |
|---|---|---|
| GnRH | ||
| Vehicle | 5.0 ± 1.2 | 0.50 ± 0.1 |
| Cortisol | 1.6 ± 0.6b | 0.86 ± 0.2 |
| LH | ||
| Vehicle | 6.3 ± 0.9 | 1.0 ± 0.2 |
| Cortisol | 2.8 ± 0.9b | 1.1 ± 0.2 |
Values are mean ± sem for 10 vehicle and eight cortisol-treated ewes.
Amplitude expressed as pg/min for GnRH and ng/ml for LH.
Treatment effect (vehicle vs. cortisol; P < 0.05).
Discussion
Our findings demonstrate that an elevation in plasma cortisol can markedly reduce GnRH pulse frequency in ewes during the follicular phase of the estrous cycle. This finding complements the previous observation that cortisol reduces LH pulse frequency in the follicular phase (11), and it extends those findings by determining that this effect of cortisol is mediated by the brain rather than the pituitary gland. The present results also indicate that cortisol lowers GnRH pulse frequency in gonadectomized ewes treated with ovarian steroids to simulate the follicular phase secretory pattern of estradiol and progesterone. This effect of cortisol has not been observed in untreated gonadectomized ewes devoid of ovarian steroids (6). To our knowledge this is the first direct demonstration that cortisol reduces the frequency of GnRH pulses in any species.
Of interest, we observed that cortisol lowered GnRH pulse frequency when it was elevated for 27 or 31 h from the time of progesterone withdrawal (experiments 2 and 3), but not when elevated for 6 h beginning 20 h after the decrease in progesterone from a luteal phase level (experiment 1). The lack of an effect of cortisol on GnRH pulse frequency in experiment 1 cannot be explained by an insufficient concentration of plasma cortisol produced by the infusion because the mean plasma cortisol value in experiment 1 was intermediate between the values observed in experiments 2 and 3, in which cortisol did lower GnRH pulse frequency (Fig. 2). Nevertheless, the duration of treatment in experiment 1 (6 h) was clearly shorter than that in experiments 2 and 3 (27 or 31 h), and a critical duration may be necessary for cortisol to reduce GnRH pulse frequency in follicular phase ewes. However, we previously observed that 4 h exposure to the same magnitude elevation in plasma cortisol, repeated every 12 h from the time of progesterone withdrawal, did reduce LH pulse frequency in follicular phase ewes (11). Thus, time of cortisol infusion relative to progesterone withdrawal, or elevated cortisol in the recent past, might also be important. Clearly, further work is warranted to examine the importance of duration and timing of cortisol treatment in determining its efficacy in lowering GnRH pulse frequency in the follicular phase of the cycle.
One major objective of this study was to test the hypothesis that the gonadal steroid milieu of the follicular phase enables cortisol to reduce GnRH pulse frequency. This hypothesis was based on our earlier finding that a stress-like elevation of cortisol over a 6-h period did not reduce GnRH pulse frequency in ovariectomized ewes devoid of gonadal steroids (6), whereas the same level of cortisol maintained for 27 h in the present study did lower GnRH pulse frequency in follicular phase ewes (experiment 2). In experiment 3, we determined that the longer exposure to cortisol profoundly decreased GnRH pulse frequency in ovariectomized ewes if they were exposed to a background of estradiol and progesterone that simulated the ovarian steroid milieu of the follicular phase. These findings may be interpreted in two ways. First, duration might be important, and cortisol would reduce GnRH pulse frequency in ovariectomized ewes devoid of gonadal steroids if infused for a longer period. Second, the hormonal milieu might be the determining factor, and the efficacy of cortisol in suppressing GnRH pulse frequency requires the presence of ovarian steroids. We distinguished between these alternatives in another experiment conducted as part of a separate study. That experiment revealed that a 29-h elevation in plasma cortisol, to a level comparable to that used here, failed to suppress LH pulse frequency in ovariectomized ewes devoid of gonadal hormones, whereas the same treatment again reduced LH pulse frequency in ovariectomized ewes in the artificial follicular phase model (31). Therefore, we may conclude that an interaction exists between cortisol and ovarian hormones, such that the presence of gonadal steroids, endogenous or exogenous, enables cortisol to exert a powerful inhibitory effect at the level of the brain to decrease GnRH pulse frequency.
It is important to consider the present findings in context of the physiological role that cortisol might play in stress-induced suppression of pulsatile GnRH and LH secretion during the follicular phase of the cycle. Previous studies indicate that transportation (a form of psychosocial stress) and endotoxin (a commonly used model of immune/inflammatory stress) can suppress pulsatile LH secretion and delay the preovulatory LH surge in follicular phase ewes (18,32). The magnitude of the plasma cortisol increments produced by infusion in the present study was within the range of that observed in response to either psychosocial stress or endotoxin (Fig. 2). However, the duration of the cortisol increments used here was greater than that observed during psychosocial stress. Thus, our findings must be interpreted cautiously with respect to the role that cortisol might play in physiological suppression of pulsatile GnRH secretion during stress. Nevertheless, the duration of the increment in plasma cortisol that reduced GnRH pulse frequency in the present study was similar to that observed previously during infusion of endotoxin in follicular phase ewes. Furthermore, the plasma cortisol concentrations in the present study were probably greater than that needed to lower GnRH pulse frequency because a sustained elevation in cortisol to only 30 ng/ml (i.e. less than half of the level used here) reduced LH pulse frequency in follicular phase ewes (11). Collectively, these observations, in conjunction with the present findings, encourage further work to assess the physiological relevance of glucocorticoids in suppression of pulsatile GnRH secretion in response to different types of stress during the follicular phase.
It is also important to place the present findings into context with respect to extensive evidence that mediators other than glucocorticoids contribute to stress-induced suppression of gonadotropin secretion. For example, other hormones of the hypothalamo-pituitary-adrenal axis (CRH and arginine vasopressin), as well as various cytokines (IL-1, IL-6, TNF-α) and prostaglandins all participate in suppression of pulsatile LH secretion in response to immune/inflammatory stress (33,34,35,36,37); cortisol is redundant and not necessary for this effect (8). CRH appears to mediate suppression of pulsatile LH secretion in rats during physical restraint or the metabolic stress of insulin-induced hypoglycemia (38,39). Endogenous opioid peptides have also been implicated in stress-induced suppression of pulsatile LH secretion (40,41,42,43,44). Thus, glucocorticoids may be considered members of a family of intermediates that suppress gonadotropin secretion in response to stress. The relative importance of particular members of this family likely depends on the type of stress and may vary with species. Our recent work in sheep indicates that cortisol is an essential mediator of the suppression of pituitary responsiveness to GnRH in ovariectomized ewes during psychosocial stress. The relative importance of cortisol and other intermediates to suppression of pulsatile GnRH secretion in response to different stress types during the follicular phase of the cycle remains an open question for future research.
The effect of cortisol to decrease GnRH pulse frequency in the follicular phase is probably not its only action on the GnRH neurosecretory system at this stage of the cycle. Cortisol also interferes with the positive feedback response to estradiol by delaying, blunting, and even blocking the estradiol-induced LH surge (45,46). These effects of cortisol likely include an action on the central nervous system, given that the positive feedback response to estradiol in ewes involves induction of a large GnRH surge (14,21). These observations fit nicely with the results of experiment 2 and previous findings (11,47) showing that cortisol delays the natural LH surge of follicular phase ewes, and they suggest that cortisol delays the preovulatory LH surge by interfering with the positive feedback action of estradiol. Cortisol might also delay the preovulatory LH surge by interfering with the follicular phase estradiol increase. This could be affected either at the ovarian level via reduced granulosa cell responsiveness to gonadotropic hormones (48,49) or at a neuroendocrine level via reduced GnRH pulse frequency (this study) and decreased pituitary responsiveness to GnRH (4,6,9), which would lower gonadotropic drive to the follicle.
The ability of cortisol to reduce pituitary responsiveness to GnRH has been well documented in ovariectomized ewes (4,6,9). However, until now, this effect had not been disclosed in the natural follicular phase due to complications occurring from the intricate feedback interplay within the hypothalamic-pituitary-ovarian axis (11). Specifically, the action of cortisol to reduce LH pulse frequency during the follicular phase is accompanied by lowered estradiol secretion (11,47), which would be expected to reduce negative feedback and allow an increase in the amplitude of LH pulses (19). This, in turn, could mask a separate action of cortisol to reduce responsiveness to GnRH. Likewise, the effect of cortisol to lower GnRH pulse frequency in follicular phase ewes could mask an independent suppressive action of cortisol on pituitary responsiveness to GnRH because decreased frequency of GnRH pulses, in itself, leads to increased LH pulse amplitude due to an inverse relationship between the two (50,51,52).
Despite these complexities, the present study provides initial evidence that cortisol reduces pituitary responsiveness to GnRH in follicular phase ewes. Specifically, our statistical analysis revealed that the amplitude of LH pulses in experiment 1 was lowered to a greater extent during the acute infusion of cortisol than during infusion of vehicle, and this occurred without any suppressive effect of cortisol on GnRH pulse frequency or amplitude. In addition, reduced pituitary responsiveness to GnRH is suggested by the effect of the sustained elevation of plasma cortisol in experiment 2, in which GnRH, but not LH, pulse amplitude was increased by cortisol. We interpret the absence of a corresponding increase in LH pulse amplitude in cortisol-treated ewes to reflect a suppressive action of the glucocorticoid at the pituitary level. Collectively, these findings provide initial evidence that the suppressive action of cortisol on pituitary responsiveness to GnRH, which is readily demonstrable in the absence of ovarian steroids (4,6,9), might also be expressed during the follicular phase of the cycle, although further work is needed to confirm this interpretation.
The foregoing observations and considerations indicate that the neuroendocrine effects of cortisol during the follicular phase are highly complex, and include at least three actions at the hypothalamic and/or pituitary level: 1) suppression of GnRH pulse frequency, 2) lowering of pituitary responsiveness to GnRH, and 3) delay of the preovulatory LH surge. Importantly, gonadal steroids are necessary for, or greatly amplify, the hypothalamic effect of cortisol that leads to a lowering of GnRH pulse frequency. This influence of ovarian steroids may help to explain why responses to stress differ between males and females, in lactating vs. nonlactating females, and between the luteal and follicular phases of the cycle (7,53,54,55,56). Furthermore, because the development of high-frequency GnRH and LH pulses is of fundamental importance for progression of the preovulatory chain of endocrine events, it is intriguing to consider the possibility that the suppressive effect of cortisol on GnRH pulse frequency might constitute one mechanism by which stress compromises fertility.
Acknowledgments
We thank Doug Doop and Gary McCalla for their exceptional animal care, Andrew V. Pytiak and Alison P. Cooper for their technical assistance, and Drs. Alain Caraty, Gordon D. Niswender, and Leo E. Reichert, Jr., for supplying RIA reagents.
Footnotes
This work was supported by National Institutes of Health Grants HD30773, T32-07048, and T32-08322.
Present address for K.M.B.: Department of Reproductive Medicine, University of California, San Diego, Leichtag Biomedical Research Building, Room 349, La Jolla, California 92093-0674.
Present address for A.E.O.: Department of Physiology and Biophysics, University of Washington, Box 356460, 1959 Northeast Pacific Street, Health Sciences Building, Room BB604, Seattle Washington 98195-6460.
The preliminary report has appeared in 2007 Biol Reprod 74 (Suppl 1, Abstract 429).
Disclosure Statement: The authors have nothing to declare.
First Published Online September 18, 2008
Abbreviations: CIDR, Controlled internal drug release; CV, coefficient of variation.
References
- Breen KM, Karsch FJ 2006 New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrinol 27:233–245 [DOI] [PubMed] [Google Scholar]
- Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, O'Byrne KT 2003 The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. J Neuroendocrinol 15:468–476 [DOI] [PubMed] [Google Scholar]
- Ferin M 1993 Neuropeptides, the stress response, and the hypothalamo-pituitary-gonadal axis in the female rhesus monkey. Ann NY Acad Sci 697:106–116 [DOI] [PubMed] [Google Scholar]
- Breen KM, Stackpole CA, Clarke IJ, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Young EA, Karsch FJ 2004 Does the type II glucocorticoid receptor mediate cortisol-induced suppression in pituitary responsiveness to gonadotropin-releasing hormone? Endocrinology 145:2739–2746 [DOI] [PubMed] [Google Scholar]
- Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ 2007 Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology 148:1882–1890 [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ 2004 Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology 145:692–698 [DOI] [PubMed] [Google Scholar]
- Stackpole CA, Turner AI, Clarke IJ, Lambert GW, Tilbrook AJ 2003 Seasonal differences in the effect of isolation and restraint stress on the luteinizing hormone response to gonadotropin-releasing hormone in hypothalamopituitary disconnected, gonadectomized rams and ewes. Biol Reprod 69:1158–1164 [DOI] [PubMed] [Google Scholar]
- Debus N, Breen KM, Barrell GK, Billings HJ, Brown M, Young EA, Karsch FJ 2002 Does cortisol mediate endotoxin-induced inhibition of pulsatile luteinizing hormone and gonadotropin-releasing hormone secretion? Endocrinology 143:3748–3758 [DOI] [PubMed] [Google Scholar]
- Breen KM, Davis TL, Doro LC, Nett TM, Oakley AE, Padmanabhan V, Rispoli LA, Wagenmaker ER, Karsch FJ 2008 Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 149:767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey AK, Plant TM 1985 A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod 33:423–431 [DOI] [PubMed] [Google Scholar]
- Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ 2005 Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology 146:2107–2115 [DOI] [PubMed] [Google Scholar]
- Saketos M, Sharma N, Santoro NF 1993 Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod 49:1270–1276 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Thomas GB, Yao B, Cummins JT 1987 GnRH secretion throughout the ovine estrous cycle. Neuroendocrinology 46:82–88 [DOI] [PubMed] [Google Scholar]
- Hauger RL, Karsch FJ, Foster DL 1977 A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology 101:807–817 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Moenter SM, Karsch FJ 1994 Sampling of hypophyseal portal blood of conscious sheep for direct monitoring of hypothalamic neurosecretory substances. Methods Neurosci 20:162–183 [Google Scholar]
- Battaglia DF, Beaver AB, Harris TG, Tanhehco E, Viguie C, Karsch FJ 1999 Endotoxin disrupts the estradiol-induced luteinizing hormone surge: interference with estradiol signal reading, not surge release. Endocrinology 140:2471–2479 [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Krasa HB, Padmanabhan V, Viguie C, Karsch FJ 2000 Endocrine alterations that underlie endotoxin-induced disruption of the follicular phase in ewes. Biol Reprod 62:45–53 [DOI] [PubMed] [Google Scholar]
- Goodman RL 1994 Neuroendocrine control of the ovine estrous cycle. In: Knobil E, Neill JD, eds. The physiology of reproduction. New York: Raven Press; 659–709 [Google Scholar]
- Van Cleeff J, Karsch FJ, Padmanabhan V 1998 Characterization of endocrine events during the periestrous period in sheep after estrous synchronization with controlled internal drug release (CIDR) device. Domest Anim Endocrinol 15:23–34 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ 1990 The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127:1375–1384 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Glover BH, Karsch FJ 1994 Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 134:1806–1811 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ 1981 Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol 89:229–240 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ 1997 Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology 138:5408–5414 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Schanbacher B 1987 [Augmentation, by naloxone, of the frequency and amplitude of LH-RH pulses in hypothalamo-hypophyseal portal blood in the castrated ram]. C R Acad Sci III 305:369–374 (French) [PubMed] [Google Scholar]
- Niswender GD, Midgley Jr AR, Reichert Jr LE 1968 Radioimmunologic studies with murine, ovine and porcine luteinizing hormone. In: Rosenborg E, ed. Gonadotropins. Los Altos, CA: Geron-X; 299–306 [Google Scholar]
- Niswender GD, Reichert Jr LE, Midgley Jr AR, Nalbandov AV 1969 Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology 84:1166–1173 [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguie C, Karsch FJ 1997 Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology 138:4273–4281 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 250(4 Pt 1):E486–E493 [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Evans NP 1996 Feedback actions of estradiol on GnRH secretion during the follicular phase of the estrous cycle. Acta Neurobiol Exp (Wars) 56:715–725 [DOI] [PubMed] [Google Scholar]
- Oakley AE, Breen KM, Tilbrook AJ, Wagenmaker ER, Karsch FJ Gonadal steroidal milieu of the estrous cycle enables cortisol to inhibit LH pulse frequency. In: Program No. 7763 2006 Abstract viewer/itinerary planner. Atlanta: Society for Neuroscience, 2006 online [Google Scholar]
- Dobson H, Tebble JE, Phogat JB, Smith RF 1999 Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J Reprod Fertil 116:1–8 [DOI] [PubMed] [Google Scholar]
- Barrell GK 2007 Immunological influences on reproductive neuroendocrinology. Soc Reprod Fertil Suppl 64:109–122 [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Battaglia DF 2002 Mechanisms for endotoxin-induced disruption of ovarian cyclicity: observations in sheep. Reprod Suppl 59:101–113 [PubMed] [Google Scholar]
- Karsch FJ, Battaglia DF, Breen KM, Debus N, Harris TG 2002 Mechanisms for ovarian cycle disruption by immune/inflammatory stress. Stress 5:101–112 [DOI] [PubMed] [Google Scholar]
- Daniel JA, Whitlock BK, Wagner CG, Sartin JL 2002 Regulation of the growth hormone and luteinizing hormone response to endotoxin in sheep. Domest Anim Endocrinol 23:361–370 [DOI] [PubMed] [Google Scholar]
- Kalra PS, Edwards TG, Xu B, Jain M, Kalra SP 1998 The anti-gonadotropic effects of cytokines: the role of neuropeptides. Domest Anim Endocrinol 15:321–332 [DOI] [PubMed] [Google Scholar]
- Cates PS, Li XF, O'Byrne KT 2004 The influence of 17β-oestradiol on corticotrophin-releasing hormone induced suppression of luteinising hormone pulses and the role of CRH in hypoglycaemic stress-induced suppression of pulsatile LH secretion in the female rat. Stress 7:113–118 [DOI] [PubMed] [Google Scholar]
- Mitchell JC, Li XF, Breen L, Thalabard JC, O'Byrne KT 2005 The role of the locus coeruleus in corticotropin-releasing hormone and stress-induced suppression of pulsatile luteinizing hormone secretion in the female rat. Endocrinology 146:323–331 [DOI] [PubMed] [Google Scholar]
- He D, Sato I, Kimura F, Akema T 2003 Lipopolysaccharide inhibits luteinizing hormone release through interaction with opioid and excitatory amino acid inputs to gonadotropin-releasing hormone neurones in female rats: possible evidence for a common mechanism involved in infection and immobilization stress. J Neuroendocrinol 15:559–563 [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M 2000 Inhibitory effects of endotoxin on LH secretion in the ovariectomized monkey are prevented by naloxone but not by an interleukin-1 receptor antagonist. Neuroimmunomodulation 7:6–15 [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Cates PS, Sandhu S, Strutton PH, McGarvey C, Coen CW, O'Byrne KT 1997 Hypoglycaemia-induced inhibition of pulsatile luteinizing hormone secretion in female rats: role of oestradiol, endogenous opioids and the adrenal medulla. J Neuroendocrinol 9:867–872 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Horton RJ, Doughton BW 1990 Investigation of the mechanism by which insulin-induced hypoglycemia decreases luteinizing hormone secretion in ovariectomized ewes. Endocrinology 127:1470–1476 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Vale W, Rivier C 1986 Opioids act centrally to modulate stress-induced decrease in luteinizing hormone in the rat. Endocrinology 119:2445–2450 [DOI] [PubMed] [Google Scholar]
- Wagenmaker ER, Breen KM, Oakley AE, Pierce BN, Tilbrook AJ, Turner AI, Karsch FJ, Cortisol interferes with the estradiol-induced surge of luteinizing hormone in the ewe. Biol Reprod, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BN, Clarke IJ, Turner AI, Rivalland ETA, Tilbrook AJ, Cortisol disrupts the ability of estradiol-17β to induce the LH surge in ovariectomized ewes. Domest Anim Endocrinol, in press [DOI] [PubMed] [Google Scholar]
- Macfarlane MS, Breen KM, Sakurai H, Adams BM, Adams TE 2000 Effect of duration of infusion of stress-like concentrations of cortisol on follicular development and the preovulatory surge of LH in sheep. Anim Reprod Sci 63:167–175 [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Erickson GF 1978 Glucocorticoid inhibition of FSH-induced estrogen production in cultured rat granulosa cells. Steroids 32:639–648 [DOI] [PubMed] [Google Scholar]
- Michael AE, Pester LA, Curtis P, Shaw RW, Edwards CR, Cooke BA 1993 Direct inhibition of ovarian steroidogenesis by cortisol and the modulatory role of 11β-hydroxysteroid dehydrogenase. Clin Endocrinol (Oxf) 38:641–644 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT, Findlay JK, Burman KJ, Doughton BW 1984 Effects on plasma luteinizing hormone and follicle-stimulating hormone of varying the frequency and amplitude of gonadotropin-releasing hormone pulses in ovariectomized ewes with hypothalamo-pituitary disconnection. Neuroendocrinology 39:214–221 [DOI] [PubMed] [Google Scholar]
- Kaynard AH, Karsch FJ 1988 Hypophyseal actions of pulsatile gonadotropin-releasing hormone in the ewe: development and application of a new experimental model. Neuroendocrinology 48:287–295 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT 1985 GnRH pulse frequency determines LH pulse amplitude by altering the amount of releasable LH in the pituitary glands of ewes. J Reprod Fertil 73:425–431 [DOI] [PubMed] [Google Scholar]
- Stackpole CA, Clarke IJ, Breen KM, Turner AI, Karsch FJ, Tilbrook AJ 2006 Sex difference in the suppressive effect of cortisol on pulsatile secretion of luteinizing hormone in sheep. Endocrinology 147:5921–5931 [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI 2006 The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31:151–178 [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Fulks R, Gerald MS 2008 Plasma cortisol responses to stress in lactating and nonlactating female rhesus macaques. Horm Behav 53:170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Ibbott MD, Clarke IJ 2006 Activation of the hypothalamo-pituitary-adrenal axis by isolation and restraint stress during lactation in ewes: effect of the presence of the lamb and suckling. Endocrinology 147:3501–3509 [DOI] [PubMed] [Google Scholar]