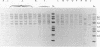
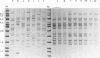
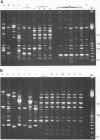
Abstract
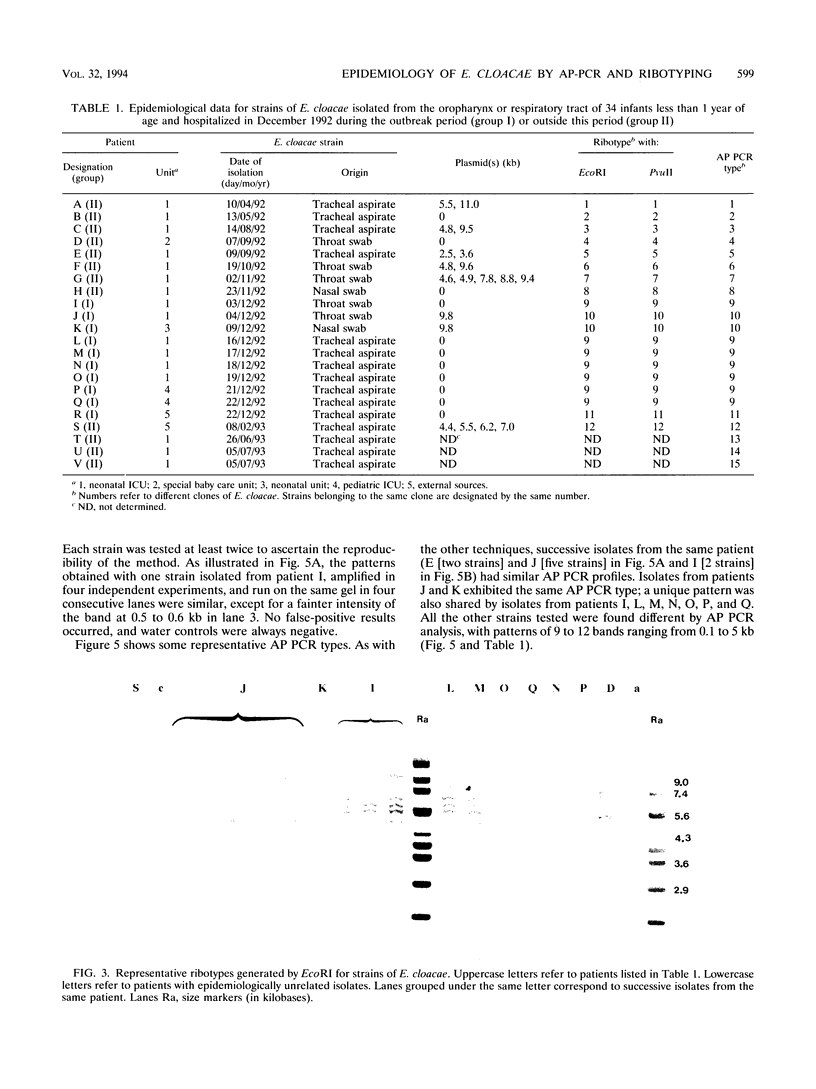
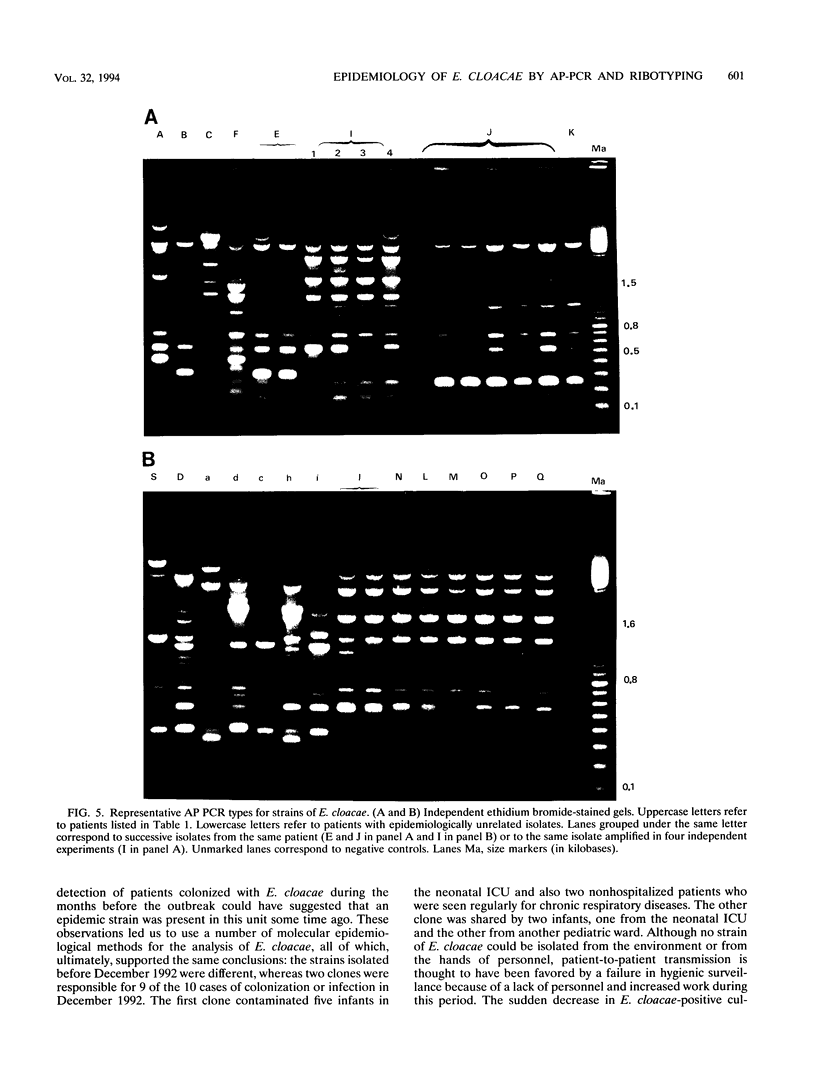
In December 1992, Enterobacter cloacae was isolated from the oropharynx and respiratory tract of six ventilated neonates hospitalized in the intensive care unit (ICU) of our hospital. To establish the spread of the outbreak, 41 strains of E. cloacae were analyzed for genotypic markers by three methods: plasmid profile analysis, ribotyping with EcoRI or PvuII endonuclease, and arbitrarily primed (AP) PCR. The tested strains included 12 isolates from the 6 epidemic cases, 4 isolates from the respiratory tract of 4 children hospitalized in other wards during the same period, 13 isolates from 12 children hospitalized in pediatric units before or after the outbreak, and 12 epidemiologically unrelated isolates. Ribotyping and AP PCR demonstrated that each of the last 12 strains exhibited distinct genomic patterns, as did each of the strains isolated from neonates hospitalized before or after the epidemic peak. Conversely, two clones of strains were found among the isolates recovered in December, with concordant results being obtained by the three typing methods: the first clone included seven strains from five ventilated children in the ICU and two children from another ward; another clone was shared by one neonate in the ICU and an infant from another ward. These results indicate that ribotyping and AP PCR-the latter applied, to our knowledge, for the first time to the genotypic analysis of E. cloacae--represent very discriminatory tools for the investigation of nosocomial outbreaks caused by this species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthelot P., Grattard F., Mahul P., Jospe R., Pozzetto B., Ros A., Gaudin O. G., Auboyer C. Ventilator temperature sensors: an unusual source of Pseudomonas cepacia in nosocomial infection. J Hosp Infect. 1993 Sep;25(1):33–43. doi: 10.1016/0195-6701(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Bingen E., Denamur E., Lambert-Zechovsky N., Brahimi N., el Lakany M., Elion J. Rapid genotyping shows the absence of cross-contamination in Enterobacter cloacae nosocomial infections. J Hosp Infect. 1992 Jun;21(2):95–101. doi: 10.1016/0195-6701(92)90028-k. [DOI] [PubMed] [Google Scholar]
- Daw M. A., Corcoran G. D., Falkiner F. R., Keane C. T. Application and assessment of cloacin typing of Enterobacter cloacae. J Hosp Infect. 1992 Mar;20(3):141–151. doi: 10.1016/0195-6701(92)90082-w. [DOI] [PubMed] [Google Scholar]
- Falkiner F. R. Enterobacter in hospital. J Hosp Infect. 1992 Mar;20(3):137–140. doi: 10.1016/0195-6701(92)90081-v. [DOI] [PubMed] [Google Scholar]
- Flynn D. M., Weinstein R. A., Nathan C., Gaston M. A., Kabins S. A. Patients' endogenous flora as the source of "nosocomial" Enterobacter in cardiac surgery. J Infect Dis. 1987 Aug;156(2):363–368. doi: 10.1093/infdis/156.2.363. [DOI] [PubMed] [Google Scholar]
- Garaizar J., Kaufmann M. E., Pitt T. L. Comparison of ribotyping with conventional methods for the type identification of Enterobacter cloacae. J Clin Microbiol. 1991 Jul;29(7):1303–1307. doi: 10.1128/jcm.29.7.1303-1307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston M. A., Crees-Morris J. A., Pitt T. L. Serotypes and biochemical profiles of British hospital strains of Enterobacter cloacae in relation to site of infection and antibiotic susceptibility. J Hosp Infect. 1987 Jul;10(1):17–27. doi: 10.1016/0195-6701(87)90028-4. [DOI] [PubMed] [Google Scholar]
- Gaston M. A. Enterobacter: an emerging nosocomial pathogen. J Hosp Infect. 1988 Apr;11(3):197–208. doi: 10.1016/0195-6701(88)90098-9. [DOI] [PubMed] [Google Scholar]
- Grattard F., Etienne J., Pozzetto B., Tardy F., Gaudin O. G., Fleurette J. Characterization of unrelated strains of Staphylococcus schleiferi by using ribosomal DNA fingerprinting, DNA restriction patterns, and plasmid profiles. J Clin Microbiol. 1993 Apr;31(4):812–818. doi: 10.1128/jcm.31.4.812-818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertl R., Bandlow G. Epidemiological fingerprinting of Enterobacter cloacae by small-fragment restriction endonuclease analysis and pulsed-field gel electrophoresis of genomic restriction fragments. J Clin Microbiol. 1993 Jan;31(1):128–133. doi: 10.1128/jcm.31.1.128-133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert-Zechovsky N., Bingen E., Denamur E., Brahimi N., Brun P., Mathieu H., Elion J. Molecular analysis provides evidence for the endogenous origin of bacteremia and meningitis due to Enterobacter cloacae in an infant. Clin Infect Dis. 1992 Jul;15(1):30–32. doi: 10.1093/clinids/15.1.30. [DOI] [PubMed] [Google Scholar]
- Markowitz S. M., Smith S. M., Williams D. S. Retrospective analysis of plasmid patterns in a study of burn unit outbreaks of infection due to Enterobacter cloacae. J Infect Dis. 1983 Jul;148(1):18–23. doi: 10.1093/infdis/148.1.18. [DOI] [PubMed] [Google Scholar]
- Mayhall C. G., Lamb V. A., Gayle W. E., Jr, Haynes B. W., Jr Enterobacter cloacae septicemia in a burn center: epidemiology and control of an outbreak. J Infect Dis. 1979 Feb;139(2):166–171. doi: 10.1093/infdis/139.2.166. [DOI] [PubMed] [Google Scholar]
- Mazurier S. I., Wernars K. Typing of Listeria strains by random amplification of polymorphic DNA. Res Microbiol. 1992 Jun;143(5):499–505. doi: 10.1016/0923-2508(92)90096-7. [DOI] [PubMed] [Google Scholar]
- McConkey S. J., Coleman D. C., Falkiner F. R., McCann S. R., Daly P. A. Enterobacter cloacae in a haematology/oncology ward--first impressions. J Hosp Infect. 1989 Nov;14(4):277–284. doi: 10.1016/0195-6701(89)90067-4. [DOI] [PubMed] [Google Scholar]
- Modi N., Damjanovic V., Cooke R. W. Outbreak of cephalosporin resistant Enterobacter cloacae infection in a neonatal intensive care unit. Arch Dis Child. 1987 Feb;62(2):148–151. doi: 10.1136/adc.62.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Chu M. L., Ho L. J., Hwang R. C. Analysis of plasmid pattern in paediatric intensive care unit outbreaks of nosocomial infection due to Enterobacter cloacae. J Hosp Infect. 1991 Sep;19(1):33–40. doi: 10.1016/0195-6701(91)90126-s. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A., Struelens M., Quint W. Typing of Legionella pneumophila strains by polymerase chain reaction-mediated DNA fingerprinting. J Clin Microbiol. 1993 Aug;31(8):2198–2200. doi: 10.1128/jcm.31.8.2198-2200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]