Introduction
Diversity of Head and Neck Squamous Cell Carcinoma (HNSCC)
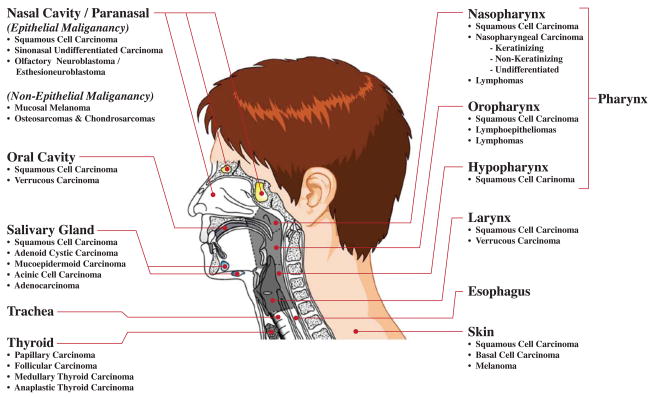
There is a remarkably diverse array of anatomy and tumor morphologies, with at least ten anatomic subsites of the head and neck, challenging all members of the multidisciplinary team to precisely define the extent of a patient’s disease. (Figure 1) While the majority of the histopathology consists of squamous cell carcinoma (SCC), there are dozens of other pathologic diagnoses. Accordingly, a broad spectrum of treatment modalities are offered, frequently in combination, including chemotherapy, radiation including Intensity Modulated Radiation Therapy (IMRT), and surgery with and without reconstruction. Historically, the treating teams have labored to join the anatomic and morphologic considerations (as well as patient preference and comorbidities) of a patient’s disease to select the appropriate range of treatment options. Increasingly clinicians are also required to consider a new set of issues, the so-called molecular determinants of head and neck cancer. In the sections below we will attempt to highlight the spectrum of these targets most likely to impact clinicians and patients in the coming years. We will touch briefly on inherited and somatic aberrations that predispose to tumorigenesis, both genetic and epigenetic, as well as a number of specific cancer pathways as targets of tumorigenesis and therapeutics. Finally, we will consider the role of new and developing molecular diagnostics in the management of patient care.
Figure 1. Diversity of Head & Neck Cancer.
Histopathologic diagnoses that present at the various subsites in the head and neck.
Molecular Basis of Risk Factors for Development of Head and Neck Cancer
The most well known risk factor for developing head and neck cancer is the deleterious effects of tobacco. Indeed, HNSCC was one of the first carcinomas to be linked with p53 mutations caused by tobacco usage [1]. Alcohol use is synergistic with tobacco in causing HNSCC. There are other cultural habit-forming risk factors that have an association with HNSCC. Betel nut, a fruit that is the basic ingredient of a stimulant chew, is used by an estimated 200 to 400 million throughout Southeast Asia [2]. Betel nut is incorporated into Asian medicines to treat a variety of complaints from headaches to rheumatism [3]. The odds ratio of developing leukoplakia and submucous fibrosis from using betel nut is five compared to one in non-chewers [4]. The addition of tobacco raises the risk 3-fold [4]. The duration and frequency of betel nut use increase the risk of developing cancer, suggesting a dose-response relation [5].
Tobacco smoke is associated with structural changes in DNA, particularly those induced by oxidative damage. Benzo[α]pyrene diol epoxide (BPDE), a known tobacco carcinogen, induces genetic damage by forming covalently bound DNA adducts throughout the genome, including p53 [6]. Damage induced by BDPE and other such carcinogens can be repaired through the nucleotide excision repair (NER) system. Along with the NER the base excision repair (BER) system is another set of multi-step enzymatic complexes involved in the repair of nonspecific DNA damage, including gamma and ultraviolet radiation, cross linking, and chemical intra-/interstrand adduct formation. The BER handles the largest number of cytotoxic and mutagenic base lesions by specifically removing alterations of a single base pair that has been methylated, oxidated, or reduced and corrects single strand interruptions in DNA [7]. Therefore, individual variations in NER/BER are one of the factors that may influence tobacco smoking related cancer risks like HNSCC.
Interestingly, several studies have demonstrated that sequence variations in NER/BER genes contribute to HNSCC susceptibility [8–11]. The ERCC1 gene product is a key enzyme in the NER system, and one particular polymorphism at the ERCC1 gene (C8092A) may affect its mRNA stability, resulting in impaired DNA repair capacity [12]. Two single nucleotide polymorphisms (SNPs) in the XPD gene (Asp312Asn and Lys751Gln), also part of the NER cascade have been associated with suboptimal DNA repair capacity [13]. There are conflicting data regarding SNPs in the BER system and the predilection for developing HNSCC. Li et al, in one of the largest case-control studies of 830 patients with HNSCC and 854 cancer-free controls, evaluated the progression to HNSCC based on polymorphisms in 3 BER non-synonymous SNPs [9]. The BER system enzyme XRCC1 (Arg399Gln), actually inconsistently increases the risk of HNSCC in Caucasians [13–16]. On the other hand, Li et al conclude that polymorphisms in the ADPRT enzyme of the BER system are associated with HNSCC and they demonstrate that individuals with the ADPRT 762Ala/Ala and Ala/Ala1Val/Ala genotypes were at lower risk of developing HNSCC compared with individuals who had the Val/Val genotypes [9]. Further studies to elucidate the genetic predisposition of developing HNSCC in the face of total tobacco burden may provide preventative health benefits to individuals with susceptible polymorphisms in the future.
Marijuana is the most commonly used illegal drug in the United States and the second most commonly smoked substance after tobacco [17]. Habitual marijuana smoking manifests with similar signs and symptoms associated with chronic tobacco use [18, 19]. Furthermore the carcinogenic properties of marijuana smoke are similar to those of tobacco and numerous studies parallel the use of cannabinoids to cancer development [20–22]. Marijuana has been shown to induce cytogenic changes consisting of chromosomal breaks, deletions, and translocations in mammalian cells in vivo [23]. Until recently, there was not enough evidence to suggest a causative relation with oropharyngeal HNSCC, especially those caused by tobacco use [24]. However, HNSCC caused by human papilloma virus (HPV) may be associated with marijuana (see below).
Clearly, normal variation in patient genotype for genes in DNA repair pathways appears to modify baseline risk for cancer development, especially when impacted by environmental toxins such as smoking. In parallel there are a range of germline variants that are much more rare than SNP’s, and accordingly called mutations. These rare heritable events can be sporadic or conserved in families and are frequently recognized due to the high penetrance of one of a number of recognized familial cancer syndromes. Fanconi’s anemia, known for the risk of developing lympho-reticular malignancies due to germline mutations in the care-taker genes FAA, FAD, and FCC, carries a risk for developing second primary cancers in the tongue, pyriform sinus and post cricoid region [25]. Patients with Bloom syndrome are characterized to have mutations in the helicase genes and are predisposed to developing solid tumors in a number of anatomical sites, 6–8% of which arise from the tongue and larynx, respectively [26]. Homozygotes with ataxia telangiectasia who survive into their 20s and 30s are at increased risk of developing chronic T-cell leukemia and solid malignancies of the oral cavity as well as breast, stomach, pancreas, ovary and bladder [27]. Xeroderma pigmentosum, an autosomal recessive disorder of one or more of the XP genes in the NER system, manifests second primaries within the oral cavity in addition to the known risks of skin malignancies [26, 28]. Other such syndromes (affected gene indicated in parentheses) with primary manifestations in the head and neck include Cowden Syndrome (PTEN), Multiple endocrine neoplasia Type I (MEN I), Multiple endocrine neoplasia Type II (MEN II), Neurofibromatosis Type II (NF-2), Retinoblastoma (Rb).
Genetic cancer syndromes are generally recognized by the early age of onset of malignancies in impacted individuals as well as specific or unusual patterns of tumors. Cancer syndrome tumors are of scientific importance out of proportion to their incidence as they point clearly at specific pathways and targets that are key to the development of malignancy, in contrast to sporadic tumors where the causative lesion may be difficult to identify. The importance of this is highlighted in the case of the RET where a number of drugs such as axitinib and vandetanib have been developed, aimed at the mutation’s effect [29, 30].
Viral Associations and New Epidemic of HNSCC caused by HPV
Recently, human papillomavirus (HPV) infection has been identified as an etiologic agent for oropharyngeal carcinoma, a subset of squamous cell carcinomas, which comprises the tongue base and tonsil. Patients with oropharyngeal squamous cell carcinomas that have the HPV genes incorporated in their tumor genome are younger in age (by 3–5 years) and are less likely to have a history of tobacco and alcohol use [31].
What is most disconcerting is that while the overall incidence of HNSCC (1973–2004) has steadily declined according to the Surveillance Epidemiology and End Results (SEER) data base, the incidence of oropharyngeal cancer is increasing among younger age groups [32–35]. The unsettling implication is that the incidence of HPV-related HNSCC of the oropharynx could overtake HPV-unrelated HNSCC, thought to be associated more with traditional risk factors.
There is substantial evidence that infection with high-risk HPV subtypes, in particular HPV-16, is a risk factor for the development of oropharyngeal cancers [36–47]. In fact, Gillison et al. purport that HPV-positive and HPV-negative HNSCC of the oropharynx should be classified as two distinct cancers based on the clinical and molecular risk factors and etiology [48]. According to their case-controlled study, patients at risk factors for HPV-16 positive oropharyngeal squamous cell carcinoma were more likely to be white (p = 0.06), married (p < 0.001), college educated (p = 0.03) and have an annual income over $50,000 (p < 0.01); while neither intensity or duration of tobacco smoking or alcohol consumption did not increase the odds ratio of HPV-16 positive HNSCC [48]. Although case control studies have not linked marijuana use to HPV-negative HNSCC, Gillison et al. demonstrate a strong association of marijuana use and HPV-16 positive HNSCC and further theorize plausible mechanisms of cannibinoid modulation of the immune system [48].
High-risk HPV strains (16 and 18) associated with oropharyngeal squamous cell carcinoma (as well as cervical cancer) manipulate cellular pathways within affected cells to activate cell growth and suppress apoptosis. Malignant transformation begins with inactivation of the p53 tumor suppressor gene by E6, while a second HPV protein, E7, inactivates the retinoblastoma tumor suppressor protein (Rb). The HPV E6 and E7 proteins, encoded in the HPV-16 genome, functionally disrupt regulatory cell-cycle and DNA-repair pathways that drives genetic or epigenetic changes during molecular progression of HNSCC [49]. E6 targets the cellular ubiquitin-protein ligase E6-AP, which then targets p53 for ubiquitination and degradation, leaving cell growth unregulated. E7 associates with Rb and p21 blocking the interaction of Rb with E2F and initiating uncontrolled cell division [50].
Not only is the nascent HPV positive tumor subject to inactivation of tumor suppression genes from the viral genome, but several genes involved in transcription and cell cycle regulation are among the most prominent up-regulated in these tumors. One such cell cycle inhibitor is CDKN2A, which encodes the p16INK4A tumor suppressor protein that functions as a cyclin-dependent kinase inhibitor in the Rb tumor suppressor pathway. Increased expression of p16INK4A may potentially reflect loss of a negative feedback loop associated with inactivation of Rb by HPV E7 [51]. Overexpression of p16INK4A is strongly correlated with HPV infection in head and neck carcinomas and has been used as a surrogate marker for HPV [52].
Detecting high-risk HPV using in situ hybridization (ISH) from ethanol-fixed and Papanicolaou-stained smears after FNA has a 93% correlation to corresponding tissue sections positive for HPV-16 with PCR [53]. Interestingly, HPV-positive tumors are associated with nonkeratinizing cytomorphology [53]. A recent meta-analysis showed that 26% of HNSCCs from all subsites contain HPV genomic DNA [54], and it is now estimated that over 50% of oropharyngeal HNSCCs are related to HPV infection [55]. Although ISH and PCR techniques are available, there are no standardized clinical tests approved by the FDA for HPV positive HNSCC tumors.
Tumorigenesis/Carcinogenesis
Whereas in the case of HPV, a virus can usurp normal cellular processes, in the case of most patients, the development of carcinoma is the result of a stepwise accumulation of genetic alterations [56]. Three main steps include initiation, promotion, and progression. For this multiple-step process to succeed, numerous cellular processes and derangements must occur. The creation of an initial, critical, early genetic change helps set into motion the carcinogenic process [57]. Exposure to carcinogenic factors may lead to the abnormal expression of tumor suppressor genes and/or proto-oncogenes, which in turn, activate pathways that lead to the malignant transformation of cells. Oftentimes, this abnormal expression may include a sporadic mutation, deletion, loss of heterozygosity, overexpression, or epigenetic modification such as hypermethylation. For example, telomerase, an enzyme involved in immortalization, has been shown to be reactivated in roughly 90% of HNSCCs, while a deletion of 9p21 is found in 70–80% of these cases. Various point mutations in TP53 and the loss of heterozygosity of 17p are shown to exist in over 50% of HNSCC lesions [58]. Once this occurs, secondary genetic changes create greater genetic instability, shifting the cell toward a more malignant phenotype (Figure 2). Specifically, inactivation of tumor-suppressor genes allow for cellular proliferation to continue with unregulated and autonomous, self-sufficient growth. Proto-oncogenes also play a key role in tumorigenesis, helping the cell attain a malignant phenotype.
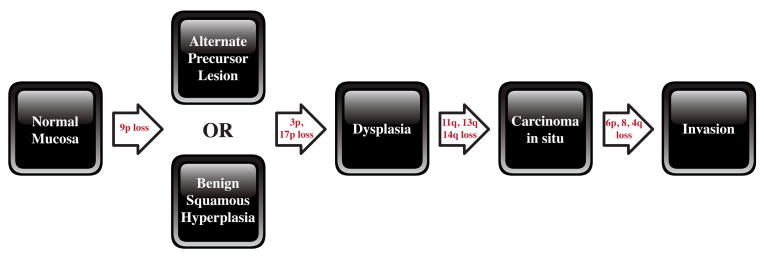
Figure 2. Genetic progression of HNSCC.
Genetic changes associated with the histological progression of HNSCC based on loss of chromosomal material (allelic loss). Genetic alterations have been placed prior to the lesion where the frequency of the particular event plateaus. It is the accumulation and not necessarily the order of genetic events that determines the progression. A small fraction of benign squamous hyperplastic lesions contain 9p21 or 3p21 loss, suggesting that an unidentified precursor lesion (or cells) may also give rise to dysplasia. Candidate tumor suppressor genes include p16 (9p21), p53 (17p), and retinoblastoma (13q), and a candidate proto-oncogene includes cyclin D1 (11q13). (figure taken and adapted with permission from Califano.)
Six hallmarks of cancer cells, distinguishing them from their normal counterparts, have been described: (i) self-sufficiency in growth signals, (ii) insensitivity to growth-inhibitory signals, (iii) evasion of programmed cell death, (iv) immortality or unlimited replicative potential, (v) sustained angiogenesis, and (vi) tissue invasion and metastasis [59]. In the following sections we will discuss major pathways, receptors, and proteins implicated in the initiation and/or progression of HNSCC as they relate to aspects of all six of these hallmarks. It is the accumulation of specific abnormalities such as those we describe, likely along with other genetic events and alterations that account for the process of carcinogenesis in HNSCC.
Field Cancerization
While it is unclear exactly why HPV appears to target certain subsites in the head and neck, the pattern is clear. In contrast, there appears to be a more general phenomenon seen in smokers where broad regions of tissue appear to be damaged, giving rise to multiple premalignant and frankly invasive tumors. In 1953, Slaughter et al. first hypothesized that primary tumors emerge from a layer of pre-cancerous tissue and coined the term “field cancerization” after demonstrating histopathologic changes consistent with genetic aberration from normal mucosa [60]. Forty years after Slaughter proposed field cancerization, Califano et al. demonstrated the molecular basis for histopathologic changes. Samples of dysplastic mucosa and benign hyperplastic lesions displayed loss of heterozygosity at specific loci (9p21 (20%), 3p21 (16%), 17p13 (11%)) [49]. In particular, loss of 9p21 or 3p21 is one of the earliest detectable events leading to the progression to dysplasia. From dysplasia, further genetic alteration in 11q, 13q, 14q creates carcinoma in situ. (Figure 2)
The high rate of recurrence in the location of the primary tumor is thought to be a result of the fact that 30% of histopathologiclly benign squamous cell epithelium consists of a clonal population with genetic alterations seen in HNSCC [61]. Studies using microsatellite analysis and X chromosome inactivation have verified that metachronous and synchronous lesions from distinct anatomic sites in HNSCC often originate from a common clone [62]. This evidence confirms that genetically altered mucosa is difficult to cure in the HNSCC patient since it is on the path to tumorigenesis. Indeed, second primaries are common in the patients with HNSCC.
Epithelial-to-Mesenchymal Transition
There is evidence to suggest fundamental changes to the programming of cells, including stem cells, may also be involved in tumorigenesis. One program that is particularly dangerous is the Epithelial-to-Mesenchymal Transition (EMT), a phenotypic change in cells that provides them with the ability to escape from constraints of surrounding tissue architecture. It has been postulated that EMT is the means by which epithelial tumors invade and metastasize to other tissues. As defined by Hugo et al, EMT is a culmination of protein modifications and transcriptional events in response to extracellular stimuli. These changes lead to long term, yet sometimes reversible, cellular changes [63]. Abnormalities in cadherins, tight junctions and desmosomes lead to a decrease in cell-cell adherence and loss of polarity in the cells, increasing the mobility of these cells. More specifically, epithelial cells disassemble their junctional structures, undergo extracellular matrix remodeling, begin to express proteins of mesenchymal origin, and subsequently become migratory [64]. This process has been postulated to be a part of normal embryogenesis, as well as the inflammatory process and wound healing [63]. When the process of EMT becomes pathological, it lacks the tight coordination and regulatory checkpoints that are normally present. Specifically, in the carcinogenic process, EMT causes changes in tumor cell properties that contribute to tumor invasion and metastasis, enabling cancer cell dissemination and self-renewal capabilities [65]. In HNSCC, EMT has been found to play a role, especially in high-risk tumor subtypes. Chung and colleagues showed that genes involved in EMT and nuclear factor-KB (NF-KB) signaling deregulation are the most prominent molecular characteristics of the high-risk tumors in the subset they examined [66]. While it is clear that EMT plays a role in tumorigenesis in many cancers, the complete clinical significance of this process is yet to be fully defined.
Epigenetic Modification
While many programs (such as those discussed in Figure 2) are the result of direct damage to the genome, there are other mechanisms of heritable somatic changes in gene expression that do not require direct alteration of the DNA sequence itself. The DNA molecule can be modified, such as by the addition or subtraction of methyl groups without a change in the base composition. Similarly, histones, the structural proteins found in close association with DNA, can be modified such as by acetylation, methylation and ubiquitylation. These non-DNA encoded modification can result in heritable changes in gene expression that are clinically significant, including in the setting of cancer. Different cancers display varying behaviors, likely due to the multiple epigenetic changes and genetic mutations that occur within a tumor environment. Hypermethylation is one such type of epigenetic modification that is increasingly well-characterized. Recently identified as a probable component in the development of carcinoma, hypermethylation in certain promoter regions of a gene can lead to repression of transcription [67]. Numerous studies have implicated this process of aberrant methylation in many tumor suppressor genes, causing them to become inactive [67].
Molecular Pathways Involved in HNSCC
Increasingly, model systems and other research techniques have helped to decipher pathways of importance for our patients (Figure 3). Knowledge of these pathways has led investigators to interrogate key pathway components for tumor-specific gene mutations, and many have been reported in head and neck tumors (Table 1). Initial clarity in the activated pathways and mutated genes of head and neck tumors has resulted in clinical trials of host of targeted therapies, such as those documented in table 2.. The most promising pathways and agents from this inventories are discussed below.
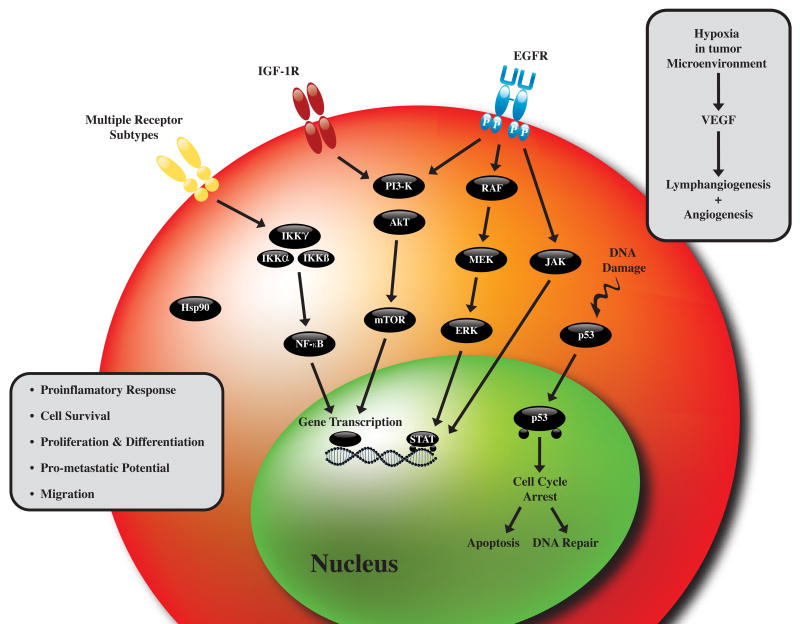
Figure 3.
Molecular pathways contributing to the promotion and progression of tumorigenesis in head and neck cancer.
Table 1. Most Common Somatic Mutations of Various Head & Neck Subsites.
Limited listing of some of the most common somatic mutations found in various anatomical regions of the head and neck. While at this time, there exists the capabilities to detect these mutations within tumor samples, their full clinical relevance has yet to be fully realized.
| HEAD & NECK SUBSITE | GENE | SAMPLE POSITIVE/TOTAL (% POSITIVE) |
|---|---|---|
| Larynx | CDKN2A | 45/262 (17%) |
| PTEN | 10/43 (23%) | |
| EGFR | 5/82 (6%) | |
| KRAS | 4/166 (2%) | |
| HRAS | 2/96 (2%) | |
|
| ||
| Oral Cavity | CDKN2A | 98/508 (19%) |
| HRAS | 67/494 (13%) | |
| FGFR3 | 44/136 (32%) | |
| PIK3CA | 18/145 (12%) | |
| KRAS | 14/497 (2%) | |
|
| ||
| Oropharynx | MET | 33/156 (21%) |
| CDKN2A | 19/173 (10%) | |
| PTEN | 7/27 (32%) | |
| KRAS | 3/105 (2%) | |
| BRAF | 3/52 (5%) | |
|
| ||
| Tonsil | EGFR | 7/45 (15%) |
| CDKN2A | 0/3 (0%) | |
| HRAS | 0/3 (0%) | |
| KRAS | 0/3 (0%) | |
| NRAS | 0/3 (0%) | |
|
| ||
| Sinonasal Cavity | KRAS | 4/121 (3%) |
| HRAS | 2/11 (18%) | |
| NRAS | 2/11 (18%) | |
| STK11 | 2/7 (28%) | |
| EGFR | 1/5 (20%) | |
|
| ||
| Esophagus (upper 1/3) | TP53 | 4/4 (100%) |
| KRAS | 1/4 (25%) | |
| CDKN2A | 1/3 (33%) | |
| PIK3CA | 1/3 (33%) | |
| CTNNB1 | 0/9 (0%) | |
|
| ||
| Thyroid | BRAF | 2013/4793 (41%) |
| RET | 274/706 (38%) | |
| NRAS | 132/1962 (6%) | |
| KRAS | 80/1878 (4%) | |
| HRAS | 56/1844 (3%) | |
|
| ||
| Salivary Gland | HRAS | 17/90 (18%) |
| PTEN | 5/13 (38%) | |
| DTNNB1 | 2/44 (4%) | |
| KRAS | 1/40 (2%) | |
| CDKN2A | 1/8 (12%) | |
Note: Squamous Cell Carcinoma (SCC) was the only histology tested for in the Larynx, Oral Cavity, Oropharynx, Tonsil, Sinonasal Cavity, and Esophagus. A wider range of histologic variants were included for the analysis of Thyroid and Salivary Gland subsites.
Table 2.
A limited listing of selected targeted agents that are currently undergoing clinical trials for the treatment of head and neck carcinoma. This partial list was obtained through an extensive and comprehensive search on www.clinicaltrial.gov.
| Drug Name (Tradename) | Target(s) | Phase of study in Head and Neck cancer |
|---|---|---|
| Cetuximab (Erbitux) | EGFR | III |
| Gefitinib (Iressa) | EGFR | I/II/III |
| Erlotinib (Tarceva) | EGFR | I/II/III |
| Panitumumab (Vectibix) | EGFR | I/II/III |
| BIBW 2992 (Tovok) | EGFR, HER-2/neu | II |
| Zalutumumab (HuMax-EGFr) | EGFR | I/II/III |
| Trastuzumab (Herceptin) | HER-2/neu | II |
| Lapatinib (Tykerb) | EGFR, HER-2/neu | I/II/III |
| Cediranib (Recentin) | VEGF | I/II |
| Sorafenib (Nexavar) | Raf, VEGF | I/II |
| Semaxanib | VEGF | I/II |
| Pazopanib | VEGF | II |
| Sunitinib (Sutent) | VEGF | I/II |
| Bevacizumab (Avastin) | VEGF | I/II/III |
| Romidepsin | Histone deacetylase | I/II |
| Vorinostat (Zolinza) | Histone deacetylase | I/II |
| Dasatinib (Sprycel) | Tyrosine kinases | II |
| Imatinib (Gleevec) | Tyrosine kinases | II |
| Pazopanib | VEGF, Tyrosine kinases | II |
| Vandetanib (Zactima) | VEGF, EGFR | I/II |
| XL880 | VEGF, Tyrosine kinases | II |
| Perifosine (KRX-0401) | Akt | II |
| Bortezomib (Velcade) | NF-kB, Tyrosine kinases | I/II |
| Lonafarnib (Serasar) | Farnesyl transferase | I/II |
| Tanespimycin (KOS-953) | Hsp90 | I/II |
| AZD0530 | Src/Abl kinase | II |
Epidermal Growth Factor Receptor (EGFR)
Epidermal growth factor receptor (EGFR) signaling has been strongly implicated in carcinogenesis, tumor progression, and response to therapy in HNSCC (reviewed in [68]). The ErbB family of proteins, a family of four structurally-related receptor tyrosine kinases, is comprised of four receptors (ErbB 1–4, also known as HER 1–4) and thirteen polypeptide extracellular ligands [69]. In the literature, ErbB2 is synonymous with HER2/neu, while ErbB1 is commonly referred to as EGFR. When ligands bind to one of the ErbB receptors, a dimer forms and the receptor’s intracellular tyrosine residues then undergo ATP-dependent autophosphorylation. Currently, there are 12 different ligands that are known to activate four known ErbB receptors.
Once phosphorylated, the receptor has the potential to trigger a number of different intracellular downstream pathways that can eventually arrest apoptosis, promote cellular proliferation, stimulate tumor-induced neovascularization, and activate carcinoma invasion and metastasis [69]. The Ras/mitogen-activated protein kinase/extracellular signal-related kinase (Ras-MAPK-ERK) pathway is known to control gene transcription, cell proliferation and cell-cycle progression, while the phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) pathway has been shown to stimulate numerous anti-apoptotic signals within the cell. The Janus kinase/signal transducers and activators of transcription (JAK/STAT) and the phospholipase-Cγ/protein kinase C (PLCγ/PKC) pathways are also activated in association with EGFR phosphorylation [70]. Thus, EGFR plays a role in carcinoma growth and survival through a multitude of oncogenic downstream signaling pathways.
EGFR mRNA and protein are known to be preferentially expressed in HNSCC compared to surrounding normal tissues, suggesting a significant role in carcinogenesis. Similarly, the vast majority of epithelial carcinomas overexpress and possess functional activation of the EGFR family of receptors [71]. In HNSCC, EGFR is overexpressed in up to 80–100% of tumors, some of the highest rates of any human carcinoma [72, 73]. Interestingly, there are regional differences among tissues in the head and neck that express EGFR, with relatively lower levels associated with laryngeal tumors as compared to those of the oral cavity and oropharynx [74].
EGFR demonstrates increased overexpression in the more advanced-staged carcinomas as well as in those carcinomas that were found to be poorly differentiated [70]. In addition, EGFR overexpression is associated with decreased patient survival rates, and has been demonstrated by some groups to confer resistance to various therapeutic modalities including targeted therapy [70, 75–79]. While the association with poor patient prognosis has not been as clearly established, specific mutations of the EGFR receptor have also been studied. The most common mutation of EGFR is likely EGFRvIII, occurring in up to 40% of HNSCC [80]. This mutant receptor is only found in cancer cells and manifests from an in-frame deletion of exons 2–7, which encodes the receptor’s extracellular domain, thus resulting in a constitutively active receptor that is completely independent of any activation via ligand binding [80]. The fact that EGFRvIII is not found in normal tissues makes this a very intriguing highly-specific target for therapy, given that it would not interfere with the normal EGFR signaling in non-cancerous tissues. (Table 1)
In addition to overexpression, other pathological manifestations of EGFR can be carried out through mutational activation, amplification, and transactivation by other tyrosine kinases [81]. The potential, constitutive activation of several different oncogenic pathways, via EGFR-independent mechanisms, likely explains the lack of response that is commonly appreciated in patients being treated with EGFR inhibitor therapy [70].
With the prominent role that EGFR is known to play in tumorigenesis, this family of proteins was a logical choice in pursuing a new class of targeted cancer therapy. Currently, there are several EGFR antagonists available for clinical utilization in the treatment of four metastatic epithelial carcinomas, including non-small cell lung cancer, colorectal cancer, pancreatic cancer, and HNSCC. The two classes of therapies that exist to date are monoclonal antibodies to EGFR receptor subunits and small-molecule EGFR tyrosine kinase inhibitors (TKIs). In simplest terms the monoclonal antibodies probably act by binding the conserved extracellular domain of EGFR and then blocking the ligand-binding region by competitive inhibition. In turn, this blocks ligand-induced autophosphorylation through the inability to stimulate tyrosine kinase. The EGFR tyrosine kinase inhibitors function via a separate mechanism. They act by reversibly competing with ATP in its binding site to the intracellular catalytic domain of tyrosine kinase, therefore inhibiting autophosphorylation of EGFR and its subsequent downstream signaling [71].
Akimoto et al. was the first to provide evidence that EGFR expression may have an effect on radiation sensitivity [82], a result that has been validated clinically [75, 83]. EGFR over-expression in head and neck cancer cell lines were found to have greater radioresistence compared to cell lines with relatively lower levels of EGFR expression. It was also found that following radiation, EGFR becomes upregulated within the tumor, leading to increased activation of its downstream signaling pathways [84, 85]. This work culminated in the landmark 2006 publication of the randomized trial by Bonner et al. showing an overall and progression-free survival advantage with the addition of cetuximab to standard radiation therapy (RT) [86].
There are currently at least 40 trials involving patients with HNSCC investigating various “targeted agents” including tyrosine kinase inhibitors and antibody therapy [87]. More than ten different EGFR-targeting agents are in development for the treatment of various carcinomas. Although a great deal of effort has gone into the development and validation of predictive biomarkers, it remains difficult to determine a priori who will benefit from these therapies. In some studies, EGFR expression is an independent predictor of response while in others no relationship is appreciated [75, 88–91]. The search for molecular predictors of clinical outcome that would potentially optimize patient selection and therapeutic efficacy continues to be an area of intense ongoing investigation.
Insulin-Like Growth Factor-1 Receptor (IGF-1R)
An emerging potential target for directed, molecular-based cancer therapy is the Insulin-Like Growth Factor (IGF) signaling axis. Numerous preclinical and clinical studies have implicated the Insulin-Like Growth Factor-1 Receptor (IGF-1R) and its ligands, Insulin Growth Factor-1 (IGF-1) and Insulin Growth Factor-2 (IGF-2), in both the development and progression of a number of human cancers [92–94]. IGF-1R is a transmembrane heterotetramer receptor that consists of two α and two β subunits. Like the insulin receptor, IGF1-R possesses tyrosine kinase (TK) activity. With activation of the receptor, downstream signaling events include phosphorylation of insulin receptor substrate-1 (IRS-1), activation of mitogen-activated protein kinases (MAPKs), and stimulation of the phosphatidylinositol-3 kinase(PI3K) pathway [95]. This activation of both the Ras-MAPK-ERK and PI3K/Akt pathways is similar to the downstream signaling seen with EGFR autophosphorylation and activation. Six IGF binding proteins (IGFBPs) are known to exist in humans. These proteins have been shown to help modulate the effects of IGF-1 via multiple unique mechanisms. In humans, IGF-1 is bound to one of the IGFBPs over 95% of the time, with IGFBP-3 accounting for roughly 85% of this binding [96].
IGF-1R has been shown, in both in vitro and in vivo studies, to encourage cellular growth, and protect cells from apoptosis [97]. This phosphorylation cascade leads to the activation of various transcription factors involved in both cellular proliferation and transformation [98–102]. Constitutive activation and/or overexpression of IGF-1R has been associated with malignant transformation, involving glioblastoma, melanoma, pancreatic, breast, colon, and ovarian carcinoma models [103–105]. The receptor has been closely linked to the metastatic properties of tumor cells, with studies showing the receptor to signal pathways linked to tumor invasiveness and angiogenesis [106, 107]. IGF-1R signaling has also been shown to influence and promote focal adhesion stability, cell-to-cell contact, and cellular motility [108]. Numerous studies have also shown that enhanced IGF-1R activation is associated with resistance to certain cytotoxic chemotherapy regimens, hormonal agents and biological anticancer therapies, as well as radiation therapy [109–117].
While Ouban et al showed only 13% immunostaining positivity for IGF-1R in 31 different human HNSCC tissue samples, other studies have shown much higher levels of expression [118]. IGF-1R is ubiquitously expressed at varying levels in cancerous tissues, and plays an intricate role in the regulation of cellular proliferation and differentiation even at very low levels of expression [95, 118].
As mentioned previously, the IGF-R1 and the EGFR signaling pathways are intricately associated with one another, regulating overlapping downstream signaling pathways. Interestingly, increased IGF-1R expression has been reported to mediate resistance to anti-EGFR-based therapies in certain solid tumors, including glioblastoma, pancreatic, and breast carcinoma [119–122]. It has been found that the use of the combination of both antibodies was more effective than either single agent alone at reducing cancer cell growth [123]. There may be a potential benefit in the use of combined anti-tyrosine kinase receptor directed therapies to treat HNSCC. Slomiany et al also demonstrated the potential for the co-targeting of both IGF-1R and EGFR signaling pathways in HNSCC [124].
Design of IGF-1R inhibitors has proven to be somewhat problematic, due to the close homology (60–70% amino acid homology) with the insulin receptor [125]. However, specific inhibitors have recently been developed. Several approaches at tumor growth inhibition have been undertaken, including IGF-1R dominant mutants, IGF-1R blocking antibodies, and oligonucleotides aimed at downregulating IGF-1R expression, little success has been achieved in terms of improved clinical outcomes. However, the first IGF-1R tyrosine kinase inhibitor showing in vivo therapeutic potential, NVP-AEW541 (Novartis), has been shown to enhance tumor cell chemosensitivity and inhibit tumor growth in human fibrosarcoma, myeloma, and Ewing’s sarcoma models [125, 126]. Studies with the inhibitor have yet to be undertaken in a HNSCC model.
Phosphatidylinositol-3-kinase/protein kinase B pathway (PI3-K/Akt)
The phosphatidylinositol-3-kinase/protein kinase B (PI3-K/Akt) signal transduction pathway has been shown to regulate numerous cellular processes, including apoptosis, proliferation, cell cycle progression, cytoskeletal stability and motility, as well as energy metabolism [127, 128]. Activated Akt induces increased expression of numerous proliferative and anti-apoptotic proteins, including Bcl-2, Bcl-x and NF-kB [127]. The pathway has been shown to be activated in up to 50–80% of HNSCCs [78]. The PI3-K/Akt pathway is one of the main downstream signaling pathways activated by the ErbB/tyrosine kinase receptor family of receptors. Upon ligand binding, the cytoplasmic domain of the EGFR undergoes tyrosine phosphorylation and subsequently activates PI3-K [129]. However, activation of the PI3-K/Akt pathway is not entirely dependent on the tyrosine kinase family of receptors. In certain carcinoma models, it has been shown to be activated through direct mutation or amplification of PI3-K, amplification of Akt, activation of the RAS oncogene, and/or decreased expression of the tumor-suppressor protein, phosphatase and tensin homolog (PTEN), a known inhibitor of the PI3-K/Akt pathway [129]. Loss of PTEN expression, along with Akt activation, correlates with worse clinical outcomes in patients with SCC of the tongue [130, 131]. This pathway has also been found to be overexpressed and activated in a number of different carcinomas, including HNSCC. In a study conducted by Massarelli et al, it was shown that disease-free survival was significantly decreased in cases of SCC of the tongue that stained positive for activated Akt (p-Akt) [132]. The comparatively poor outcome that was associated with p-Akt expression was also found to be independent of cancer stage and nodal status. Akt activation has also been correlated with the squamous cell progression and transformation: from normal epithelial tissue, to dysplasia, and then even to invasive squamous cell carcinoma [133].
Following radiation therapy, the PI3kK/Akt pathway has also been shown to be upregulated. Bussink et al described how the pathway is intricately involved with resistance to radiation therapy via multiple mechanisms [129]. The RAS oncogene is a well-known contributor to the intrinsic radioresistance of tumor cells, mediated at least partially, through its downstream signaling through the PI3-K/Akt pathway [129]. Additionally, this pathway is involved in DNA repair via EGFR signaling, with multiple studies showing that with EGFR blockade, the PI3-K/Akt pathway–mediated DNA repair process is altered. When combined with radiation therapy, this decreased DNA repair leads to greater levels of tumor cell apoptosis and subsequent improved locoregional control when compared to radiation therapy alone [129].
While this pathway is most often activated through EGFR, EGFR-independent activation is quite common and of clinical relevance as well [129]. Molino et al showed, utilizing tissue microarray technology, that the PI3-K/Akt/mTOR pathway was frequently activated in HNSCC samples and that this was often independent of any associated EGFR activation [134]. There has also been a strong and independent correlation between expression of activated Akt (pAkt) and treatment outcome in laryngeal and oropharyngeal HNSCC [132, 135]. Similarly, enhanced Akt activity has been independently associated with more advanced tumor stage and progression in a number of different malignancies [136]. Knowing this information, further study of this pathway, independent of EGFR, is needed so that it can be utilized in regards to treatment as well as prognostic markers for HNSCC.
A recent phase II trial conducted by Argiris and colleagues involved the use of an Akt phosphorylation/activation inhibitor, perifosine, in a small group of patients with incurable recurrent and/or metastatic HNSCC [137]. While the inhibitor had some preclinical anti-tumor activity in vivo, no objective clinical responses were appreciated, with 18/19 patients having disease that progressed at 8 weeks follow-up [137]. However, Akt activation has also been associated with resistance to EGFR inhibition in a non-small cell lung cancer model [138], and therefore, benefits from combined targeted molecular therapy are still of potential interest. The potential clinical usefulness and of the PI3-K/Akt pathway as a therapeutic target and/or prognostic marker in HNSCC is promising.
Mammalian Target of Rapamycin (mTOR)
One of the downstream cell-growth regulators associated with the PI3-K/Akt pathway is an atypical serine/threonine kinase named mammalian target of rapamycin (mTOR). While Akt helps control cellular proliferation and growth through the coordination of mitogenic signaling with energy and nutrient-sensing pathways that control protein synthesis, it requires mTOR to fully exert these effects [139]. mTOR is involved in modulation of the cell cycle and ribosomal function to subsequently participate in both cell growth and apoptosis [140]. When activated through Akt, mTOR then phosphorylates the translation regulator p70-S6 kinase, which in turn activates the ribosomal S6 protein, a protein involved in translation and one of the most downstream targets of the PI3-K/Akt/mTOR pathway. This activation of S6 ribosomal protein thus adds to the control of cell growth through increased manipulation of mRNA translation [141].
Amornphimoltham et al found that in clinical specimens from patients with HNSCC, as well as in HNSCC-derived cell lines, aberrant accumulation of activated S6 (p-S6) was a frequent occurrence in both early dsyplastic lesions and carcinomas [139]. They also showed that the activated ribosomal protein was decreased when HNSCC cell lines were treated with rapamycin, a macrolide antibiotic and a known inhibitor of mTOR [139]. Similar findings were seen when rapamycin was used in a HNSCC xenograft model. In vivo, rapamycin’s effects included induction of apoptosis and inhibition of cellular growth of the HNSCC cells with subsequent tumor regression [139]. It has also been shown that rapamycin sensitivity of tumor cells is at least partially dependent on the dysregulation of the tumor suppressor gene, PTEN, the well-known inhibitor of the PI3-K/Akt pathway [142, 143]. Interestingly, in some of the HNSCC cell lines, EGFR inhibition had no effect on the activity of the mTOR pathway, indicating a potential clinical and therapeutic benefit if both EGFR inhibitors and mTOR inhibitors were used in combination [139]. The study did reveal the Akt/mTOR pathway to be a potential therapeutic target for the treatment of HNSCC, particularly in terms of development of analogues to rapamycin.
Nathan et al showed that one of the main downstream effectors of the Akt/mTOR pathway, eIF4E, was overexpressed in histologically “tumor-free” surgical margins of resected HNSCC samples, and was also an independent predictor of tumor recurrence [144]. In a later study, the same group utilized an eIF4E-overexpressing, PTEN-mutant HNSCC cell line, FaDu, in a minimal residual disease murine model, and showed that the rapamycin analog, Temsirolimus (CCI-779), was effective in prolonging survival, including improved tumor-free survival [145]. It was concluded that CCI-779 represented a new potential targeted therapy for the treatment of HNSCC, since overexpression and activation of mTOR occurs in many of these tumors [139, 146].
Nuclear factor - kappa B (NF-kB)
Nuclear factor–kB (NF-kB) transcription factors are the final downstream mediators of many of the above-mentioned pathways and therefore play a key role in head and neck cancers. It has become clear that NF-kB–mediated inflammatory signaling is overexpressed in many HNSCCs. Expression has also been shown to be associated with tumorigenesis and metastasis [147, 148]. Constitutive NF-kB activation has been shown to be common when induced by carcinogen exposure or stimulation with oncogenic viruses, with induced levels of activation being seen in tissue specimens of HNSCC [149–151]. Increases in nuclear localization of NF-kB has been detected in roughly 85% of patients with HNSCC, with increased immunostaining correlating with worse prognosis of the disease [151].
NF-kB is known to be an important factor in regulating the expression of genes associated with angiogenesis (IL-8), apoptosis (Bcl-xL), cellular proliferation (cyclin D1), and proinflammatory cytokine cascades (IL-6, IL-1α) [148, 150, 152–156]. In terms of cell survival and apoptosis, NF-kB, in close association with STAT3 and p53, has been found to directly affect the balance of pro-apoptotic and anti-apoptotic proteins in squamous cell carcinoma (SCC) cell lines [157]. It is also known be involved in attenuated sensitivity to cytotoxic anticancer therapy [155, 158]. Similarly, the reactive oxygen species (ROS) that ionizing radiation creates in the body have been shown to induce NF-kB as a cytoprotective mechanism within tumor cells, rendering the radiation therapy less effective [159]. There is even evidence that NF-kB has direct carcinogenic effects that give tumor cells the ability to evade the regulatory functions of the immune system [148]. It has also been shown that both tobacco smoke condensate and betel nut extract activate NF-kB indirectly via degradation of inhibitor kB proteins (IkB) [160, 161].
With its constitutive activation and proven role in radiation therapy resistance, its inhibition is a logical target for therapy. Salicylates, antioxidants, nonsteroidal anti-inflammatory drugs (NSAIDs), prostaglandins, and glucocorticoids have all shown effectiveness in various settings to inhibit NF-kB [162]. IkB kinase beta (IKKβ) inhibitors have also shown some effectiveness in preclinical studies in various carcinomas, yet no head and neck carcinoma studies are currently underway [163]. Further development of these novel therapies continues in the hopes that through inhibition of NF-kB, a decrease in tumor invasion, aggressiveness, and metastasis will be realized.
Heat Shock Protein 90
Heat Shock Protein 90 (Hsp90) is a molecular chaperone that induces conformational changes in numerous protein substrates, including transcription factors and protein kinases [164, 165]. Hsp90 has been shown to be involved with proteins in many signaling pathways important for tumor cell survival, proliferation, and metastasis. Specifically, Hsp90 is associated with the EGFR pathway, IGF-R, Akt, ERK, Ikβ kinases, p53, and STAT3 [165]. It has been shown to be constitutively expressed at up to 10 times higher levels in tumor cells when compared to normal cells [166]. Therefore, therapeutic inhibition of Hsp90 offers the potential to simultaneously disrupt numerous pathways known to be involved in the progression toward malignant phenotypes.
A Geldanamycin derivative and semisynthetic analogue, 17-AAG (Tanespimycin), became the first Hsp90 inhibitor to enter clinical testing [167]. Recently, Shintani and colleagues investigated the effects of 17-AAG on radiation sensitivity on oral SCC cell lines in vitro. They found that the radiation response was enhanced in 17-AAG treated cells, but only in cell lines with wild-type p53 expression. Yin and colleagues recently used a novel Hsp90 inhibitor, EC5, in their study of eight HNSCC cell lines [168]. EC5, a benzoquinone ansamycin antibiotic derivative, was shown to have anti-tumor effects in xenograft models [168]. They also compared these results with 17-AAG, and it was shown that EC5 caused more potent anti-tumor effects in these models [168].
Prognostic and Diagnostic Markers, Genetic Profiling
With the advent of increasingly sophisticated molecular detection techniques and technologies such as DNA microarrays, large numbers of genetic markers are able to be tested with greater ease. In a study by Roepman and colleagues, predictor gene sets were found to have greater predictive power in the detection of local nodal metastases from primary tumor samples than the current clinical diagnosis and staging systems [169]. While this technology offers excellent opportunities to further dissect the molecular and genetic interactions that participate in carcinogenesis, it is clear that conflicting data and findings will persist, as continued improvement in analysis and interpretation occurs.
Early in the molecular study of head and neck cancer, several studies demonstrated an association between p53 abnormalities and poor outcome. Yet, other large follow-up studies failed to demonstrate such an association [78]. The prognostic significance of p16 aberrations has also been variable. A majority of studies conducted on the significance of EGFR and cyclin D1 overexpression have shown them to be associated with a worse prognosis in patients with HNSCC [78]. Similarly, genetic alterations of Cox-2, p27, p53, CCDN1 and VEGF, among others, have all been shown in various studies to be associated with an increased risk of metastasis, disease recurrence, and/or overall worse prognosis [78, 170]. However, like many other markers, there have been other studies that fail to confirm these significant findings.
As single molecular markers, most that have been studied to date have failed to show sufficient predictive potential in terms of the course of disease, prognosis, and survival. However, while single markers may not prove to have the clinical applicability that many had hoped for, combinations of different molecular markers and genetic expression patterns may offer more promising diagnostic and prognostic value. Utilizing cDNA microarray technology, Chung and colleagues looked at 60 HNSCC tumor samples and categorized them into four distinct subtypes of HNSCC, each showing clinically unique behavior [171]. Not only did they show that these subtypes showed distinct behavior clinically, they also concluded that the status of possible regional metastases could be predicted by genetic microarray analysis of the primary lesion [171]. Other combinatorial approaches, using biomarkers and traditional clinical markers have been proposed and will likely gain increasing interest [172]. Selection from among the competing markers, and incorporation into clinical practice will be one of the major challenges on the horizon for physicians.
Conclusion
While cancer is generally thought of as a disease of DNA, the risk for any individual of developing a tumor based on his or her genomic makeup cannot be explicitly determined given our current scope of knowledge. However, the risk is certainly a function of germline DNA composition, interacting with the environment (especially toxins and infections), and perhaps an arbitrary component of statistical probability applied over the years of a normal lifespan. The types of genetic damage incurred are probably primarily to DNA in the form of deletions, amplifications, or focal damaging mutations, although other mechanisms are increasingly noted to be important including epigenetic changes to chromatin. No matter what the mechanism, the ultimate event is a perturbation of normal cellular biomolecules and homeostasis leading to the hallmark processes of cancer characterized by pathways such as those described in the preceding sections.
Certainly most scientists and clinicians emphasize models of carcinogenesis similar to the ones just described, heavily focused on molecular events and cancer pathways. Yet while our research colleagues rely heavily on an increasingly sophisticated set of molecular assays and reagents, the clinical care of the head and neck cancer patient is currently almost devoid of any molecular diagnostic. Likewise, the set of targeted cancer therapies for our patients remains limited and prognosis or response to therapy has rarely if every been assigned based on molecular testing. However, the landscape appears quite likely to change in the near future. One has only to look at the therapies listed in table 2 to witness the increasing need to identify which specific genes and pathways are altered in a particular tumor. It is likely that patients at differential risk of developing cancer are increasingly likely to be identified from germline DNA, either in the form of mutations or SNP’s. Tumors that are primarily attributable to viral etiologies are likely to be identified and with potentially altered treatment paradigms, or perhaps averted entirely through vaccines. The integration of molecular diagnostics and molecular-risk-based approach to cancer treatment that is pathway driven is likely to be both a major success and challenge that our generation faces over the coming years.
Synopsis
Patients present with differential baseline risk of cancer based on normal and expected variation in genes associated with cancer. The baseline risk of developing cancer is acted on throughout life as the genome of different cells interacts with the environment in the form of exposures such as toxins or infections. As genetic damage is incurred throughout a lifetime, either directly to DNA sequences or to the epigenome, events are set in motion to progressively disrupt normal cellular pathways towards tumorigenesis. This manuscript attempts to characterize broad categories of genetic aberrations and pathways in a light that might be useful for the clinician to understand the risk of developing cancer, the pathways that are disrupted, and the potential for molecular-based diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brennan JA, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332(11):712–7. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 2.Zain RB, et al. Oral lesions associated with betel quid and tobacco chewing habits. Oral Dis. 1997;3(3):204–5. doi: 10.1111/j.1601-0825.1997.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 3.Norton SA. Betel: consumption and consequences. J Am Acad Dermatol. 1998;38(1):81–8. doi: 10.1016/s0190-9622(98)70543-2. [DOI] [PubMed] [Google Scholar]
- 4.Warnakulasuriya S, Trivedy C, Peters TJ. Areca nut use: an independent risk factor for oral cancer. BMJ. 2002;324(7341):799–800. doi: 10.1136/bmj.324.7341.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu CT, et al. A case-control study of oral cancer in Changhua County, Taiwan. J Oral Pathol Med. 1996;25(5):245–8. doi: 10.1111/j.1600-0714.1996.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 6.Denissenko MF, et al. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274(5286):430–2. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DM, 3rd, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6(4):544–59. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Hung RJ, et al. Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am J Epidemiol. 2005;162(10):925–42. doi: 10.1093/aje/kwi318. [DOI] [PubMed] [Google Scholar]
- 9.Li C, et al. Genetic polymorphisms in DNA base-excision repair genes ADPRT, XRCC1, and APE1 and the risk of squamous cell carcinoma of the head and neck. Cancer. 2007;110(4):867–75. doi: 10.1002/cncr.22861. [DOI] [PubMed] [Google Scholar]
- 10.Cheng L, et al. Expression of nucleotide excision repair genes and the risk for squamous cell carcinoma of the head and neck. Cancer. 2002;94(2):393–7. doi: 10.1002/cncr.10231. [DOI] [PubMed] [Google Scholar]
- 11.Handra-Luca A, et al. Excision repair cross complementation group 1 immunohistochemical expression predicts objective response and cancer-specific survival in patients treated by Cisplatin-based induction chemotherapy for locally advanced head and neck squamous cell carcinoma. Clin Cancer Res. 2007;13(13):3855–9. doi: 10.1158/1078-0432.CCR-07-0252. [DOI] [PubMed] [Google Scholar]
- 12.Shen MR, I, Jones M, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998;58(4):604–8. [PubMed] [Google Scholar]
- 13.Lunn RM, et al. XPD polymorphisms: effects on DNA repair proficiency. Carcinogenesis. 2000;21(4):551–5. doi: 10.1093/carcin/21.4.551. [DOI] [PubMed] [Google Scholar]
- 14.Duell EJ, et al. Polymorphisms in the DNA repair genes XRCC1 and ERCC2 and biomarkers of DNA damage in human blood mononuclear cells. Carcinogenesis. 2000;21(5):965–71. doi: 10.1093/carcin/21.5.965. [DOI] [PubMed] [Google Scholar]
- 15.Lunn RM, et al. XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res. 1999;59(11):2557–61. [PubMed] [Google Scholar]
- 16.Lei YC, et al. Effects on sister chromatid exchange frequency of polymorphisms in DNA repair gene XRCC1 in smokers. Mutat Res. 2002;519(1–2):93–101. doi: 10.1016/s1383-5718(02)00127-4. [DOI] [PubMed] [Google Scholar]
- 17.Johnston LD, O’Malley PM. The recanting of earlier reported drug use by young adults. NIDA Res Monogr. 1997;167:59–80. [PubMed] [Google Scholar]
- 18.Tashkin DP. Pulmonary complications of smoked substance abuse. West J Med. 1990;152(5):525–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Tashkin DP. Is frequent marijuana smoking harmful to health? West J Med. 1993;158(6):635–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Hashibe M, Ford DE, Zhang ZF. Marijuana smoking and head and neck cancer. J Clin Pharmacol. 2002;42(11 Suppl):103S–107S. doi: 10.1002/j.1552-4604.2002.tb06010.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZF, et al. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1071–8. [PubMed] [Google Scholar]
- 22.Donald PJ. Marijuana smoking--possible cause of head and neck carcinoma in young patients. Otolaryngol Head Neck Surg. 1986;94(4):517–21. doi: 10.1177/019459988609400420. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman S, Zimmerman AM. Genetic effects of marijuana. Int J Addict. 1990;25(1A):19–33. doi: 10.3109/10826089009067003. [DOI] [PubMed] [Google Scholar]
- 24.Rosenblatt KA, et al. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004;64(11):4049–54. doi: 10.1158/0008-5472.CAN-03-3425. [DOI] [PubMed] [Google Scholar]
- 25.Lustig JP, et al. Head and neck carcinoma in Fanconi’s anaemia--report of a case and review of the literature. Eur J Cancer B Oral Oncol. 1995;31B(1):68–72. doi: 10.1016/0964-1955(94)00044-5. [DOI] [PubMed] [Google Scholar]
- 26.Prime SS, et al. A review of inherited cancer syndromes and their relevance to oral squamous cell carcinoma. Oral Oncol. 2001;37(1):1–16. doi: 10.1016/s1368-8375(00)00055-5. [DOI] [PubMed] [Google Scholar]
- 27.Hecht F, Hecht BK. Cancer in ataxia-telangiectasia patients. Cancer Genet Cytogenet. 1990;46(1):9–19. doi: 10.1016/0165-4608(90)90003-s. [DOI] [PubMed] [Google Scholar]
- 28.Keukens F, et al. Xeroderma pigmentosum: squamous cell carcinoma of the tongue. Acta Derm Venereol. 1989;69(6):530–1. [PubMed] [Google Scholar]
- 29.Cohen EE, et al. Axitinib Is an Active Treatment for All Histologic Subtypes of Advanced Thyroid Cancer: Results From a Phase II Study. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells SAGJ, Jr, RF Gagel, et al. Vandetanib in metastatic hereditary medullary thyroid cancer: Follow-up results of an open-label phase II trial. J Clin Oncol. 2007;25:303s. [Google Scholar]
- 31.Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck. 2007;29(8):779–92. doi: 10.1002/hed.20573. [DOI] [PubMed] [Google Scholar]
- 32.Canto MT, Devesa SS. Oral cavity and pharynx cancer incidence rates in the United States, 1975–1998. Oral Oncol. 2002;38(6):610–7. doi: 10.1016/s1368-8375(01)00109-9. [DOI] [PubMed] [Google Scholar]
- 33.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103(9):1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 34.Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128(3):268–74. doi: 10.1001/archotol.128.3.268. [DOI] [PubMed] [Google Scholar]
- 35.Chaturvedi AK, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 36.Syrjanen S. HPV infections and tonsillar carcinoma. J Clin Pathol. 2004;57(5):449–55. doi: 10.1136/jcp.2003.008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods KV, et al. Analysis of human papillomavirus DNA in oral squamous cell carcinomas. J Oral Pathol Med. 1993;22(3):101–8. doi: 10.1111/j.1600-0714.1993.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 38.Mork J, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 39.Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982–1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(6):622–35. doi: 10.1067/moe.2001.115392. [DOI] [PubMed] [Google Scholar]
- 40.Luo CW, Roan CH, Liu CJ. Human papillomaviruses in oral squamous cell carcinoma and pre-cancerous lesions detected by PCR-based gene-chip array. Int J Oral Maxillofac Surg. 2007;36(2):153–8. doi: 10.1016/j.ijom.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Herrero R, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 42.Gillison ML, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 43.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–45. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86(2):104–14. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 45.Kreimer AR, et al. HPV16 semiquantitative viral load and serologic biomarkers in oral and oropharyngeal squamous cell carcinomas. Int J Cancer. 2005;115(2):329–32. doi: 10.1002/ijc.20872. [DOI] [PubMed] [Google Scholar]
- 46.Klussmann JP, et al. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001;92(11):2875–84. doi: 10.1002/1097-0142(20011201)92:11<2875::aid-cncr10130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 47.D’Souza G, et al. Case-Control Study of Human Papillomavirus and Oropharyngeal Cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 48.Gillison ML, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 49.Califano J, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56(11):2488–92. [PubMed] [Google Scholar]
- 50.Tran N, Rose BR, O’Brien CJ. Role of human papillomavirus in the etiology of head and neck cancer. Head Neck. 2007;29(1):64–70. doi: 10.1002/hed.20460. [DOI] [PubMed] [Google Scholar]
- 51.Slebos RJ, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12(3 Pt 1):701–9. doi: 10.1158/1078-0432.CCR-05-2017. [DOI] [PubMed] [Google Scholar]
- 52.Ansari-Lari MA, et al. Distinction of endocervical and endometrial adenocarcinomas: immunohistochemical p16 expression correlated with human papillomavirus (HPV) DNA detection. Am J Surg Pathol. 2004;28(2):160–7. doi: 10.1097/00000478-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Zhang MQ, El-Mofty SK, Davila RM. Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: a tool for identifying the site of an occult head and neck primary. Cancer. 2008;114(2):118–23. doi: 10.1002/cncr.23348. [DOI] [PubMed] [Google Scholar]
- 54.Kreimer AR, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 55.Fakhry C, Gillison ML. Clinical Implications of Human Papillomavirus in Head and Neck Cancers. J Clin Oncol. 2006;24(17):2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 57.Weinberg R. Oncogenes, Antioncogenes, and the Molecular Bases of Multistep Carcinogenesis. Cancer Res. 1989;49:3713–3721. [PubMed] [Google Scholar]
- 58.Argiris A, et al. Head and neck cancer. Lancet. 2008;371(9625):1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 60.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 61.Sidransky D, et al. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992;355(6363):846–7. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- 62.Bedi GC, et al. Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res. 1996;56(11):2484–7. [PubMed] [Google Scholar]
- 63.Hugo H, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 64.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–20. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung CH, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006;66(16):8210–8. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 67.Worsham MJ, et al. Epigenetic events of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132(6):668–77. doi: 10.1001/archotol.132.6.668. [DOI] [PubMed] [Google Scholar]
- 68.Thariat J, Milas L, Ang KK. Integrating Radiotherapy With Epidermal Growth Factor Receptor Antagonists and Other Molecular Therapeutics for the Treatment of Head and Neck Cancer. International Journal of Radiation Oncology*Biology*Physics. 2007;69(4):974–984. doi: 10.1016/j.ijrobp.2007.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 70.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 71.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 72.Herbst RS, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22(5):785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 73.Grandis JR, Tweardy DJ. TGF-alpha and EGFR in head and neck cancer. J Cell Biochem Suppl. 1993;17F:188–91. doi: 10.1002/jcb.240531027. [DOI] [PubMed] [Google Scholar]
- 74.Takes RP, et al. Differences in expression of oncogenes and tumor suppressor genes in different sites of head and neck squamous cell. Anticancer Res. 1998;18(6B):4793–800. [PubMed] [Google Scholar]
- 75.Ang KK, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–6. [PubMed] [Google Scholar]
- 76.Kim S, et al. Emerging perspectives in epidermal growth factor receptor targeting in head and neck cancer. Head Neck. 2008;30(5):667–74. doi: 10.1002/hed.20859. [DOI] [PubMed] [Google Scholar]
- 77.Ford AC, Grandis JR. Targeting epidermal growth factor receptor in head and neck cancer. Head Neck. 2003;25(1):67–73. doi: 10.1002/hed.10224. [DOI] [PubMed] [Google Scholar]
- 78.Lothaire P, et al. Molecular markers of head and neck squamous cell carcinoma: promising signs in need of prospective evaluation. Head Neck. 2006;28(3):256–69. doi: 10.1002/hed.20326. [DOI] [PubMed] [Google Scholar]
- 79.Reuter CW, Morgan MA, Eckardt A. Targeting EGF-receptor-signalling in squamous cell carcinomas of the head and neck. Br J Cancer. 2007;96(3):408–16. doi: 10.1038/sj.bjc.6603566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sok JC, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12(17):5064–73. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 81.Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007;298(1):70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- 82.Akimoto T, et al. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5(10):2884–90. [PubMed] [Google Scholar]
- 83.Liang K, et al. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57(1):246–54. doi: 10.1016/s0360-3016(03)00511-x. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt–Ullrich RK, et al. Altered expression of epidermal growth factor receptor and estrogen receptor in MCF–7 cells after single and repeated radiation exposures. Int J Radiat Oncol Biol Phys. 1994;29(4):813–9. doi: 10.1016/0360-3016(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt-Ullrich RK, et al. ERBB receptor tyrosine kinases and cellular radiation responses. Oncogene. 2003;22(37):5855–65. doi: 10.1038/sj.onc.1206698. [DOI] [PubMed] [Google Scholar]
- 86.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 87. [cited 2007 September 21];ClinicalTrials.gov. Available from: http://www.clinicaltrials.gov/
- 88.Burtness B, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23(34):8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 89.Soulieres D, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22(1):77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 90.Eriksen JG, et al. The prognostic value of epidermal growth factor receptor is related to tumor differentiation and the overall treatment time of radiotherapy in squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2004;58(2):561–6. doi: 10.1016/j.ijrobp.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 91.Bentzen SM, et al. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol. 2005;23(24):5560–7. doi: 10.1200/JCO.2005.06.411. [DOI] [PubMed] [Google Scholar]
- 92.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 93.Mitsiades CS, Mitsiades N. Treatment of hematologic malignancies and solid tumors by inhibiting IGF receptor signaling. Expert Rev Anticancer Ther. 2005;5(3):487–99. doi: 10.1586/14737140.5.3.487. [DOI] [PubMed] [Google Scholar]
- 94.Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92(12):2097–101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khandwala HM, et al. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21(3):215–44. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 96.Baxter RC. The role of insulin-like growth factors and their binding proteins in tumor hypoglycemia. Horm Res. 1996;46(4–5):195–201. doi: 10.1159/000185023. [DOI] [PubMed] [Google Scholar]
- 97.Resnicoff M, et al. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995;55(11):2463–9. [PubMed] [Google Scholar]
- 98.DeAngelis T, Ferber A, Baserga R. Insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the platelet-derived growth factor receptor. J Cell Physiol. 1995;164(1):214–21. doi: 10.1002/jcp.1041640126. [DOI] [PubMed] [Google Scholar]
- 99.Sell C, et al. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14(6):3604–12. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Resnicoff M, et al. Insulin-like growth factor-1 and its receptor mediate the autocrine proliferation of human ovarian carcinoma cell lines. Lab Invest. 1993;69(6):756–60. [PubMed] [Google Scholar]
- 101.Trojan J, et al. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;259(5091):94–7. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- 102.Sell C, et al. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993;90(23):11217–21. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reinmuth N, et al. Impact of insulin-like growth factor receptor-I function on angiogenesis, growth, and metastasis of colon cancer. Lab Invest. 2002;82(10):1377–89. doi: 10.1097/01.lab.0000032411.41603.c2. [DOI] [PubMed] [Google Scholar]
- 104.Zeng H, et al. Requirement of different signaling pathways mediated by insulin-like growth factor-I receptor for proliferation, invasion, and VPF/VEGF expression in a pancreatic carcinoma cell line. Biochem Biophys Res Commun. 2003;302(1):46–55. doi: 10.1016/s0006-291x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 105.Zhang X, et al. Multiple signaling pathways are activated during insulin-like growth factor-I (IGF-I) stimulated breast cancer cell migration. Breast Cancer Res Treat. 2005;93(2):159–68. doi: 10.1007/s10549-005-4626-8. [DOI] [PubMed] [Google Scholar]
- 106.Long L, et al. Loss of the metastatic phenotype in murine carcinoma cells expressing an antisense RNA to the insulin-like growth factor receptor. Cancer Res. 1995;55(5):1006–9. [PubMed] [Google Scholar]
- 107.Zeigler ME, et al. Growth factor-induced epidermal invasion of the dermis in human skin organ culture: expression and role of matrix metalloproteinases. Invasion Metastasis. 1996;16(1):11–8. [PubMed] [Google Scholar]
- 108.Goel HL, et al. beta1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005;65(15):6692–700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- 109.Abe S, et al. Increased expression of insulin-like growth factor i is associated with Ara-C resistance in leukemia. Tohoku J Exp Med. 2006;209(3):217–28. doi: 10.1620/tjem.209.217. [DOI] [PubMed] [Google Scholar]
- 110.Allen GW, et al. Insulin-like growth factor-I receptor signaling blockade combined with radiation. Cancer Res. 2007;67(3):1155–62. doi: 10.1158/0008-5472.CAN-06-2000. [DOI] [PubMed] [Google Scholar]
- 111.Camirand A, Lu Y, Pollak M. Co-targeting HER2/ErbB2 and insulin-like growth factor-1 receptors causes synergistic inhibition of growth in HER2-overexpressing breast cancer cells. Med Sci Monit. 2002;8(12):BR521-6. [PubMed] [Google Scholar]
- 112.Desbois-Mouthon C, et al. Impact of IGF-1R/EGFR cross-talks on hepatoma cell sensitivity to gefitinib. Int J Cancer. 2006;119(11):2557–66. doi: 10.1002/ijc.22221. [DOI] [PubMed] [Google Scholar]
- 113.Gee JM, et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 114.Knowlden JM, et al. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146(11):4609–18. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 115.Wan X, Helman LJ. Effect of insulin-like growth factor II on protecting myoblast cells against cisplatin-induced apoptosis through p70 S6 kinase pathway. Neoplasia. 2002;4(5):400–8. doi: 10.1038/sj.neo.7900242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wiseman LR, et al. Type I IGF receptor and acquired tamoxifen resistance in oestrogen-responsive human breast cancer cells. Eur J Cancer. 1993;29A(16):2256–64. doi: 10.1016/0959-8049(93)90218-5. [DOI] [PubMed] [Google Scholar]
- 117.Yin D, et al. Insulin-like growth factor-I decreased etoposide-induced apoptosis in glioma cells by increasing bcl-2 expression and decreasing CPP32 activity. Neurol Res. 2005;27(1):27–35. doi: 10.1179/016164105X18151. [DOI] [PubMed] [Google Scholar]
- 118.Ouban A, et al. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol. 2003;34(8):803–8. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 119.Jones HE, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11(4):793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 120.Ueda S, et al. Potential crosstalk between insulin-like growth factor receptor type 1 and epidermal growth factor receptor in progression and metastasis of pancreatic cancer. Mod Pathol. 2006;19(6):788–96. doi: 10.1038/modpathol.3800582. [DOI] [PubMed] [Google Scholar]
- 121.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62(1):200–7. [PubMed] [Google Scholar]
- 122.Tao Y, et al. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway--therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4(10):591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 123.Barnes CJ, et al. Insulin-like growth factor receptor as a therapeutic target in head and neck cancer. Clin Cancer Res. 2007;13(14):4291–9. doi: 10.1158/1078-0432.CCR-06-2040. [DOI] [PubMed] [Google Scholar]
- 124.Slomiany MG, et al. Insulin-like growth factor-1 receptor and ligand targeting in head and neck squamous cell carcinoma. Cancer Lett. 2007;248(2):269–79. doi: 10.1016/j.canlet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 125.Riedemann J, V, Macaulay M. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13(Suppl 1):S33–43. doi: 10.1677/erc.1.01280. [DOI] [PubMed] [Google Scholar]
- 126.Garcia-Echeverria C, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5(3):231–9. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 127.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29(5):233–42. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 128.Kada F, Saji M, Ringel MD. Akt: a potential target for thyroid cancer therapy. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4(3):181–5. doi: 10.2174/1568008043339857. [DOI] [PubMed] [Google Scholar]
- 129.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9(3):288–96. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 130.Oc P, et al. Vascular endothelial growth factor family members are differentially regulated by c-erbB signaling in head and neck squamous carcinoma cells. Clin Exp Metastasis. 2000;18(2):155–61. doi: 10.1023/a:1006764100867. [DOI] [PubMed] [Google Scholar]
- 131.Vokes EE, Cohen EE, Mauer AM, et al. A phase I study of erlotinib snd bevacizumab for recurrent or metastatic squamous cell carcinoma of the head and neck (HNC) J Clin Oncol. 2005;23:5504. [Google Scholar]
- 132.Massarelli E, et al. Akt activation correlates with adverse outcome in tongue cancer. Cancer. 2005;104(11):2430–6. doi: 10.1002/cncr.21476. [DOI] [PubMed] [Google Scholar]
- 133.Amornphimoltham P, et al. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res. 2004;10(12 Pt 1):4029–37. doi: 10.1158/1078-0432.CCR-03-0249. [DOI] [PubMed] [Google Scholar]
- 134.Molinolo AA, et al. Dissecting the Akt/mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13(17):4964–73. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 135.Lim J, et al. Prognostic value of activated Akt expression in oral squamous cell carcinoma. J Clin Pathol. 2005;58(11):1199–205. doi: 10.1136/jcp.2004.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim D, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–87. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 137.Karamouzis MVFD, Johnson R, et al. Phase II trial of pemetrexed (P) and Bevacizumab (B) in patients with recurrent metastatic head and neck squamous cell carcinoma (HNSCC): an interim analysis [abstract] J Clin Oncol. 2007;25:6049. [Google Scholar]
- 138.Williamson SKMJ, Huang CH, et al. A phase II trial of BAY 43-9006 in patients with recurrent and/or metastatic head and neck squmaous cell carcinoma (HNSCC): a Southwest Oncology Group (SWOG) trial. J Clin Oncol. 2007;25:3766–3773. [Google Scholar]
- 139.Amornphimoltham P, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65(21):9953–61. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 140.Elser C, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25(24):3766–73. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 141.Wendel HG, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428(6980):332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 142.Neshat MS, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98(18):10314–9. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 144.Nathan CO, et al. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J Clin Oncol. 1999;17(9):2909–14. doi: 10.1200/JCO.1999.17.9.2909. [DOI] [PubMed] [Google Scholar]
- 145.Nathan CO, et al. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 2007;67(5):2160–8. doi: 10.1158/0008-5472.CAN-06-2449. [DOI] [PubMed] [Google Scholar]
- 146.Nathan CO, et al. Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2004;10(17):5820–7. doi: 10.1158/1078-0432.CCR-03-0483. [DOI] [PubMed] [Google Scholar]
- 147.Dong G, et al. The host environment promotes the constitutive activation of nuclear factor-kappaB and proinflammatory cytokine expression during metastatic tumor progression of murine squamous cell carcinoma. Cancer Res. 1999;59(14):3495–504. [PubMed] [Google Scholar]
- 148.Loercher A, et al. Nuclear factor-kappaB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res. 2004;64(18):6511–23. doi: 10.1158/0008-5472.CAN-04-0852. [DOI] [PubMed] [Google Scholar]
- 149.Chen Z, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5(6):1369–79. [PubMed] [Google Scholar]
- 150.Ondrey FG, et al. Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinog. 1999;26(2):119–29. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 151.Zhang PL, et al. Overexpression of phosphorylated nuclear factor-kappa B in tonsillar squamous cell carcinoma and high-grade dysplasia is associated with poor prognosis. Mod Pathol. 2005;18(7):924–32. doi: 10.1038/modpathol.3800372. [DOI] [PubMed] [Google Scholar]
- 152.Dong G, et al. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res. 2001;61(12):4797–808. [PubMed] [Google Scholar]
- 153.Duan J, et al. Nuclear factor-kappaB p65 small interfering RNA or proteasome inhibitor bortezomib sensitizes head and neck squamous cell carcinomas to classic histone deacetylase inhibitors and novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2007;6(1):37–50. doi: 10.1158/1535-7163.MCT-05-0285. [DOI] [PubMed] [Google Scholar]
- 154.Duffey DC, et al. Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res. 1999;59(14):3468–74. [PubMed] [Google Scholar]
- 155.Kato T, et al. Cisplatin and radiation sensitivity in human head and neck squamous carcinomas are independently modulated by glutathione and transcription factor NF-kappaB. Head Neck. 2000;22(8):748–59. doi: 10.1002/1097-0347(200012)22:8<748::aid-hed2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 156.Van Waes C, et al. Inhibition of nuclear factor-kappaB and target genes during combined therapy with proteasome inhibitor bortezomib and reirradiation in patients with recurrent head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;63(5):1400–12. doi: 10.1016/j.ijrobp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 157.Lee TL, et al. A signal network involving coactivated NF-kappaB and STAT3 and altered p53 modulates BAX/BCL-XL expression and promotes cell survival of head and neck squamous cell carcinomas. Int J Cancer. 2008;122(9):1987–98. doi: 10.1002/ijc.23324. [DOI] [PubMed] [Google Scholar]
- 158.Wang CY, et al. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5(4):412–7. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 159.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci U S A. 1998;95(22):13012–7. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Anto RJ, et al. Cigarette smoke condensate activates nuclear transcription factor-kappaB through phosphorylation and degradation of IkappaB(alpha): correlation with induction of cyclooxygenase-2. Carcinogenesis. 2002;23(9):1511–8. doi: 10.1093/carcin/23.9.1511. [DOI] [PubMed] [Google Scholar]
- 161.Lin SC, et al. Areca (betel) nut extract activates mitogen-activated protein kinases and NF-kappaB in oral keratinocytes. Int J Cancer. 2005;116(4):526–35. doi: 10.1002/ijc.21104. [DOI] [PubMed] [Google Scholar]
- 162.Allen CT, et al. Role of activated nuclear factor-kappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck. 2007;29(10):959–71. doi: 10.1002/hed.20615. [DOI] [PubMed] [Google Scholar]
- 163.Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13(4):1076–82. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 164.Morimoto RI, et al. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- 165.Stravopodis DJ, Margaritis LH, Voutsinas GE. Drug-mediated targeted disruption of multiple protein activities through functional inhibition of the Hsp90 chaperone complex. Curr Med Chem. 2007;14(29):3122–38. doi: 10.2174/092986707782793925. [DOI] [PubMed] [Google Scholar]
- 166.Ferrarini M, et al. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51(4):613–9. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- 167.Solit DB, Rosen N. Hsp90: a novel target for cancer therapy. Curr Top Med Chem. 2006;6(11):1205–14. doi: 10.2174/156802606777812068. [DOI] [PubMed] [Google Scholar]
- 168.Yin X, et al. Potent activity of a novel dimeric heat shock protein 90 inhibitor against head and neck squamous cell carcinoma in vitro and in vivo. Clin Cancer Res. 2005;11(10):3889–96. doi: 10.1158/1078-0432.CCR-04-2272. [DOI] [PubMed] [Google Scholar]
- 169.Roepman P, et al. An expression profile for diagnosis of lymph node metastases from primary head and neck squamous cell carcinomas. Nat Genet. 2005;37(2):182–6. doi: 10.1038/ng1502. [DOI] [PubMed] [Google Scholar]
- 170.Thomas GR, Nadiminti H, Regalado J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol. 2005;86(6):347–63. doi: 10.1111/j.0959-9673.2005.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chung CH, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5(5):489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 172.Kumar B, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]