Abstract
ErbB signaling has long been implicated in cancer formation and progression and is shown to regulate cell division, migration and death during tumorigenesis. The functions of the ErbB pathway during early vertebrate embryogenesis, however, are not well understood. Here we report characterization of ErbB activities during early frog development. Gain-of-function analyses show that EGFR, ErbB2 and ErbB4 induce ectopic tumor-like cell mass that contains increased numbers of mitotic cells. Both the muscle and the neural markers are expressed in these ectopic protrusions. ErbBs also induce mesodermal markers in ectodermal explants. Loss-of-function studies using carboxyl terminal-truncated dominant-negative ErbB receptors demonstrate that blocking ErbB signals leads to defective gastrulation movements and malformation of the embryonic axis with a reduction in the head structures in early frog embryos. These data, together with the observation that ErbBs are expressed early during frog embryogenesis, suggest that ErbBs regulate cell proliferation, movements and embryonic patterning during early Xenopus development.
Keywords: ErbB, epidermal growth factor receptor, receptor tyrosine kinase, proliferation, cell differentiation, gastrulation, Xenopus
Introduction
ErbB family of receptor tyrosine kinases (RTKs) consists of four closely-related transmembrane proteins that mediate signals from a group of growth factors containing the epidermal growth factor (EGF) domain, such as EGF, transforming growth factor-α (TGF-α) and neuregulins (NRGs; Olayioye et al., 2000; Yarden and Sliwkowski, 2001; Carpenter, 2003; Citri et al., 2003; Jorissen et al., 2003). Among the receptors, ErbB2 (HER2/Neu) has not been shown to bind to any ligands with high affinity, while the other three receptors bind to eleven EGF-like growth factors with distinct specificity and affinity (Riese II and Stern, 1998; Jones et al., 1999; Burgess et al., 2003). Similar to other RTKs, ligand association induces receptor homo- and hetero-dimerization and activation. ErbB2, though lacking the ability to bind to growth factors, is poised to form heterodimers with ligand-activated ErbBs and is a favored partner for all other ErbBs. Activation of ErbB receptors leads to trans-phosphorylation of several tyrosine residues in the cytoplasmic tails of ErbBs, which then function as docking sites for various adaptor/scaffold proteins and signaling molecules. ErbB3 (HER3), unlike other ErbBs, does not have intrinsic tyrosine kinase activity and therefore needs to form heterodimers with other ErbBs to transmit signals (Citri et al., 2003). Multiple signaling cascades are stimulated downstream of ErbB RTKs, including the mitogen-activated protein kinase (MAPK) pathway, the phosphatidylinositol-3 kinase (PI3K) pathway, and the phospholipase C gamma (PLCγ)/protein kinase C (PKC) pathway (Olayioye et al., 2000; Yarden and Sliwkowski, 2001). Each ErbB receptor has its preferred repertoire of downstream signaling molecules, and heterodimer formation helps to augment signaling diversity and potency (Olayioye et al., 2000; Sweeney and Carraway III, 2000).
Consistent with their diverse signaling capacities, ErbBs control a variety of cell functions during both embryogenesis and adult homeostasis. The most noted role of ErbBs is their involvement in cancer formation and progression (Olayioye et al., 2000; Yarden and Sliwkowski, 2001; Alaoui-Jamali et al., 2003; Holbro et al., 2003). Activating mutations and amplification or overexpression of ErbB signaling components, including both ligands and receptors, have been implicated in multiple tumor types, such as breast, lung, pancreatic, prostate and ovarian cancers. ErbB1 (HER1/EGFR) and ErbB2 are especially associated with many high grade malignant tumors with poor prognosis. In these cases, ErbBs have been shown to regulate cell survival, proliferation and migration during tumor production and metastasis.
Besides their function in tumorigenesis, ErbBs are reported to regulate multiple processes during early invertebrate development. In C. elegans, a single EGF-like growth factor and a single EGFR mediate several cell fate determination events, such as induction of vulval cells and specification of anterior spicules. EGFR signal is also employed in myoepithelial contractions during worm ovulation (Moghal and Sternberg, 2003). In Drosophila, one inhibitory and four activating ligands interact with a single EGFR to control cell proliferation, differentiation and motility in different contexts during fly development. The activities of EGFR are required for, for example, correct patterning of neuroectoderm, specification of muscle precursor cells, cell division and fate determination in eye imaginal disc and migration of border cells during oogenesis (Schweitzer and Shilo, 1997; Casci and Freeman, 1999; Shilo 2003). In both C. elegans and Drosophila, MAPK pathway is the predominant downstream signal activated by EGFR, though PI3K and maybe other pathways are also used in specific processes (Moghal and Sternberg, 2003; Shilo 2003). Genetic studies in these invertebrate models also demonstrate that various regulatory factors exist to modulate presentation and activation of the EGF-like ligands, as well as the range, strength, duration and outcome of EGFR signals (Moghal and Sternberg, 2003; Shilo 2003).
In contrast to invertebrate models, we know relatively little about ErbB functions during early vertebrate development. All four ErbB receptors have been cloned from mouse, and each member has been knocked out individually. ErbB2 and ErbB4 null mice share a common defect in lacking the myocardial trabeculae in the heart ventricle; in addition, each mutant also displays specific phenotypes, such as reduction in cranial neural crest-derived sensory neurons in ErbB2 null mice and alteration in connections of the cranial ganglia with the hindbrain region in ErbB4 null mice (Gassmann et al., 1995; Lee et al., 1995). Both ErbB2 and ErbB3 are required for formation of the sympathetic nervous system, and ErbB3 also functions in a cell-autonomous fashion for Schwann cell development (Riethmacher et al., 1997; Britsch et al., 1998). EGFR knockout mice show strain-specific defects in placenta, skin, lung, hair follicles and gastrointestinal track, but all mutant mice demonstrate postnatal neurodegeneration in a strain-independent manner (Miettinen et al., 1995; Sibilia and Wagner, 1995; Threadgill et al., 1995; Sibilia et al., 1998). Currently, no double or multiple ErbB knockout mice have been described, thus excluding further analysis of potentially overlapping and redundant functions of ErbB receptors in early mouse development. In addition, potential mechanisms for the mutant mice phenotypes, such as defects in cell proliferation or cell fate determination, are not completely understood. The activities of ErbBs in development of other vertebrate systems are less studied, with only a handful reports on EGFR function in zebrafish and in chick (Atit et al., 2003; Goishi et al., 2003).
To further comprehend the roles of ErbB signaling in early vertebrate embryogenesis, we undertook characterization of ErbB activities during early frog development. We report here that overexpression of EGFR, ErbB2 and ErbB4 induces ectopic tail-like structures in early Xenopus embryos and stimulates mesodermal marker expression in ectodermal explants. Inhibition of ErbB signals results in impairment of gastrulation movements and defects in embryonic axis formation. Our data thus suggest that ErbB signals play crucial roles during early Xenopus embryogenesis.
Results
Xenopus ErbBs are expressed during early developmental stages
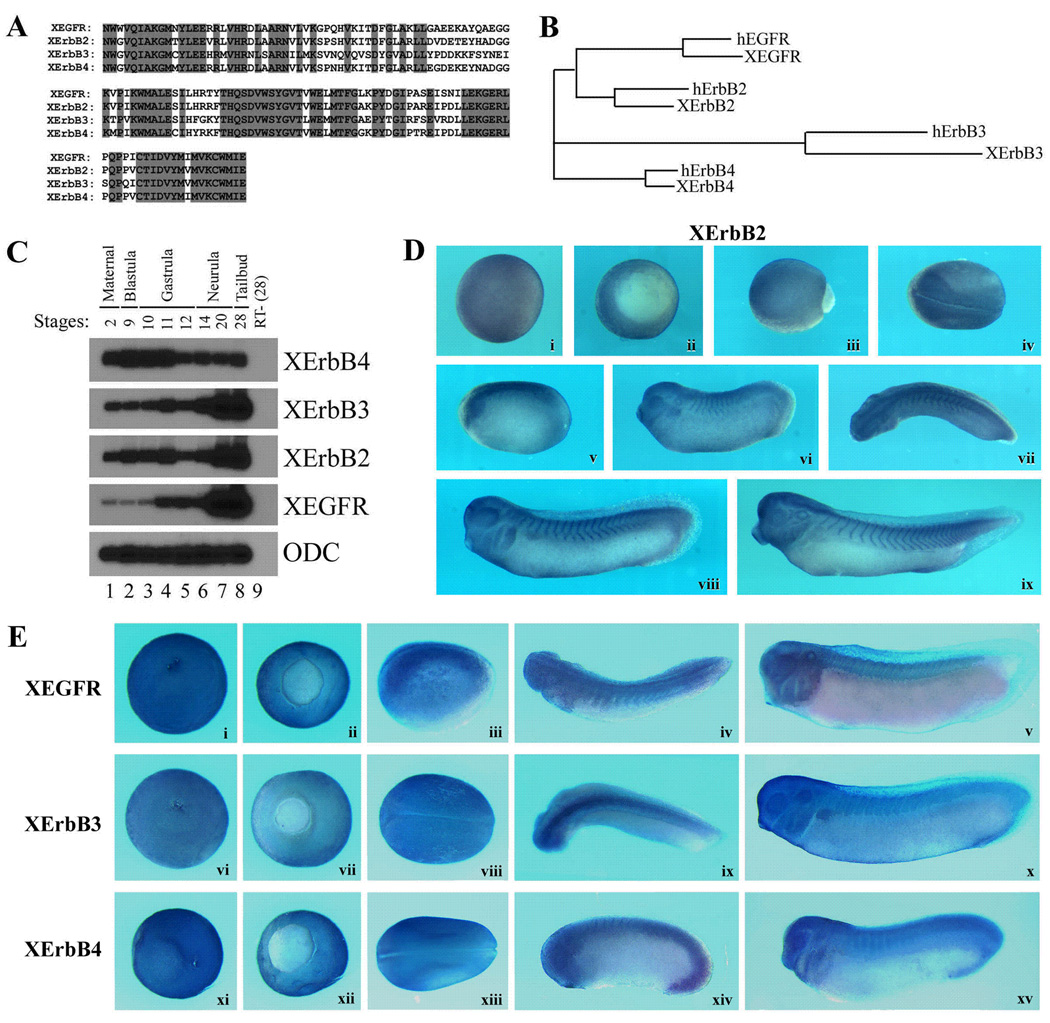
In Xenopus, the ErbB ligand Neuregulin is expressed from maternal stages onward and is distributed widely in early embryos (Yang et al., 1998a; 1999), suggesting that ErbB pathway may regulate early frog development. The expression of ErbB receptors during frog embryogenesis, however, has not been described. To see whether ErbBs are expressed at the appropriate time and place to modulate early frog development, we undertook a degenerate RT-PCR cloning strategy and obtained the sequences encoding the conserved domain of all four Xenopus ErbBs (Fig. 1A). Sequence alignment with human ErbBs shows that the Xenopus ErbBs share 94%, 93%, 82% and 96% homology with the corresponding region of human EGFR, ErbB2, 3 and 4 (Fig. 1B). RT-PCR analyses using specific primer pairs for each individual ErbBs demonstrated that all ErbBs were expressed in early frog embryos from maternal to at least tailbud stages, though EGFR was expressed at a significantly lower level than other ErbBs during early development and its transcripts were only increased from mid-gastrula stages (Fig. 1C). Dissection of early gastrula embryos revealed that ErbBs 2, 3 and 4 were expressed at high levels in the animal and marginal zone regions, while EGFR was enriched in the animal region only (not shown). Library screening using the Xenopus ErbB PCR products as probes yielded several partial Xenopus ErbB clones, with ErbB2 recovered most frequently (not shown). In situ hybridization using a 5kb Xenopus ErbB2 probe encoding a N-terminally truncated protein showed that XErbB2 was expressed at gastrula stages in ectodermal and mesodermal regions (Fig. 1D, panels i and ii). During neurulation, its expression was gradually elevated in the neural tissues (Fig. 1D, iii to v). Starting from early tailbud stages, XErbB2 was also expressed in myotome as well as neural and neural crest-derived tissues, such as brain, spinal cord, eyes and branchial arches (Fig. 1D, vi to ix). Using Xenopus EST database and X. tropicalis genome information, we also obtained long fragments of Xenopus laevis EGFR, ErbB3 and ErbB4 sequences. In situ analyses with these fragments as probes showed that like ErbB2, all other ErbBs were expressed uniformly in both ectodermal and mesodermal regions at gastrula stages (Fig. 1E, i, ii, vi, vii, xi and xii). The expression was increased in the neural tissues during neurulation (Fig. 1E, iii, viii and xiii). At tailbud stages, ErbBs were seen in the brain, spinal cord, branchial arches, somites, eyes and otic vesicles (Fig. 1E, iv, v, ix, x, xiv, xv). The expression pattern of Xenopus ErbBs thus indicates that ErbBs are expressed at right time and place to potentially regulate early frog development.
Figure 1. ErbB receptors are expressed during early Xenopus embryogenesis.
A) Alignment of the four Xenopus ErbBs in the conserved domain shows that they are homologous to each other, with 65% to 88% identical residues in this region. Shaded residues are identical in all four ErbBs. B) The Xenopus ErbB fragments are highly homologous to their human counterparts in the conserved region in the tyrosine kinase domain, with 94%, 93%, 82% and 96% similarity between Xenopus EGFR, ErbBs 2, 3, 4 and their corresponding human homologs, respectively. C) RT-PCR analysis shows that all ErbBs are expressed maternally, and the expression persists to at least tailbud stages during early Xenopus development. D) Expression of XErbB2 detected by in situ hybridization. XErbB2 was expressed widely in both ectodermal and mesodermal regions during gastrula and early neurula stages (i–iii). During neurulation (iv) and early tailbud stages (v), its expression was elevated in the neural tissues. Starting from early tailbud stages, its transcripts were also seen in myotomal muscles in addition to neural and neural crest-derived structures, such as brain, spinal cord, eyes and branchial arches (v–viii). This expression pattern persists to at least tadpole stages (ix). Panels i and ii are animal and vegetal views of mid-gastrula embryos respectively; panels iii. iv, and vii are dorsal views of late gastrula, neurula and tailbud embryos respectively; panels v, vi, viii and ix are lateral views of tailbud and tadpole stage embryos. E) Xenopus EGFR (i to v), ErbB3 (vi to x) and ErbB4 (xi to xv) are expressed widely during early development. At gastrula stages, they were expressed uniformly in ectodermal and mesodermal regions (i, ii, vi, vii, xi, xii); and the expression was increased in the neural tissues during neurulation (iii, viii and xiii). At tailbud stages, the expression was seen in the brain, spinal cord, brachial arches, somites, eyes and otic vesicles (iv, v, ix, x, xiv, xv). The embryos were viewed from animal (i, vi, xi) or vegetal (ii, vii, xii) sides at gastrula stages; and from lateral (iii, v, x, xiv, xv) or dorsal (iv, viii, ix, xiii) sides with the anterior to the left for neurula and tailbud embryos.
Overexpression of ErbBs in early frog embryos induces ectopic protrusions
To understand the function of ErbB signals during early frog development, we first employed a gain-of-function approach by overexpression of ErbB receptors in early Xenopus embryos. RNAs encoding mammalian EGFR, ErbBs 2, 3 and 4 were injected into both animal poles of 2-cell stage embryos and the phenotypes of the injected embryos were observed at tailbud to tadpole stages. Though overexpression of ErbB3, which is defective in tyrosine kinase activity, did not significantly alter the morphology of the embryos, expression of EGFR, ErbB2 and ErbB4 all induced ectopic protrusions in Xenopus tadpoles in a dose dependent manner (e.g. the incidence of embryos with protrusions increased from 68%, n=94, to 83%, n=54, when the doses of ErbB4 was increased from 250pg to 500pg; Fig. 2A). The sites of the protrusions could be observed at the head, the trunk or the tail regions, possibly reflecting the sites of RNA injection. Coexpression of ErbBs with a lineage tracer, the gene coding for nuclear beta-galactosidase (nβGal), showed that ErbBs induced ectopic tail-like structure in a cell-autonomous fashion (Fig. 2B). In addition to ectopic protrusions, the head structures of many injected embryos were defective, with malformation in the eyes and reduction of the forebrain tissues (Fig. 2 and data not shown). The phenotypes induced by ErbB overexpression were similar to those induced by injection of the gene encoding the EGF domain of Xenopus neuregulin ligand (not shown), indicating that overexpression of ErbBs mimics the activation of endogenous ErbBs by the ligand. In addition, we observed that activation of fibroblast growth factor (FGF) signaling pathway also induced similar tail-like protrusions (e.g. 85% of embryos, n=85, formed the ectopic structures when 1ng FGFR1 was used, data now shown; also see Weinstein et al., 1998; Bottcher et al., 2004). The data suggest that ErbBs and FGF receptors stimulate common downstream signal(s) to induce ectopic protrusions.
Figure 2. Overexpression of EGFR, ErbB2 and ErbB4 induces ectopic protrusions.
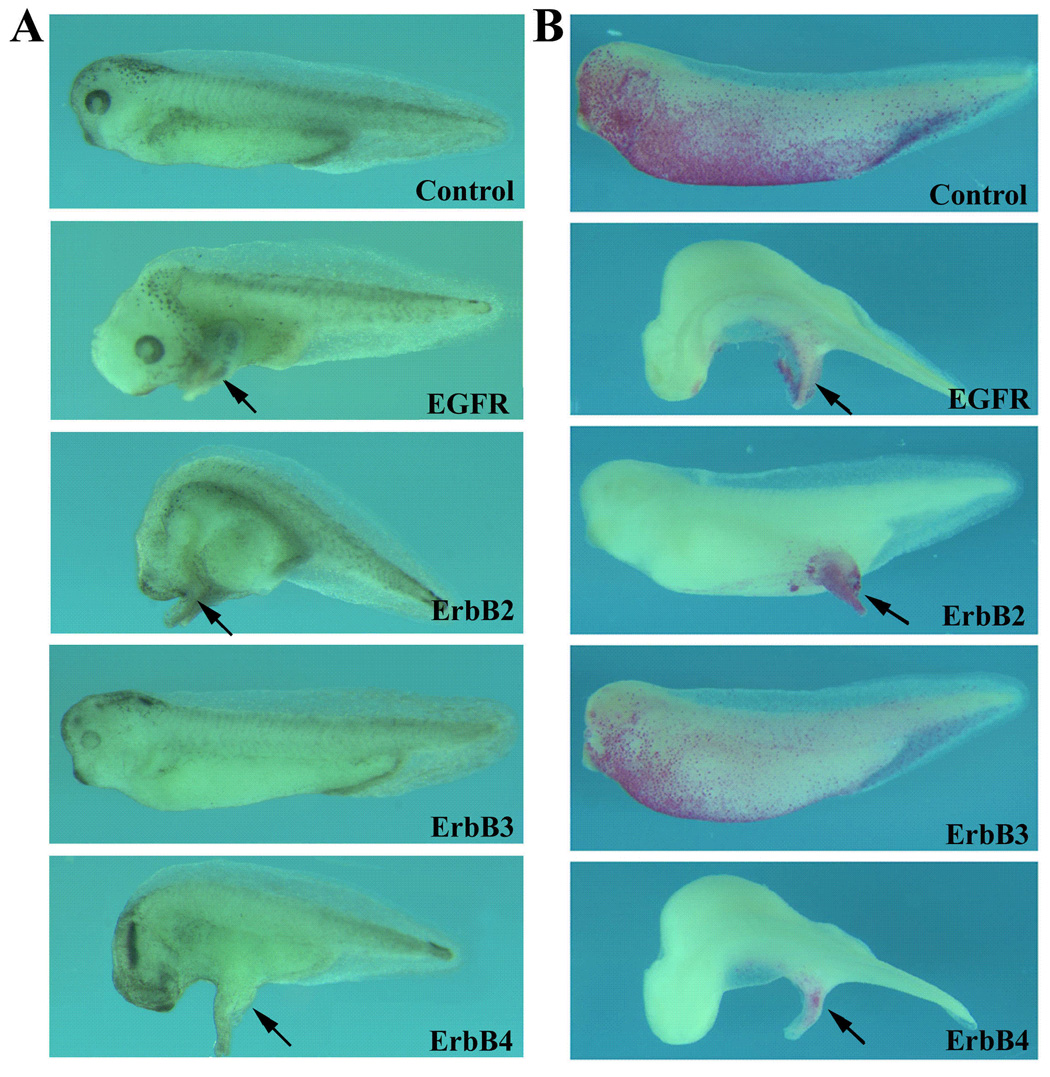
RNAs encoding ErbBs were injected into the animal poles of 2-cell stage embryos and the resulting tadpoles were examined at stages 34–38. A) EGFR (1ng), ErbB2 (500pg) and ErbB4 (500pg), but not the kinase-defective ErbB3 (2ng), induced ectopic protrusions when overexpressed in the ectoderm of early frog embryos. B) Lineage tracing with nuclear beta-galactosidase (200pg RNA) indicated that ErbBs induced ectopic structures cell-autonomously. Black arrows point to the ectopic structures. All embryos were oriented with anterior to the left.
Activation of ErbB signaling stimulates cell proliferation
Injection of ErbBs into animal poles but not ventral marginal zones of early frog embryos induced ectopic tail-like structure (Fig. 2 and data not shown), indicating that ErbBs may not act as Wnt or BMP inhibitors to convert ventral to dorsal mesoderm to form a partial secondary axis. Since activation of ErbB signaling in epidermal-derived tissues leads to dysregulated cell proliferation and carcinoma formation in human and mouse (Olayioye et al., 2000; Yarden and Sliwkowski, 2001; Muller 2002; Holbro et al., 2003), we hypothesized that the ectopic structures induced by ErbB overexpression in frogs might represent a tumor-like cell mass formed by increased cell division. We therefore examined the number and the distribution of the mitotic cells in these embryos by anti-phosphorylated histone H3 antibody staining in our immunohistochemistry studies. Consistent with the previous report (Saka and Smith, 2001), we observed a high number of mitotic cells in the dorsal region, including the neural and the neural crest tissues. Scattered mitotic cells were also seen in the lateral and the ventral regions (Fig. 3A and data not shown). In embryos overexpressing ErbBs, dramatic increase in mitotic cells was observed, and these cells were located both inside and immediately-surrounding the ectopic structures. Many of these structures were found at the lateral or the ventral regions, places where relatively few mitotic cells were normally detected in control embryos (Fig. 3B–D, and data not shown). Counting the mitotic cells present in the protrusions and in the rest of the embryos in 60–100 transverse sections from 9–12 different embryos for each ErbB and the uninjected control samples showed that there was an 8.4 to 8.9 fold increase in mitotic cells per unit area in the protrusions induced by EGFR, ErbB2 and ErbB4 (see Experimental Procedures). Our results thus demonstrate that like in mammalian species, ErbB signals can stimulate cell proliferation in early Xenopus embryos.
Figure 3. ErbBs stimulate cell proliferation in the ectopic protrusions.
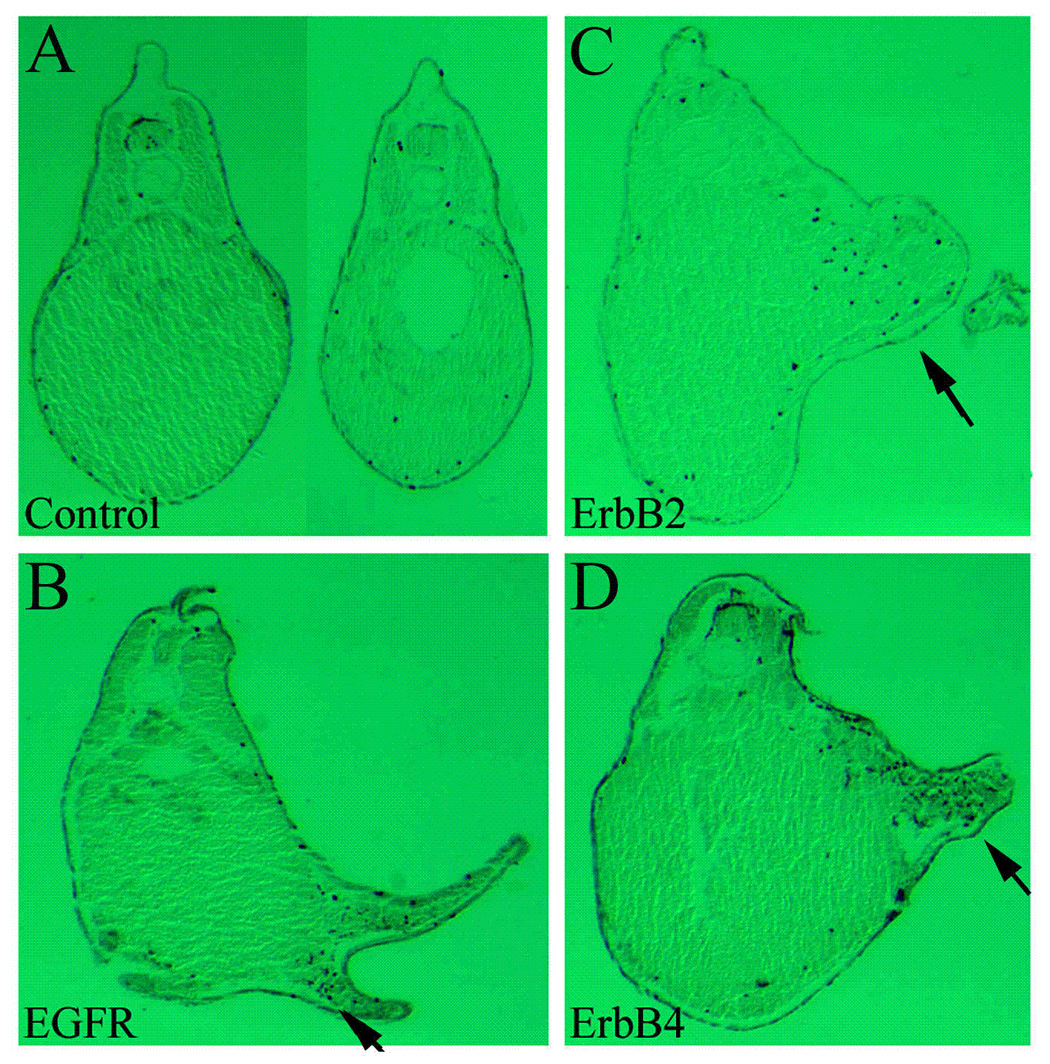
RNAs encoding ErbBs were injected into the animal poles of 2-cell stage embryos as in Figure 2, and the resulting tadpoles (stages 32–36) were analyzed by whole mount immunohistochemistry with anti-phosphorylated histone H3 antibody for detection of mitotic cells. Transverse microtomal sections of the embryos are shown here, with the dorsal side up. While most mitotic cells were scattered in wild type embryos, a dramatic increase in mitotic cells was seen in the ectopic protrusions induced by overexpression of EGFR, ErbB2 and ErbB4 (compare panels B, C and D with A; black arrows, ectopic structures). Cell counting indicated that there was an average of 8-fold increase in mitotic cells in the protrusions.
ErbBs stimulate expression of differentiated cell markers in ectopic structures
To further analyze whether the ectopic structures induced by ErbBs contained only undifferentiated ectodermal cells or a change of cell fate might also occur in addition to increased cell proliferation, we examined the expression of the mesodermal and the neural markers. The antibodies 12/101 and Xen2, which recognize muscle- and neural-specific epitopes respectively, were used in our immunohistochemistry assays. As shown in Fig. 4, both the muscle and the neural markers were detected in the ectopic structures induced by ErbBs. The ectopic domains of muscle and neural markers were often (though not always) disconnected with the primary regions that express these markers (Fig. 4 and data not shown, black arrows: ectopic protrusions). As our lineage tracing data indicated that ErbBs induced ectopic protrusion autonomously (Fig. 2B), the result suggested that the presence of these markers in the ectopic structures might not be due to proliferative expansion of muscle or neural tissues from the primary axis, but might represent a de novo induction of these genes in the protrusions. Our data therefore indicate that in addition to regulate cell division, ErbB signals may also participate in cell differentiation and embryonic patterning processes.
Figure 4. ErbBs promote expression of differentiated cell markers in the ectopic protrusions.
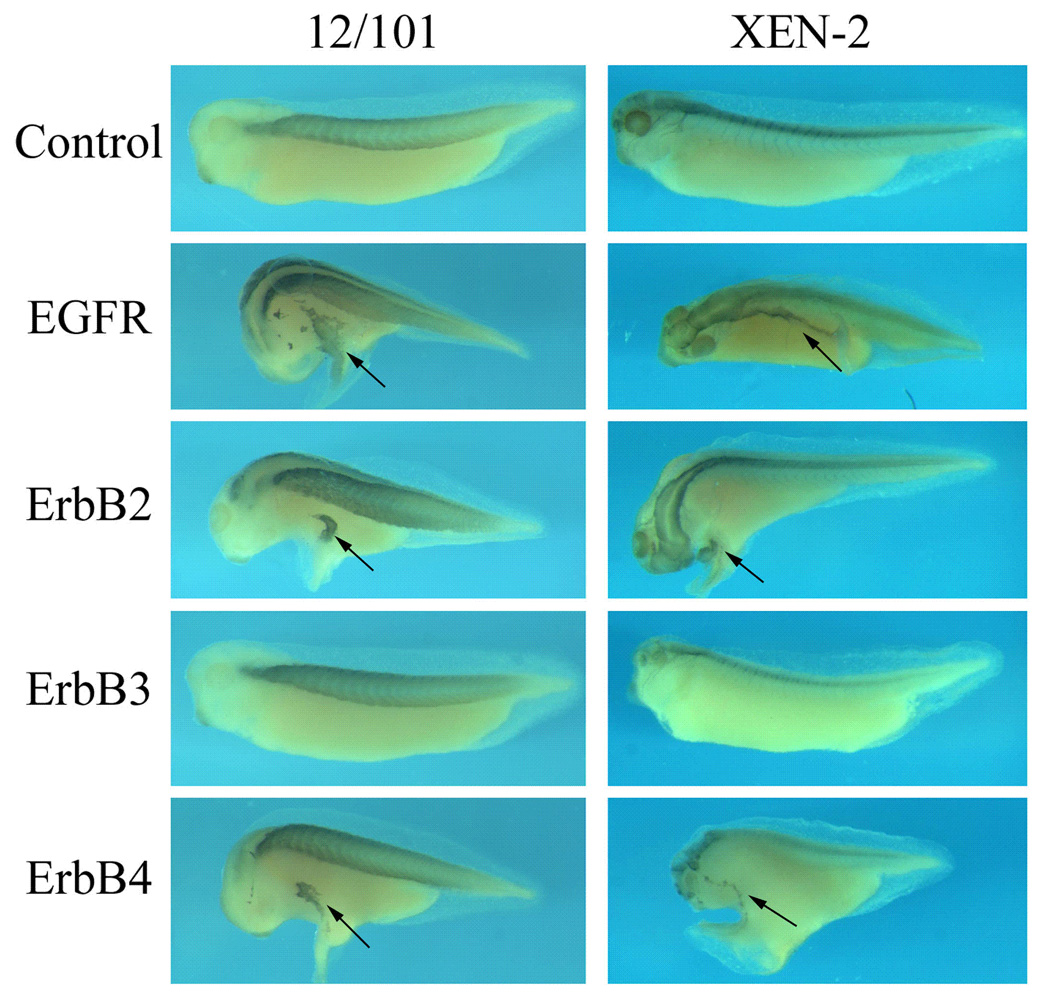
Tadpoles injected with ErbBs (same as in Figure 2) were analyzed with immunohistochemistry studies using antibodies against a muscle epitope (12/101) and a neural epitope (Xen2). In the ectopic structures induced by EGFR, ErbB2 and ErbB4, both markers were expressed; the new sites of marker expression were often separated from the endogenous expression domains, suggesting a de novo induction of these differentiated genes in the protrusions. Black arrows point to the ectopic structures.
ErbBs induce mesodermal markers in Xenopus ectodermal explants
The activation of muscle and neural markers in ErbB-induced ectopic structures suggests that ErbB signals may regulate cell fate determination. To test this hypothesis in a more direct way, we examined the ability of the ErbB receptors to induce mesodermal markers in Xenopus ectodermal explants (animal caps). RNAs encoding ErbBs were injected into the animal poles of two-cell stage embryos. Animal caps were dissected from the injected embryos at blastula stages and incubated to gastrula or tailbud stages before total RNA was extracted and expression of the marker genes was analyzed by reverse-transcription PCR (RT-PCR). As shown previously, uninjected control animal caps did not express any mesodermal markers at meaningful levels (lane 1, Fig. 5). While the caps injected with the kinase-deficient ErbB3 were also devoid of most markers, the other ErbBs all induced the expression of several mesodermal genes with different efficiency. Both ErbB2 and ErbB4 induced the pan-mesodermal markers Brachyury (Xbra, Smith et al., 1991) and VegT (Lustig et al., 1996; Stennard et al., 1996; Zhang and King, 1996; Horb and Thomsen, 1997), the ventrolateral marker MyoD (Hopwood et al., 1989), the ventral marker Xhox3 (Ruiz i Altaba and Melton, 1989) and the posterior marker Derriere (Sun et al., 1999) efficiently at gastrula stages (lanes 3 and 5, Fig. 5). At tailbud stages, ErbB2 strongly induced the muscle marker muscle actin (Gurdon et al., 1985), the posterior marker HoxB9 (Wright et al., 1990), and the heart marker Nkx2.5 (Tonissen et al., 1994), while ErbB4 was more efficient in stimulating the expression of the blood marker globin (Battaglia and Melli, 1977; Patient et al., 1982; Fig. 5, lanes 3 and 5). EGFR, in contrast, was weaker than ErbB2 and ErbB4 in inducing the mesodermal markers. Not only higher doses of EGFR were required to induce mesoderm, but also EGFR did not induce the whole range of markers seen by ErbB2 and ErbB4 overexpression. At gastrula stages, EGFR induced VegT and Derriere, but not Brachyury; while at tailbud stages, EGFR stimulated the expression of muscle actin and XhoxB9, but not the heart marker Nkx2.5 (lane 2, Fig. 5). The dorsal mesodermal markers and the endodermal markers, such as goosecoid, chordin and mixer, were not activated significantly by any of the ErbBs (Fig. 5). The observation that ErbBs were able to induce mesodermal genes in animal caps was consistent with a previous report which showed that the ErbB ligand neuregulin could induce many mesodermal markers in animal caps of Xenopus embryos (Chung and Chung, 1999). Our results thus confirm that activation of ErbB signals in Xenopus ectodermal tissues can indeed change cell from epidermal to mesodermal fate.
Figure 5. ErbBs induce mesodermal markers in Xenopus ectodermal explants.
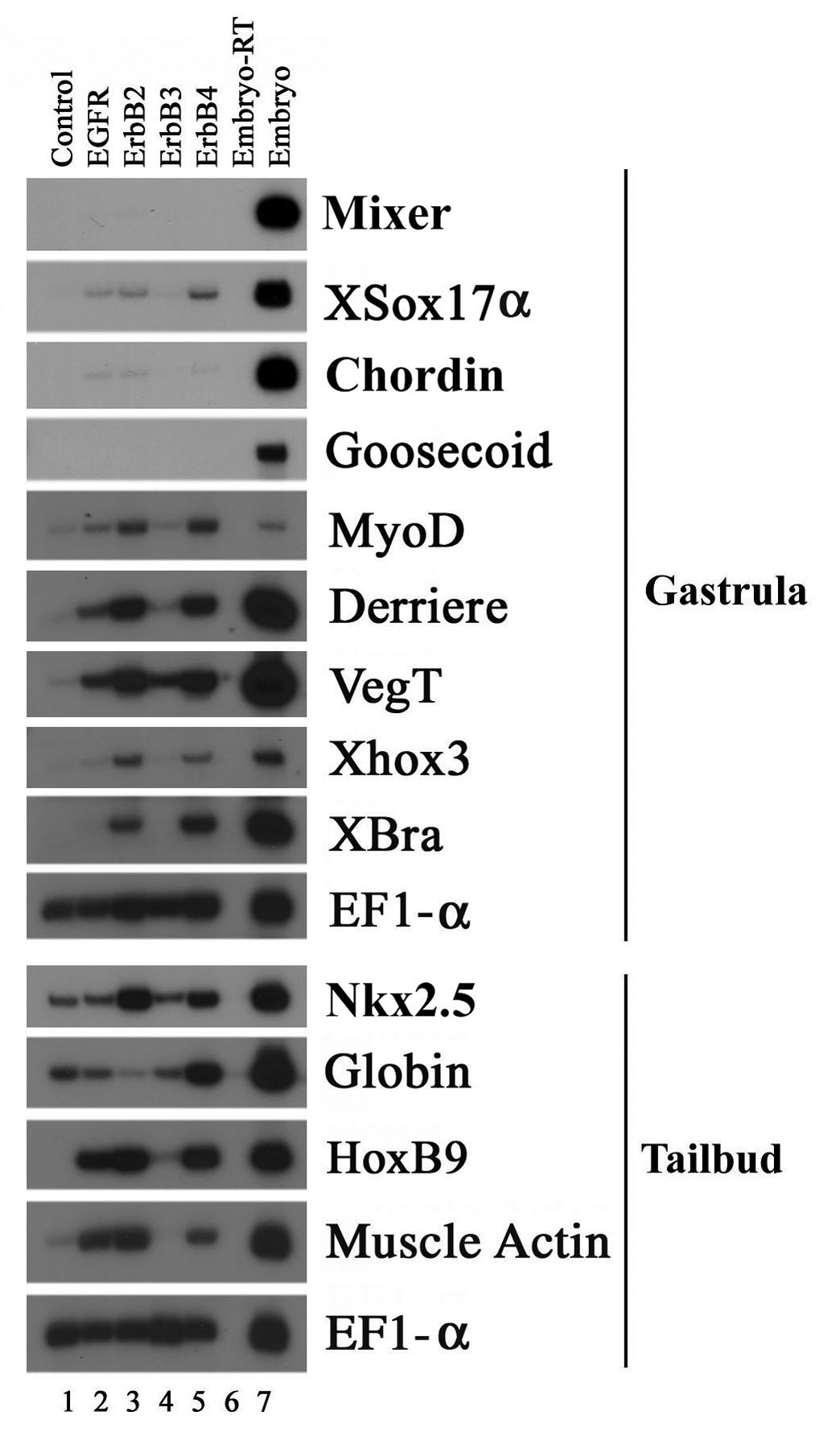
RNAs encoding ErbB receptors were injected into the animal poles of two-cell stage embryos. Animal caps were dissected at blastula stages and incubated to gastrula (stage 11) or tailbud (stages 28–32) stages before total RNA was extracted for RT-PCR assay. Though the kinase-defective ErbB3 did not significantly induce any mesodermal markers, EGFR, ErbB2 and ErbB4 induced the expression of different mesodermal markers with different efficiencies in animal caps. While 1ng of ErbB2 and ErbB4 RNAs were sufficient to induce multiple mesodermal genes, 4ng of EGFR RNA was required to induce a subset of the mesodermal markers.
Blocking ErbB signals leads to axial defects associated with disrupted gastrulation and head malformation in Xenopus embryos
Gain-of-function studies suggest that ErbBs may be involved in cell proliferation and differentiation processes during early frog development. To gain further insight into the functions of ErbB signals in early Xenopus embryogenesis, we employed a loss-of-function approach by using the truncated ErbB receptors to block the endogenous ErbB pathways. In these experiments, we constructed the mutant ErbB receptors in which most of the tyrosine kinase domains and the entire carboxyl terminal tails of the ErbBs were deleted (Fig. 6A). The resulting truncated receptors (DN-ErbBs) lost the ability to phosphorylate their partner ErbBs in receptor dimers and could not be phosphorylated by wild type ErbBs in the C-terminal region to recruit downstream signaling components. The mutant receptors, however, retained their ability to bind to ligands and dimerize with other ErbBs, and therefore could act in a dominant fashion to inhibit the endogenous ErbB signals. DN-ErbBs have been successfully used in cell culture and mouse studies to block the ErbB pathway (e.g. Ram et al., 2000; Chen et al., 2003); and in our experiments, coexpression of any of the four DN-ErbBs with the wild type ErbB2 efficiently suppressed the mesodermal induction by ErbB2 (Fig. 6A), confirming that DN-ErbBs acted as dominant negative receptors. When overexpressed in early frog embryos, DN-ErbBs induced severe gastrulation and axial defects (Fig. 6B). While the onset of gastrulation was normal (panels a to e, Fig. 6B), the closure of the blastopore was delayed at the end of gastrulation (panels f to o, Fig. 6B), so that at the tadpole stages a considerable number of the embryos had exposed endodermal tissues and showed open back and split trunk phenotype (Fig 6B; 12/78 embryos or 15.4% for DN-EGFR, 96/250 embryos or 38% for DN-ErbB2, 22/133 embryos or 16.5% for DN-ErbB3, and 82/242 embryos or 34% for DN-ErbB4). In addition, a variety of defects in axial structures were apparent in embryos that succeeded in closure of the blastopore after the initial delay. Many embryos displayed curved and/or shortened body axis (35/78 embryos or 44.9% for DN-EGFR, 53/250 embryos or 21% for DN-ErbB2, 65/133 embryos or 48.9% for DN-ErbB3, and 74/242 embryos or 31% for DN-ErbB4), and quite a significant number of the embryos showed head defects with eye malformation and reduction in brain structures (38/78 embryos or 48.7% for DN-EGFR, 96/250 embryos or 38% for DN-ErbB2, 41/133 embryos or 30.8% for DN-ErbB3, and 80/242 embryos or 33% for DN-ErbB4; Fig. 6B). Furthermore, sectioning of the embryos showed that while somites were formed in the injected embryos, the segmentation of the somites was often impaired, resulting in continuous somitic tissues (Fig. 6C). The data imply that ErbB signals are crucial for normal gastrulation movements in early Xenopus embryos and may also regulate axial patterning, including head formation and somite segmentation, during early frog development.
Figure 6. Blocking ErbB signaling with dominant negative ErbB receptors induces gastrulation and axial patterning defects.
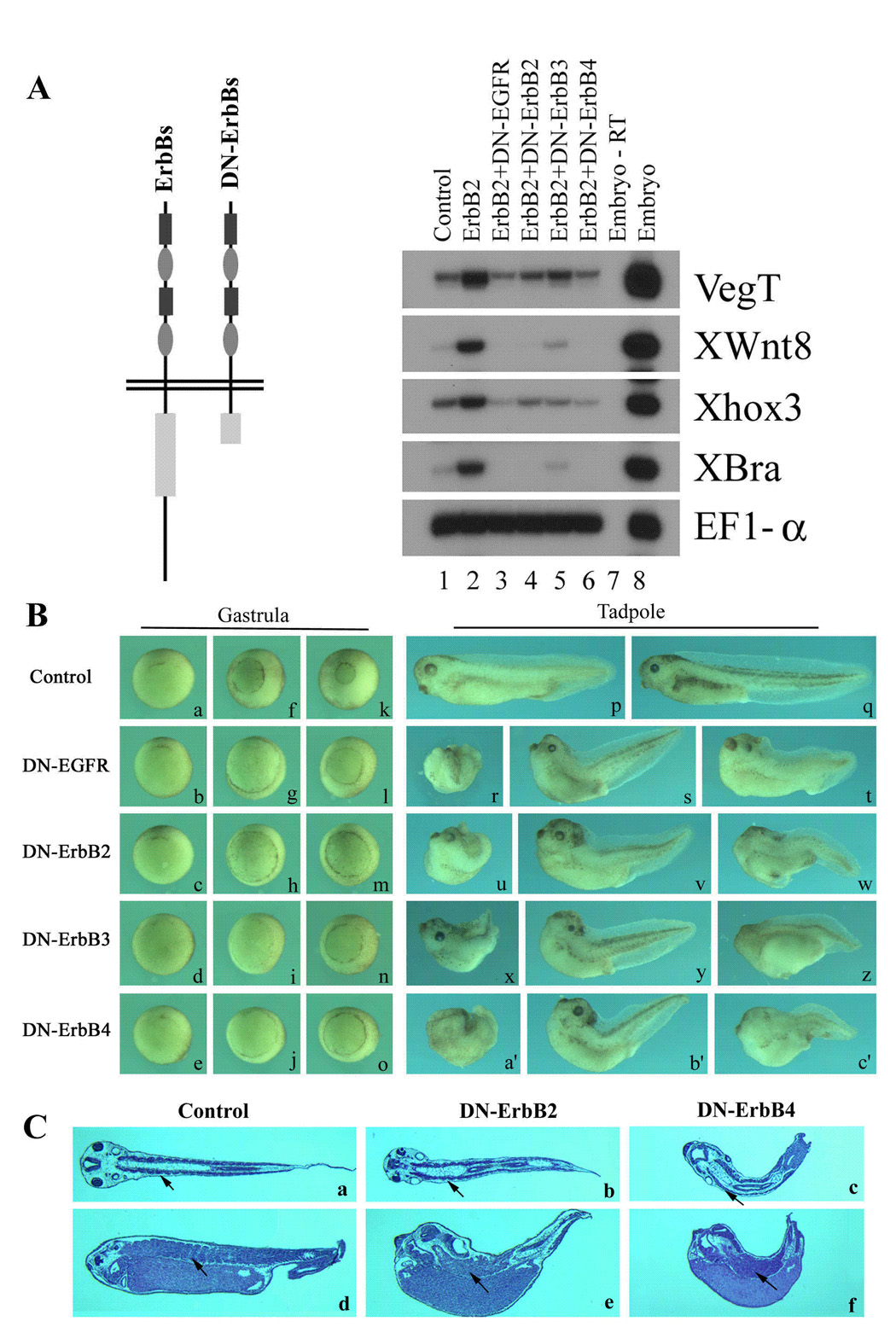
A) Schematic representation of the truncated ErbB receptors (DN-ErbBs) and the evidence supporting their actions in a dominant negative fashion to inhibit ErbB signaling. 2ng ErbB2 RNA was coinjected with 4ng RNAs encoding DN-ErbBs into both animal poles of 2-cell stage embryos. Animal caps were explanted at blastula stages and RNA was extracted at gastrula stage (stage 11) for RT-PCR assay. Coexpression of any of the DN-ErbBs with ErbB2 (lanes 3 to 6) efficiently blocked the mesodermal induction by ErbB2 (lane 2), suggesting that DN-ErbBs function as dominant negative receptors. B) Inhibition of ErbB signaling leads to gastrulation and axial patterning defects. 250pg to 1ng RNAs encoding DN-ErbBs were injected into the marginal zones of 2- to 4-cell stage embryos, and the morphology of the injected embryos was observed at gastrula (stages 10 to 12) or tadpole (stages 34–38) stages. In this panel, embryos injected with 1ng DN-ErbBs were shown. Though the onset of gastrulation was normal in embryos injected with DN-ErbBs (stage 10, panels a to e), the closure of the blastopore was severely delayed during gastrulation (stage 11, panels f to j; stage 12, panels k to o). At tadpole stages (stages 34 to 38), embryos expressing DN-ErbBs showed a variety of defects, including open back (panels r, u, x and a’), reduced head structures (panels r to c’), shortened and curved body axis (panels s, t, v, w, y, z, b’ and c’) and somite malformation (panels t, w, z and c’). C) Microtomal sections (horizontal sections, panels a to c; parasagittal sections, panels d to f) of the embryos showed that while muscle differentiation occurred in the absence of ErbB signaling, somite segmentation was impaired in embryos lacking ErbB signals. The arrows point to the segmented and the continuous somites in wild type and DN-ErbB embryos. The embryos were orientated with the head to the left.
Since delay in gastrulation may affect the interaction between head mesoderm and the overlying ectoderm that can result in head malformation, we next addressed whether the head defect induced by DN-ErbBs was direct or indirect due to impaired gastrulation. We thus injected RNAs encoding DN-ErbBs together with the tracer nβGal into one animal blastomere of 16- to 32-cell stage embryos and observed the phenotypes of the injected tadpoles (Fig. 7). In this experiment, targeted expression of DN-ErbBs in regional ectodermal tissues greatly reduced the incidence of gastrulation defect and allowed us to examine the requirement for ErbB signaling in head formation directly. While about 80% of uninjected control embryos (n=60) and 69% of βGal-injected embryos (n=49) displayed normal morphology, among the DN-ErbB-expressing embryos that showed βGal staining in the head region and normal gastrulation pattern, the percentage of the embryos with normal head structures was much reduced (14/31, or 45%, for DN-EGFR embryos; 7/28 or 25% for DN-ErbB2 samples; 9/23 or 39% in DN-ErbB3 embryos; and 11/30 or 37% of DN-ErbB4 embryos showed normal head morphology). Our results thus indicate that independent of its regulation of gastrulation movements, ErbB signaling is also required directly for head formation.
Figure 7. Inhibition of ErbB signaling in the head ectoderm leads to malformation of the head structures.
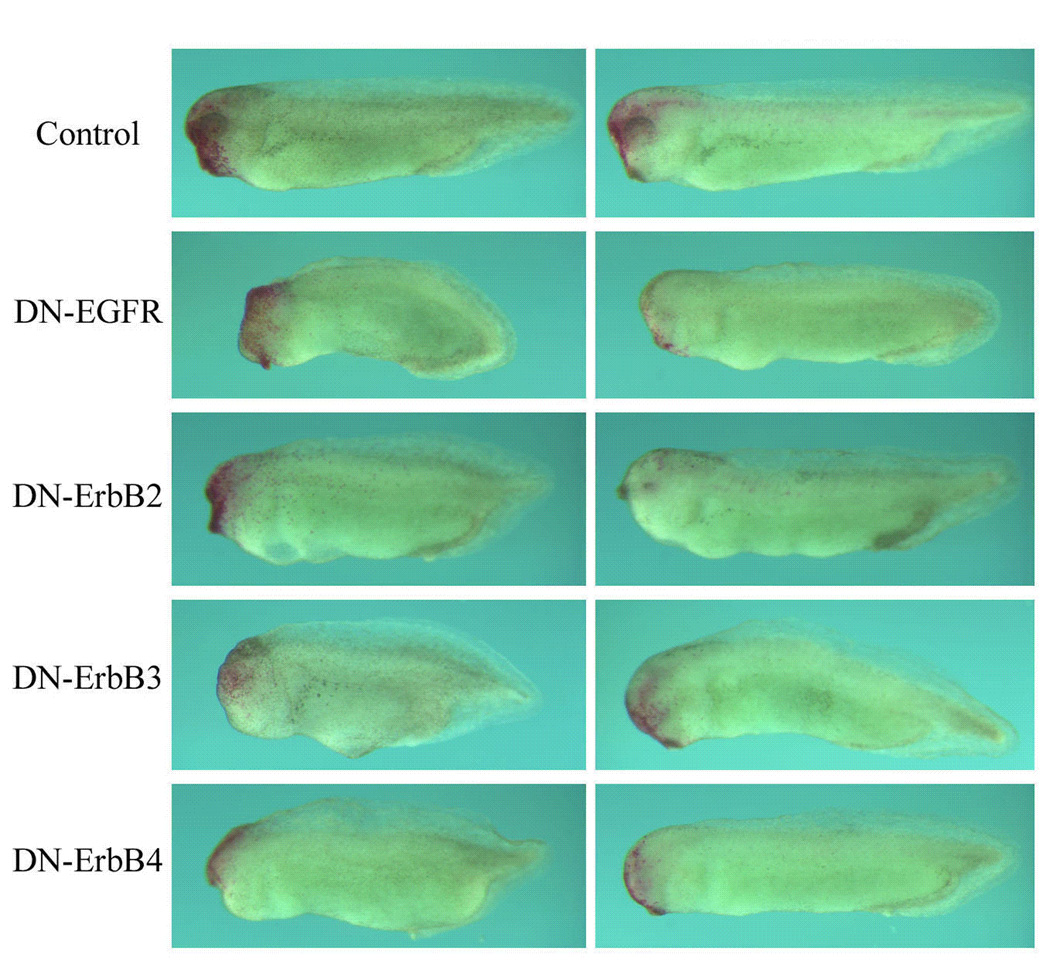
RNAs encoding DN-ErbBs (1ng) were coinjected with the lineage tracer nβGal (200pg) into one animal blastomere of 16- to 32- cell stage embryos. This targeted expression of DN-ErbBs in the ectoderm greatly reduced the incidence of gastrulation defects. The resulting tadpoles (stages 32–36) were stained with the Red-Gal substrate and the embryos with the labeling in the head were scored for their head morphology. While the majority of nβGal-injected embryos showed normal head phenotype, more than half of the embryos injected with DN-ErbBs displayed malformation of the head structures, including reduction of the eyes and the forebrain. The data suggest that ErbBs may regulate head development directly independent of their effects on gastrulation.
Inhibition of ErbB signaling affects marker gene expression in early frog embryos
Overexpression of DN-ErbBs results in gastrulation defects and an open back phenotype in early frog embryos that is similar to that induced by blocking the FGF signaling pathway (e.g. with DN-FGFR1, data now shown; also see Amaya et al., 1991, 1993; Kroll and Amaya, 1996; Conlon and Smith, 1999; Nutt et al., 2001; Yokota et al., 2003). In the case of FGF-dependent regulation of gastrulation, the immediate downstream gene Brachyury (Xbra) plays a critical role in controlling the expression of Wnt11 to modulate convergent extension movements (Tada and Smith, 2000). Blocking FGF signals leads to down-regulation of Xbra expression and impairment of gastrulation. To see whether ErbBs act in a similar way to FGFRs to regulate gastrulation via controlling Xbra transcription, we examined the expression of Xbra at gastrula stages by in situ hybridization (Fig. 8A). As all four DN-ErbBs behaved similarly in all the assays we performed (see above and data not shown), we chose to analyze gene expression in embryos expressing DN-ErbB2 and DN-ErbB4 only. Despite the delay in blastopore closure, we detected a similar pattern and level of Xbra expression in the wild type and in the DN-ErbB expressing embryos (Fig. 8A). The data suggest that unlike FGFRs, ErbBs modulate gastrulation independent of Xbra expression. In addition, as Xbra is a pan-mesodermal marker at early developmental stages, our results also imply that ErbBs may not participate in early mesodermal induction.
Figure 8. Blocking the ErbB pathway affects the pattern of marker gene expression.
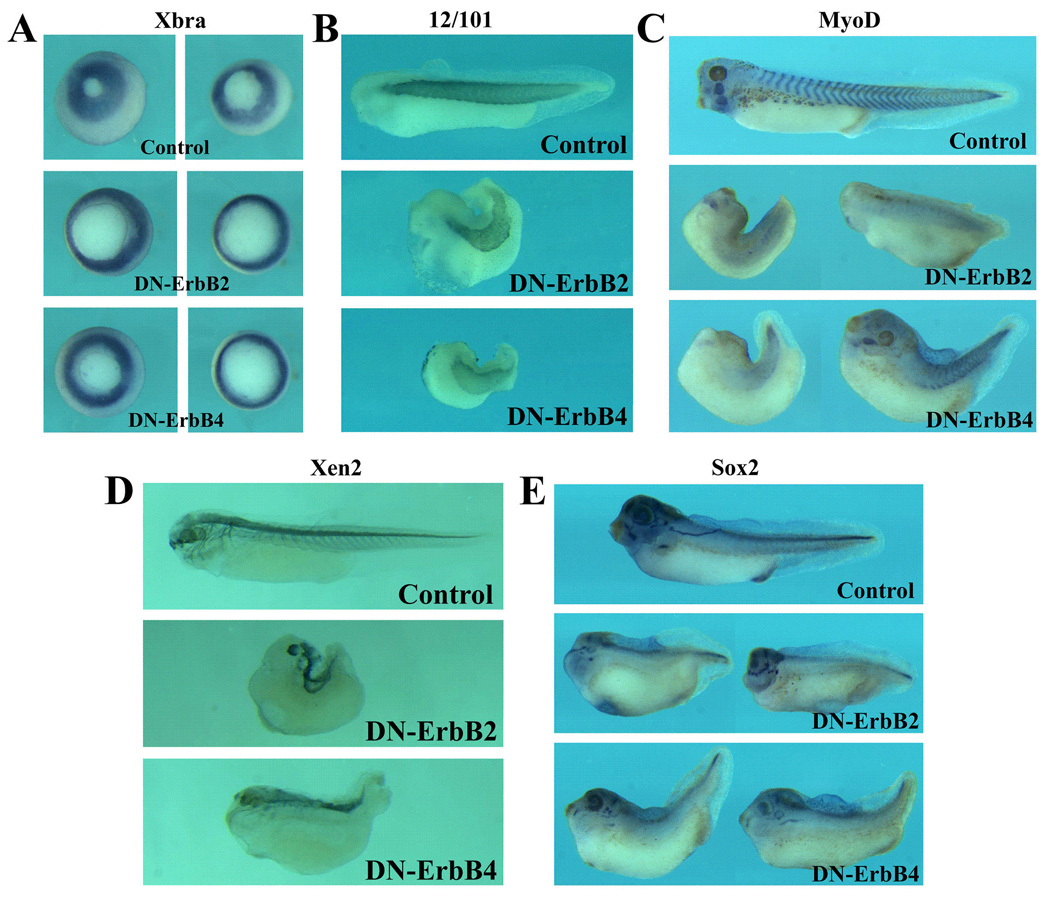
RNAs encoding DN-ErbB2 or DN-ErbB4 (total 2ng) were injected into the marginal zones of 4-cell stage embryos, and marker gene expression was examined by in situ hybridization or immunohistochemistry at gastrula (stages 11–12) or tadpole (stages 36– 40) stages. A) Inhibition of ErbB signaling does not lead to repression of early pan-mesodermal gene Brachyury (Xbra) expression, though the delay in gastrulation results in larger blastopore in embryos injected with DN-ErbBs. The embryos are at stages 11–12 and viewed from the vegetal side. B) The muscle marker 12/101 epitope was expressed in embryos expressing DN-ErbBs, but the repeated expression pattern of the epitope in distinct somites was not apparent. C) In situ hybridization with the MyoD probe illustrated that expression of MyoD in embryos injected with DN-ErbBs was reduced, and the reiterated pattern of expression in each somites was indistinct. D) The neural marker Xen2 epitope was expressed in the trunk region in embryos expressing DN-ErbBs, but the forebrain and the brachial arch neuronal expression domains were missing. E) The pan-neural marker Sox2 was expressed at normal levels in embryos injected with DN-ErbBs at gastrula stages (not shown); at tadpole stages, its expression in the head region was severely reduced, though the trunk expression was relatively normal. The embryos in panels B-E are orientated with the head on the left side.
To examine whether the axial defects we observed were associated with a failure in mesodermal and/or neural differentiation, we next analyzed the expression of the muscle and the neural markers in tadpoles with the axial defects (Fig. 8). Though the muscle marker 12/101 epitope was detected in embryos injected with DN-ErbBs, its expression was often indistinct that lacked the somitic boundaries seen in the wild type embryos (Fig. 8B). In addition, the expression of MyoD was reduced, especially at the trunk level, and the pattern of MyoD expression was also indistinct in many embryos (Fig. 8C). The results indicate that muscle differentiation was not impaired in embryos where the ErbB signals were blocked, but the segmentation of somites might be affected. When the neural marker Xen2 epitope was examined, we found that it was still expressed in embryos with the axial defects, but the expression in the head region was reduced. The forebrain expression domain was often absent, and the neuronal processes extending into the brachial arches were missing in many embryos (Fig. 8D). Similarly, the pan-neural marker Sox2 (Mizuseki et al., 1998) was expressed at comparable levels in the wild type and the DN-ErbB injected embryos at gastrula stages (not shown); but at tadpole stages, its expression in the head region, including the brain and the placodal derived tissues, was reduced or lost in DN-ErbB expressing embryos (Fig. 8E). The expression of both the Xen2 epitope and Sox2 in the trunk region was relatively normal (Fig. 8D, E). Taken together, our data demonstrate that muscle and neural differentiation proceeds normally in frog embryos in the absence of ErbB signals, but patterning of the somites and formation of the head are impaired, resulting in altered expression pattern of the muscle and the neural markers.
Discussion
The EGFR/ErbB signaling pathway plays essential roles during invertebrate development. It has been implicated in diverse processes ranging from cell proliferation and apoptosis to fate determination and migration. They control almost all aspects of development during Drosophila embryogenesis. In addition, activation of ErbB signals has been associated with multiple tumors in human. The conserved nature of this signal pathway and the common employment of homologous signals in similar developmental processes during animal evolution imply that ErbB signals may be crucial in regulation of vertebrate embryogenesis as well. To date, however, the functions of ErbB signaling during early vertebrate development are not well understood. In this study, we undertake analyses of the ErbB activities during early frog embryogenesis, using both gain- and loss-of-function approaches. Our results reveal that ErbB signals control gastrulation movement, cell proliferation and embryonic patterning in early frog embryos, thus providing evidence for the first time that the ErbB pathway plays key roles during early Xenopus development.
One main function described for ErbB signaling is regulation of cell division. During Drosophila eye development, ommatidia form from posterior to anterior eye disc following the move of the morphogenetic furrow. Control of cell proliferation by the EGFR pathway is critical at multiple steps for specification and determination of the correct numbers of specialized cell types in the fly eye (Baker, 2001; Yang and Baker, 2003; Jones and Moses, 2004). In mouse and human, activation of ErbB signals leads to deregulated cell division and formation of various tumors (Muller, 2002; Holbro et al., 2003). The capacity to stimulate cell proliferation by ErbBs seems to hold true in Xenopus as well. When overexpressed in the ectoderm of early frog embryos, ErbBs (EGFR, ErbB2 an ErbB4) induce ectopic protrusions with an increased number of mitotic cells in them (Fig. 3). This induction of tumor-like cell mass in Xenopus has previous been reported when several other genes are overexpressed in early frog embryos, including a dominant negative p53, Gli1 oncogene, and the Rel3 transcription factor (Dahmane et al., 1997; Wallingford et al., 1997; Yang et al., 1998b). Like in other cases, ErbBs induce tumor-like protrusion most efficiently when overexpressed in the ectoderm (Fig. 2 and data not shown). This differential response of different cell types to ErbB activation may reflect an intrinsic difference in extracellular signal-regulated cell cycle control mechanisms in various cells. Along the same line, it is interesting to note that most tumors associated with ErbB activation are of ectodermal and epithelial origins (Holbro et al., 2003). Our results support the notion that the Xenopus model system can be used to study the molecular mechanisms in tumorigenesis (Wallingford, 1999). One unresolved issue is whether endogenous ErbB signals participate in regulation of cell proliferation during frog embryogenesis. Our preliminary studies using truncated ErbB receptors show that injection of DN-ErbBs into the marginal zone of early frog embryos did not significantly alter the cell division pattern at tailbud to tadpole stages (not shown). The region of DN-ErbB expression in this case, however, is the region where normally low cell division is observed (Saka and Smith, 2001; Leise III and Mueller, 2004; Murakami et al., 2004). It will be important in future to determine whether blocking ErbB signals in other regions, especially in the ectoderm, can reduce cell proliferation in developing embryos.
In addition to stimulate cell division, ErbBs can induce differentiated cell markers in ectopic protrusions and in animal caps (Fig. 4 and 5). The induction of mesodermal genes in animal caps, however, required two to four fold higher doses of ErbBs than that required for induction of ectopic protrusions. This is especially true for EGFR, which is a weak mesodermal inducer. The data imply that a gradient of ErbB signals may regulate different cellular processes, with low ErbB signaling sufficient for regulation of cell proliferation and high ErbB signaling required for cell differentiation. When the ErbB pathway is inhibited by overexpression of the dominant negative receptors, we do not observe repression of the pan-mesodermal marker Brachyury at gastrula stages, though the muscle marker MyoD is reduced at late stages. In addition, somite segmentation is indistinct in embryos where ErbB signals are blocked. The data indicate that ErbBs may not be involved in mesodermal induction during early frog development, but may be required for differentiation of particular tissues and/or patterning and organization of embryonic tissues. The role of ErbBs in mesoderm formation and muscle development seems to be different from that of FGFs, which have been shown to be essential for Brachyury and MyoD expression in early Xenopus embryos (Conlon and Smith, 1999; Fisher et al., 2002). ErbBs, in comparison, seems to play an important role during somitogenesis to set up inter-somitic boundaries. It will be interesting to determine in future whether and how ErbBs interact with other signals known to control somite development, such as Notch, Wnt and FGF pathways (Hoppler et al., 1996; Jen et al., 1997, 1999; Iulianella et al., 2003; Pourquie, 2003), during frog somitogenesis. In addition to muscle defects, we also observed a reduction in the head structures in embryos where ErbB signaling was inhibited. This phenotype was not due to a general reduction in neural tissues, as the pan-neural marker Sox2 was expressed at the normal level during gastrula stages, and the expression of both Sox2 and the Xen2 epitope were relatively normal at the trunk level of the affected embryos. ErbBs may therefore participate specifically in the formation of Xenopus head. Since targeted inhibition of ErbB signaling in head ectoderm led to malformation of tadpole head (Fig. 7), the data support the idea that ErbBs can regulate head formation directly without affecting general anterior-posterior patterning of the nervous system.
One main developmental defect induced by blocking the ErbB pathway is the impaired gastrulation movements. Though the onset of gastrulation is normal as judged by the timing of the appearance of the dorsal lip, the closure of the blastopore is greatly delayed or even incomplete, resulting in “open back” phenotype. A similar open back morphology has previously been reported when using truncated versions of other receptor tyrosine kinases (RTKs), including FGFRs and PDGFRs (Amaya et al., 1991, 1993; Ataliotis et al., 1995; Kroll and Amaya, 1996; Conlon and Smith, 1999; Nutt et al., 2001; Yokota et al., 2003). However, these receptor tyrosine kinases seem to regulate different aspects of the morphogenetic movements. Activation of FGFR, for example, leads to expression of the downstream gene Brachyury, whose function is required for convergent extension movements during gastrulation (Conlon and Smith, 1999; Nutt et al., 2001). Blocking the PDGF pathway, however, does not down-regulate Brachyury expression or affect the convergent extension movements (Nagel et al., 2004). Instead, PDGF signaling controls directional migration of mesodermal cells along the blastocoel roof (Nagel et al., 2004). It is currently unclear whether ErbB signals regulate mesodermal cell involution and migration or convergent extension behaviors, and how ErbB signals may modulate these cell movements during gastrulation. In other situations, ErbBs have been implicated in modulating cell motility directly, such as during tumor progression (Holbro et al., 2003), in neuronal cell migration (Threadgill et al., 1995) and in Drosophila border cell migration during oogenesis (Lehmann, 2001). Though the MAPK pathway is one of the preferred downstream signals activated by RTKs, during gastrulation and in other contexts, PI3K pathway may also play a crucial role in regulation of cell motility. In Xenopus it has been reported that inhibition of PI3K signaling leads to impaired gastrulation (Carballada et al., 2001), and PI3K can mediate PDGF-induced mesodermal cell spreading (Symes and Mercola, 1996). It is therefore possible that PI3K, in conjunction with MAPK, functions downstream of ErbBs to regulate gastrulation movements. Further analyses are required to distinguish the functions of FGFR, PDGFR and ErbB pathways during Xenopus gastrulation morphogenesis.
In summary, we have performed gain- and loss-of-function studies to understand the functions of ErbB signaling during early frog embryogenesis. We show for the first time that ErbBs regulate important developmental processes in frogs, including gastrulation, muscle development and head formation. In addition, ErbBs can control cell proliferation in early frog embryos. Due to extensive homo- and hetero-dimerization among ErbB receptors, we cannot distinguish potential overlapping and unique roles of each individual ErbBs at this time. The differences in inducing various mesodermal markers by EGFR, ErbB2 and ErbB4 suggest that different ErbBs may have distinct activities in vivo. In future, antisense morpholino oligonucleotide-based loss-of-function approach should be used to help us resolve the overlapping and distinct activities of each ErbBs.
Experimental Procedures
Plasmids construction and RNA synthesis
The constructs for mammalian EGFR and ErbBs and Xenopus FGFRs were kindly provided by Dr. Eugene Chin (Brown Univ.), Dr. Lin Mei (UAB) and Dr. Okamoto (NIBHT, Japan). The coding regions of EGFR and ErbBs were cloned into pCS105. DN-ErbBs were subsequently constructed using PCR cloning strategy and cloned into pCS105. The templates were linearized with AscI and in vitro transcription was performed using mMessage mMachine kit (Ambion). For library screening, the PCR products of Xenopus ErbBs were used as probes to screen a stage 28–32 head library (kindly provided by Dr. Harland) and clones encoding partial Xenopus ErbB2 were recovered from the screening. XErbB3 fragment used for in situ hybridization was PCR-cloned according to the EST sequence for ErbB3 (accession number CB209506). The Xenopus EGFR and ErbB4 long fragments were obtained by PCR, using the X. tropicalis genome information for annotated ErbBs. The primers used for PCR-cloning are: XtEGFR-U: 5’-ATTCAGTGGTGGCCAGATAATTACACAG-3’, XtEGFR-D: 5’-GCC ATCCACTTGATAGGCACTTTGCCCCCTT-3’, XtErbB4-U: 5’-TGCTATTACCATA CCGTCAACTGGACA-3’ and XtErbB4-D: 5’-TCCAAAGTCGGTGATCTTCACATG ATT-3’.
RNA microinjection, embryo manipulations and RT-PCR
Embryos were obtained, maintained and microinjected with capped RNAs as described (Chang et al., 1997). Animal caps were dissected from injected or control embryos at blastula stages (stages 8–9) and total RNA was extracted from the caps at gastrula (stages 10–11) or tailbud (stages 28–32) stages for RT-PCR. For assay of the ErbB expression, the following specific primer pairs were used: XEGFR-U: 5’-AGACGTTTGGTGCATCGTGA-3’and XEGFR-D: 5’-TCACTGGCTGGAATTCCATC-3’; XErbB2-U: 5’-GACAACAGCCAAGTGTACAT-3’ and XErbB2-D: 5’-CCAGTAAGCAGCCGTACGGC-3’; XErbB3-U: 5’-GAGGAACATCGAATGGTTCA-3’ and XErbB3-D: 5’-TATTTGTGGTTGGGATAGCC-3’; XErbB4-U: 5’-CTGGTTCACAGAGATCTGGC-3’ and XErbB4-D: 5’-CAGACAGGTGGCTGAGGAAG-3’.
In situ hybridization (ISH) and whole mount immunohistochemistry (WMIHC)
ISH was performed as described by Harland (1991). MyoD and Sox2 probes were kindly provided by Dr. Blitz. WMIHC was performed as described (Chang et al., 1997). 12/101 and Xen-2 antibodies were obtained from DSHB (Univ. Iowa and NICHD), and anti-phosphorylated histone H3 (Upstate) antibody was used at 5µg/ml. The secondary antibodies (Jackson Laboratories) were used at 1:500 to 1:2000 dilutions. Some of the stained embryos were cleared in benzyl benzoate/benzyl alcohol solution and imaged directly, or embedded in paraffin and sectioned via microtome before imaging. For mitotic cell counting, 59 sections from 9 EGRFR-injected embryos, 99 sections from 11 ErbB2-injected embryos, 79 sections from 12 ErbB4-injected embryos and 68 sections from 10 control embryos were used. The number of mitotic cells in the ectopic protrusions and in the rest of the embryos was counted separately, and the average mitotic cells per arbitrary unit area were compared. For all samples, the density of mitotic cells in the ectopic protrusions was more than 8 fold higher than that in other regions of the embryos or in control embryos (8.86, 8.71 and 8.38 fold higher mitotic cells for EGFR-, ErbB2- and ErbB4-injected samples).
Acknowledgements
We thank Drs. Lin Mei, Harumasa Okamoto, Alan Wells, Eugene Chin, Ira Blitz and Ken Cho for providing constructs used in this study; Drs. Tim Games and Richard Harland for providing stage 28–32 head library. The work is supported by NIH grant.
Grant: NIH R01HD43345
References
- Alaoui-Jamali MA, Song DJ, Benlimame N, Yen L, Deng X, Hernandez-Perez M, Wang T. Regulation of multiple tumor microenvironment markers by overexpression of single or paired combinations of ErbB receptors. Cancer Res. 2003;63:3764–3774. [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. doi: 10.1242/dev.118.2.477. [DOI] [PubMed] [Google Scholar]
- Ataliotis P, Symes K, Chou MM, Ho L, Mercola M. PDGF signalling is required for gastrulation of Xenopus laevis. Development. 1995;121:3099–3110. doi: 10.1242/dev.121.9.3099. [DOI] [PubMed] [Google Scholar]
- Atit R, Conlon RA, Niswander L. EGF signaling patterns the feather array by promoting the interbud fate. Dev. Cell. 2003;4:231–240. doi: 10.1016/s1534-5807(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Baker NE. Cell proliferation, survival, and death in the Drosophila eye. Semin. Cell Dev. Biol. 2001;12:499–507. doi: 10.1006/scdb.2001.0274. [DOI] [PubMed] [Google Scholar]
- Battaglia P, Melli M. Isolation of globin messenger RNA of Xenopus laevis. Dev. Biol. 1977;60:337–350. doi: 10.1016/0012-1606(77)90132-4. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Pollet N, Delius H, Niehrs C. The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nature Cell Biol. 2004;6:38–44. doi: 10.1038/ncb1082. [DOI] [PubMed] [Google Scholar]
- Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulins-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AW, Cho H-S, Eigenbrot C, Ferguson KM, Garrett TPJ, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- Carballada R, Yasuo H, Lemaire P. Phosphatidylinositol-3 kinase acts in parallel to the ERK MAP kinase in the FGF pathway during Xenopus mesoderm induction. Development. 2001;128:35–44. doi: 10.1242/dev.128.1.35. [DOI] [PubMed] [Google Scholar]
- Carpenter G. ErbB4: mechanism of action ad biology. Exp. Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Casci T, Freeman M. Control of EGF receptor singalling: Lessons from fruitflies. Cancer Metastasis Rev. 1999;18:181–201. doi: 10.1023/a:1006313122373. [DOI] [PubMed] [Google Scholar]
- Chang C, Wilson PW, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat. Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Chung HG, Chung HM. Neuregulin induces the expression of mesodermal genes in the ectoderm of Xenopus laevis. Mol. Cells. 1999;9:497–503. [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp. Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Smith JC. Interference with brachyury function inhibits convergent extension, causes apoptosis, and reveals separate requirements in the FGF and activin signalling pathways. Dev. Biol. 1999;213:85–100. doi: 10.1006/dbio.1999.9330. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signaling pathway in skin tumors. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- Fisher ME, Isaacs HV, Pownall ME. eFGF is required for activation XmyoD expression in the myogenic cell lineage of Xenopus laevis. Development. 2002;129:1307–1315. doi: 10.1242/dev.129.6.1307. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Goishi K, Lee P, Davidson AJ, Nishi E, Zon LI, Klagsbrun M. Inhibition of zebrafish epidermal growth factor receptor activity results in cardiovascular defects. Mech. Dev. 2003;120:811–822. doi: 10.1016/s0925-4773(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Fairman S, Mohun TJ, Brennan S. Activation of muscle-specific actin genes in Xenopus development by an induction between animal and vegetal cells of a blastula. Cell. 1985;41:913–922. doi: 10.1016/s0092-8674(85)80072-6. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp. Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Brown JD, Moon RT. Expression of a dominat-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 1989;8:3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development. 1997;124:1689–1698. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- Iulianella A, Melton KR, Trainor PA. Somitogenesis: breaking new boundaries. Neuron. 2003;40:11–14. doi: 10.1016/s0896-6273(03)00604-4. [DOI] [PubMed] [Google Scholar]
- Jen WC, Wettstein D, Turner D, Chitnis A, Kintner C. The Notch ligand, X-Delta-2, mediates segmentation of the paraxial mesoderm in Xenopus embryos. Development. 1997;124:1169–1178. doi: 10.1242/dev.124.6.1169. [DOI] [PubMed] [Google Scholar]
- Jen WC, Gawantka V, Pollet N, Niehrs C, Kintner C. Periodic repression of Notch pathway genes governs the segmentation of Xenopus embryos. Genes & Dev. 1999;13:1486–1499. doi: 10.1101/gad.13.11.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Moses K. Cell-cycle regulation and cell-type specification in the developing Drosophila compound eye. Semin, Cell Dev. Biol. 2004;15:75–81. doi: 10.1016/j.semcdb.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 1999;447:227–231. doi: 10.1016/s0014-5793(99)00283-5. [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signaling. Exp. Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Lee K-F, Simon H, Chen H, Bates B, Hung M-C, Hauser C. Requirement for neuregulins receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Lehmann R. Cell migration in invertebrates: clues from border and distal tip cells. Curr. Opin. Genet. Dev. 2001;11:457–463. doi: 10.1016/s0959-437x(00)00217-3. [DOI] [PubMed] [Google Scholar]
- Leise WF, III, Mueller PR. Inhibition of the cell cycle is required for convergent extension of the paraxial mesoderm during Xenopus neurulation. Development. 2004;131:1703–1715. doi: 10.1242/dev.01054. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Kroll KL, Sun EE, Kirschner MW. Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodermal patterning and blastopore lip formation. Development. 1996;122:4001–4012. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Moghal N, Sternberg PW. The epidermal growth factor system in Caenorhabditis elegans. Exp. Cell Res. 2003;284:150–159. doi: 10.1016/s0014-4827(02)00097-6. [DOI] [PubMed] [Google Scholar]
- Muller WJ. General keynote: expression of epidermal growth factor receptor family in transgenic mouse models of human breast cancer. Gynecol. Oncol. 2002;88:S43–S46. doi: 10.1006/gyno.2002.6682. [DOI] [PubMed] [Google Scholar]
- Murakami MS, Moody SA, Darr IO, Morrison DK. Morphogenesis during Xenopus gastrulation requires Wee1-mediated inhibition of cell proliferation. Development. 2004;131:571–580. doi: 10.1242/dev.00971. [DOI] [PubMed] [Google Scholar]
- Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- Nutt SL, dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient RK, Banville D, Brewer AC, Elkington JA, Greaves DR, Lloyd MM, Williams X. The organization of the tadpole and adult alpha globin genes of Xenopus laevis. Nucl. Acids Res. 1982;10:7935–7945. doi: 10.1093/nar/10.24.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquie O. The segmentation clock: converting embryonic time into spatial pattern. Sci. 2003;301:328–330. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- Ram TG, Schelling ME, Hosick HL. Blocking HER-2/HER-3 function with a dominant negative form of HER-3 in cells stimulated by heregulin and in breast cancer cells with HER-2 gene amplification. Cell Growth Differ. 2000;11:173–183. [PubMed] [Google Scholar]
- Riese DJ, II, Stern D. Specificity within the EGF family/ErbB receptor family signaling network. BioEssays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewins GR, Birchmeier C. Severe neuropathies in mice with taegeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Ruiz i Alataba A, Melton DA. Involvement of the Xenopus homeobox gene Xhox3 in pattern formation along the anterior-posterior axis. Cell. 1989;57:317–326. doi: 10.1016/0092-8674(89)90969-0. [DOI] [PubMed] [Google Scholar]
- Saka Y, Smith JC. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev. Biol. 2001;229:307–318. doi: 10.1006/dbio.2000.0101. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Shilo B-Z. A thousand and one roles for the Drosophila EGF receptor. Trends. Genet. 1997;13:191–196. doi: 10.1016/s0168-9525(97)01091-3. [DOI] [PubMed] [Google Scholar]
- Shilo B-Z. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp. Cell Res. 2003;284:140–149. doi: 10.1016/s0014-4827(02)00094-0. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Sci. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Silibia M, Steinbach JP, Stingl L, Aguzzi A, Wagner EF. A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J. 1998;17:719–731. doi: 10.1093/emboj/17.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Stennard F, Carnac G, Gurdon JB. The Xenopus T-box gene, Antipodean, encodes a vegetally localised maternal mRNA and can trigger mesoderm formation. Development. 1996;122:4179–4188. doi: 10.1242/dev.122.12.4179. [DOI] [PubMed] [Google Scholar]
- Sun BI, Bush SM, Collins-Racie LA, LaVallie ER, DiBlasio-Smith EA, Wolfman NM, McCoy JM, Sive HL. derriere: a TGF-beta family member required for posterior development in Xenopus. Development. 1999;126:1467–1482. doi: 10.1242/dev.126.7.1467. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Carraway KL., III Ligand discrimination by ErbB receptors: differential signaling through differential phosphorylation site usage. Oncogene. 2000;19:5568–5573. doi: 10.1038/sj.onc.1203913. [DOI] [PubMed] [Google Scholar]
- Symes K, Mercola M. Embryonic mesoderm cells spread in response to platelet-derived growth factor and signaling by phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA. 1996;93:9641–9644. doi: 10.1073/pnas.93.18.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, Bernard JA, Yuspa SH, Coffey RJ, Magnuson T. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Sci. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Tonissen KF, Drysdale TA, Lints TJ, Harvey RP, Krieg PA. XNkx-2.5, a Xenopus gene related to Nkx-2.5 and tinman: evidence for a conserved role in cardiac development. Dev. Biol. 1994;162:325–328. doi: 10.1006/dbio.1994.1089. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Seufert DW, Vitra VC, Vize PD. p53 activity is essential for normal development in Xenopus. Curr. Biol. 1997;7:747–757. doi: 10.1016/s0960-9822(06)00333-2. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Tumors in tadpoles, the Xenopus embryo as a model system for the study of tumorigenesis. Trends Genet. 1999;15:385–388. doi: 10.1016/s0168-9525(99)01800-4. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Marden J, Carnevali F, Hemmati-Brivanlou A. FGF-mediated mesoderm induction involves the Src-family kinase Laloo. Nature. 1998;394:904–908. doi: 10.1038/29808. [DOI] [PubMed] [Google Scholar]
- Wright CV, Morita EA, Wilkin DJ, De Robertis EM. The Xenopus XIHbox 6 homeo protein, a marker of posterior neural induction, is expressed in proliferating neurons. Development. 1990;109:225–234. doi: 10.1242/dev.109.1.225. [DOI] [PubMed] [Google Scholar]
- Yang JF, Zhou H, Pun S, Ip NY, Peng HB, Tsim KWK. Cloning of cDNAs encoding Xenopus neuregulins: expression in myotomal muscle during embryo development. Mol. Brain Res. 1998a;58:59–73. doi: 10.1016/s0169-328x(98)00085-0. [DOI] [PubMed] [Google Scholar]
- Yang JF, Zhou H, Choi RC, Ip NY, Peng HB, Tsim KWK. A cysteine-rich form of Xenopus neuregulin induces the expression of acetylcholine receptors in cultured myotubes. Mol. Cell. Neurosci. 1999;13:415–429. doi: 10.1006/mcne.1999.0759. [DOI] [PubMed] [Google Scholar]
- Yang L, Baker NE. Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev. Cell. 2003;4:359–369. doi: 10.1016/s1534-5807(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Yang S, Lockwood A, Hollett P, Ford R, Kao K. Overexpression of a novel Xenopus Rel mRNA induces tumors in early embryos. J. Biol. Chem. 1998b;273:13746–13752. doi: 10.1074/jbc.273.22.13746. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nature Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yeo W, Gautier J. A role for programmed cell death during early neurogenesis in Xenopus. Dev. Biol. 2003;260:31–45. doi: 10.1016/s0012-1606(03)00222-7. [DOI] [PubMed] [Google Scholar]
- Yokota C, Kofron M, Zuck M, Houston DW, Isaacs H, Asashima M, Wylie CC, Heasman J. a novel role for a nodal-related protein; Xnr3 regulates convergent extension movements via the FGF receptor. Development. 2003;130:2199–2212. doi: 10.1242/dev.00434. [DOI] [PubMed] [Google Scholar]
- Zhang J, King ML. Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development. 1996;122:4149–4129. doi: 10.1242/dev.122.12.4119. [DOI] [PubMed] [Google Scholar]