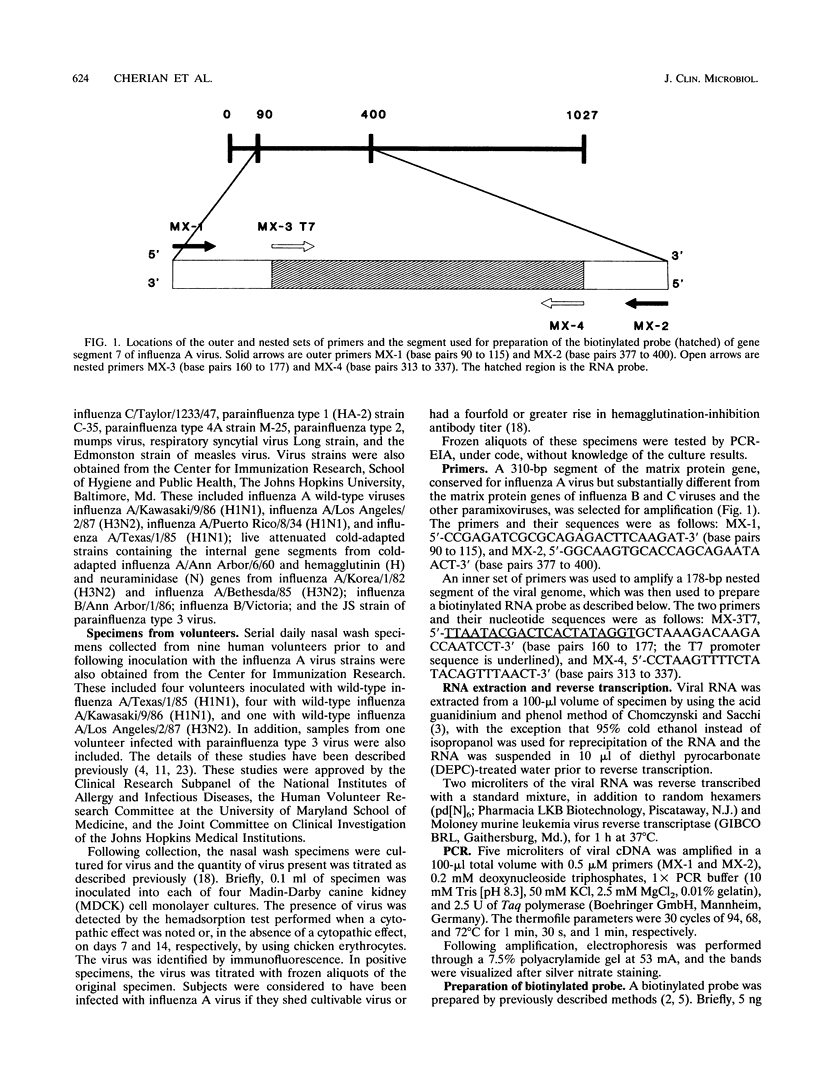
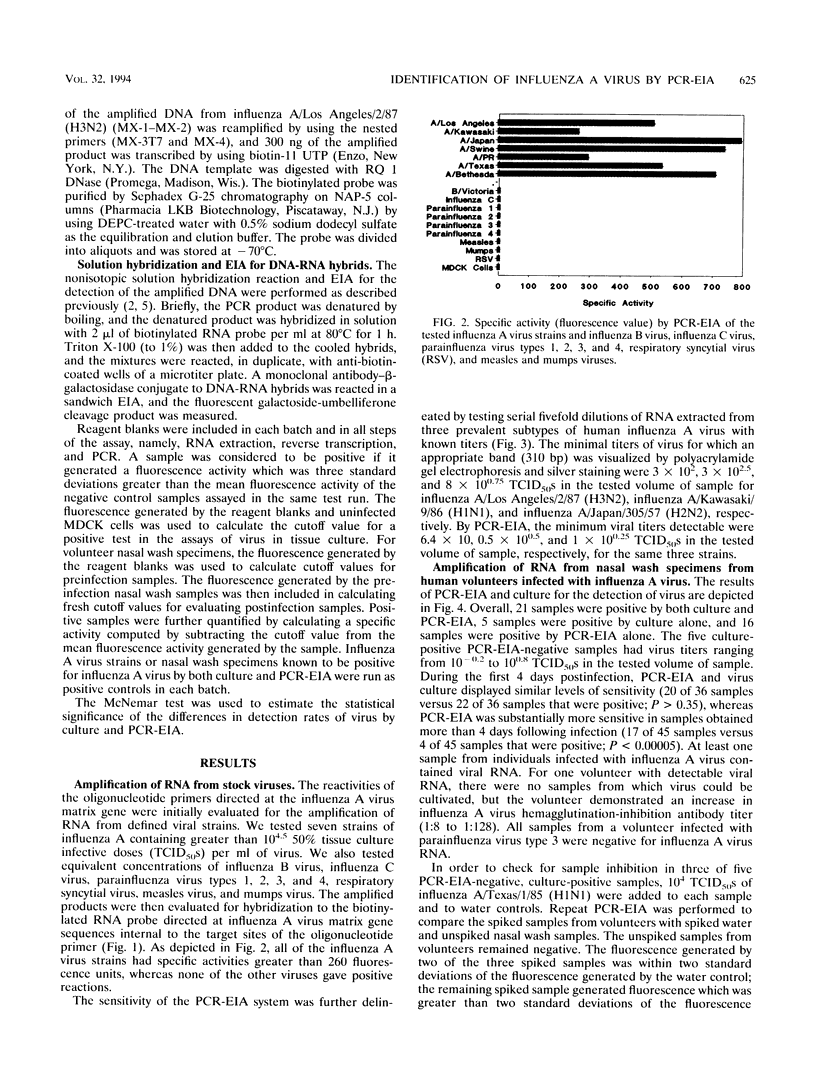
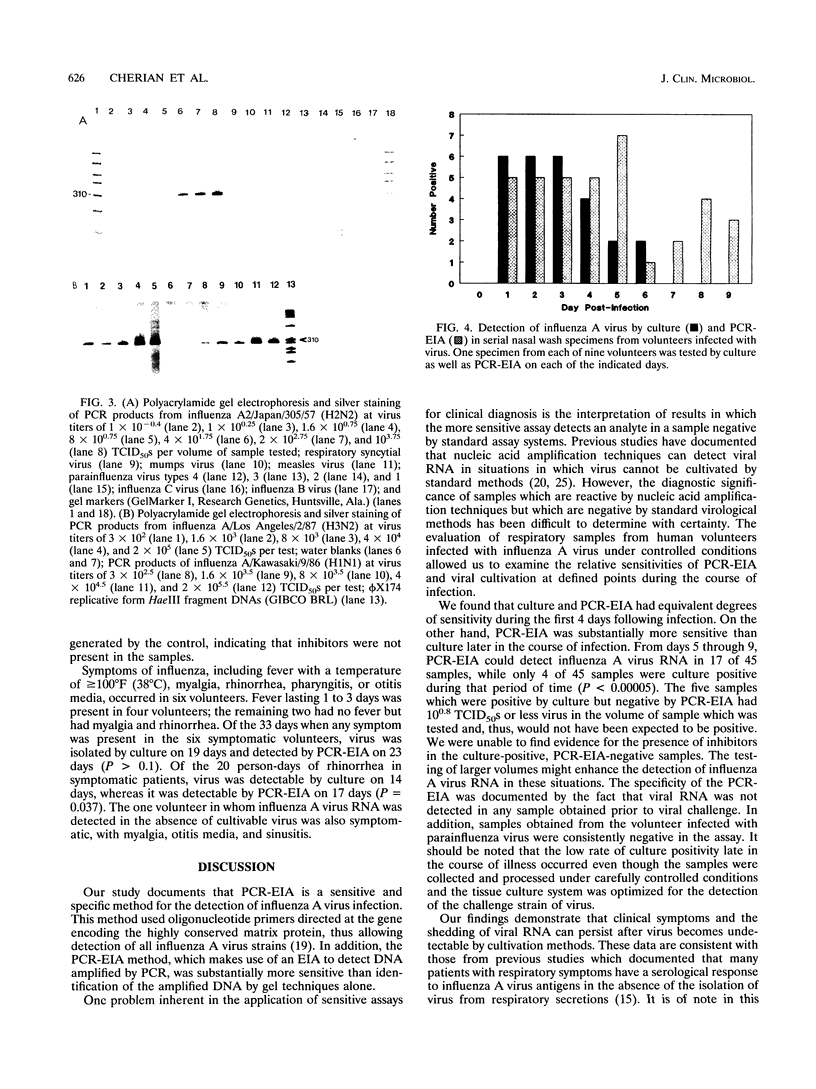
Abstract
Influenza A virus infections are a major cause of morbidity and mortality worldwide. Standard diagnostic methods either are not efficient in identifying infected individuals in a timely manner or lack sensitivity. We developed a PCR-enzyme immunoassay (PCR-EIA) for the detection of influenza A virus RNA in respiratory secretions. A reverse transcription PCR was performed with oligonucleotide primers directed at a highly conserved area of the influenza A matrix gene. Amplified DNA was identified by hybridization in solution to a nested biotinylated RNA probe and quantitated in an EIA. PCR-EIA detected small quantities of RNA from the three prevalent subtypes of human influenza A virus. Influenza B and C, parainfluenza, measles, mumps, and respiratory syncytial viruses tested negative. The potential efficiency of PCR-EIA for use in clinical diagnosis was determined by testing 90 nasal wash specimens obtained daily over a 10-day period from nine human volunteers infected with influenza A virus. Thirty-seven of the postinfection samples had detectable influenza A virus RNA by PCR-EIA, whereas only 26 postinfection samples were positive by culture. PCR-EIA was particularly efficient for the identification of influenza A virus in samples obtained more than 4 days after infection. Seventeen of 45 such samples were positive, whereas virus was cultivated from 4 samples (P < 0.00005). All preinfection samples from volunteers subsequently infected with influenza A virus were negative by PCR-EIA, as were samples from a volunteer infected with parainfluenza virus type 3. Nucleic acid amplification techniques represent important tools for the timely and sensitive diagnosis of influenza A virus infections and, therefore, their management and control.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisno A. L., Griffin J. P., Van Epps K. A., Niell H. B., Rytel M. W. Pneumonia and Hong Kong influenza: a prospective study of the 1968-1969 epidemic. Am J Med Sci. 1971 May;261(5):251–263. doi: 10.1097/00000441-197105000-00004. [DOI] [PubMed] [Google Scholar]
- Bobo L., Coutlee F., Yolken R. H., Quinn T., Viscidi R. P. Diagnosis of Chlamydia trachomatis cervical infection by detection of amplified DNA with an enzyme immunoassay. J Clin Microbiol. 1990 Sep;28(9):1968–1973. doi: 10.1128/jcm.28.9.1968-1973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Subbarao E. K., Fries L. F., Karron R. A., London W. T., Murphy B. R. Use of single-gene reassortant viruses to study the role of avian influenza A virus genes in attenuation of wild-type human influenza A virus for squirrel monkeys and adult human volunteers. J Clin Microbiol. 1992 Mar;30(3):655–662. doi: 10.1128/jcm.30.3.655-662.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlée F., Bobo L., Abbass H., Dalabetta G., Hook N. E., Shah K., Viscidi R. P. Detection of HPV-16 in cell lines and cervical lavage specimens by a polymerase chain reaction-enzyme immunoassay assay. J Med Virol. 1992 May;37(1):22–29. doi: 10.1002/jmv.1890370105. [DOI] [PubMed] [Google Scholar]
- Daisy J. A., Lief F. S., Friedman H. M. Rapid diagnosis of influenza A infection by direct immunofluorescence of nasopharyngeal aspirates in adults. J Clin Microbiol. 1979 Jun;9(6):688–692. doi: 10.1128/jcm.9.6.688-692.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döller G., Schuy W., Tjhen K. Y., Stekeler B., Gerth H. J. Direct detection of influenza virus antigen in nasopharyngeal specimens by direct enzyme immunoassay in comparison with quantitating virus shedding. J Clin Microbiol. 1992 Apr;30(4):866–869. doi: 10.1128/jcm.30.4.866-869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med. 1990 Jan 18;322(3):178–183. doi: 10.1056/NEJM199001183220307. [DOI] [PubMed] [Google Scholar]
- Frank A. L., Couch R. B., Griffis C. A., Baxter B. D. Comparison of different tissue cultures for isolation and quantitation of influenza and parainfluenza viruses. J Clin Microbiol. 1979 Jul;10(1):32–36. doi: 10.1128/jcm.10.1.32-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Dillon S. B., Hildreth J. E., Karron R. A., Funkhouser A. W., Friedman C. J., Jones C. S., Culleton V. G., Clements M. L. Safety and immunogenicity of a recombinant protein influenza A vaccine in adult human volunteers and protective efficacy against wild-type H1N1 virus challenge. J Infect Dis. 1993 Mar;167(3):593–601. doi: 10.1093/infdis/167.3.593. [DOI] [PubMed] [Google Scholar]
- Guatelli J. C., Whitfield K. M., Kwoh D. Y., Barringer K. J., Richman D. D., Gingeras T. R. Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1874–1878. doi: 10.1073/pnas.87.5.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. W., Brandt C. D., Arrobio J. O., Murphy B., Chanock R. M., Parrott R. H. Influenza A and B virus infection in infants and young children during the years 1957-1976. Am J Epidemiol. 1979 Apr;109(4):464–479. doi: 10.1093/oxfordjournals.aje.a112704. [DOI] [PubMed] [Google Scholar]
- Lui K. J., Kendal A. P. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987 Jun;77(6):712–716. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A. S., Maassab H. F., Bryan E. R. Relative efficacy of embryonated eggs and cell culture for isolation of contemporary influenza viruses. J Clin Microbiol. 1981 Jan;13(1):233–235. doi: 10.1128/jcm.13.1.233-235.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton A. W., Paton J. C., Lawrence A. J., Goldwater P. N., Harris R. J. Rapid detection of respiratory syncytial virus in nasopharyngeal aspirates by reverse transcription and polymerase chain reaction amplification. J Clin Microbiol. 1992 Apr;30(4):901–904. doi: 10.1128/jcm.30.4.901-904.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R. M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990 Aug 16;346(6285):629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Seki S., Hatsushika M., Watanabe S., Akiyama K., Nagao K., Tsutsui K. cDNA cloning, sequencing, expression and possible domain structure of human APEX nuclease homologous to Escherichia coli exonuclease III. Biochim Biophys Acta. 1992 Jul 15;1131(3):287–299. doi: 10.1016/0167-4781(92)90027-w. [DOI] [PubMed] [Google Scholar]
- Snyder M. H., Clements M. L., Betts R. F., Dolin R., Buckler-White A. J., Tierney E. L., Murphy B. R. Evaluation of live avian-human reassortant influenza A H3N2 and H1N1 virus vaccines in seronegative adult volunteers. J Clin Microbiol. 1986 May;23(5):852–857. doi: 10.1128/jcm.23.5.852-857.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. E. Neurological complications of so-called "influenza". A winter study in South-east Wales. Br Med J. 1971 Feb 13;1(5745):369–373. doi: 10.1136/bmj.1.5745.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde J., Yolken R., Willoughby R., Eiden J. Improved detection of rotavirus shedding by polymerase chain reaction. Lancet. 1991 Feb 9;337(8737):323–326. doi: 10.1016/0140-6736(91)90945-l. [DOI] [PubMed] [Google Scholar]
- Zhang W. D., Evans D. H. Detection and identification of human influenza viruses by the polymerase chain reaction. J Virol Methods. 1991 Jun;33(1-2):165–189. doi: 10.1016/0166-0934(91)90017-t. [DOI] [PubMed] [Google Scholar]