Abstract
We previously reported that aging BNXF344 rats are more vulnerable to disruptions of memory consolidation processes following an injection of E. coli than are young rats. Furthermore, this disruption was specific to hippocampal-dependent memory. In the present study we examined the time course of the level of the proinflammatory cytokine IL-1β in young and old rats following a peripheral injection of E. coli. Compared to young rats, aging rats treated with E. coli showed an exaggerated and prolonged up-regulation of IL-1β protein in the hippocampus, but not in hypothalamus, parietal cortex, prefrontal cortex, serum or spleen. Aging rats showed greater hippocampal IL-1β protein levels than their young counterparts 4h after E. coli, and these levels remained significantly elevated for 8 but not 14 days after E. coli. In a second experiment, aging rats exhibited anterograde memory consolidation impairments 4 and 8 days after an E. coli injection, but not after 14 days. A third experiment revealed that following an E. coli injection, bacterial clearance from the spleen and peritoneum was not impaired in aged rats, suggesting that elevations in hippocampal IL-1β were not mediated by impaired clearance in the periphery in aging rats. These data suggest that the exaggerated and prolonged elevation of IL-1β, specifically in the hippocampus, may be responsible for hippocampal-dependent memory impairments observed in aging rats following a bacterial infection.
Keywords: normal aging, learning, memory, glial priming, hippocampus, E. coli, peripheral administration, interleukin-1, pneumonia
1. Introduction
There has been increasing interest in the role of proinflammatory cytokines in modulating learning and memory processes (Barrientos et al., 2006a; Barrientos et al., 2002; Bilbo et al., 2005; Cunningham et al., 1996; Godbout et al., 2005; Hauss-Wegrzyniak et al., 1998; Pugh et al., 1998; Yirmiya et al., 2002). In large part, this attention comes from findings that Alzheimer’s disease is associated with significantly elevated levels of the proinflammatory cytokines interleukin-1 beta (IL-1β), IL-6 and TNF-α and/or decreased levels of the anti-inflammatory cytokines IL-1ra, and IL-10 (Griffin et al., 1989; Lombardi et al., 1999; Lue et al., 2001; Mrak and Griffin, 2005; Paganelli et al., 2002; Tarkowski et al., 2001). These altered cytokine levels have been found both peripherally and centrally. This is important because peripheral cytokines are capable of signaling the brain via both blood-borne and neural routes (Dantzer, 2004) and can lead to de novo production of pro-inflammatory cytokines within the brain (Van Dam et al., 1995). Thus, bacterial infections that are initiated in the periphery have the potential to result in inflammation in the brain.
Elevated levels of IL-1β in the brain, particularly in the hippocampus, are especially relevant to memory function because it has been shown to impair hippocampal long term potentiation (LTP) (Cunningham et al., 1996; Vereker et al., 2000a; Vereker et al., 2000b), a form of synaptic plasticity that is characterized by a persistent increase in synaptic efficacy following titanic stimulation (Bliss and Lomo, 1973). Importantly, inhibition of LTP results in hippocampal-dependent memory impairments (Morris et al., 1986; Shimizu et al., 2000), and elevated levels of IL-1β in the hippocampus have been repeatedly shown to impair hippocampal-dependent memory (Barrientos et al., 2002; Barrientos et al., 2003; Barrientos et al., 2004; Bilbo et al., 2005; Hauss-Wegrzyniak et al., 1998; Hauss-Wegrzyniak et al., 2002; Hauss-Wegrzyniak et al., 1999; Pugh et al., 1998; Pugh et al., 1999). It should be emphasized that we are referring here to IL-1β elevated above basal levels, as basal levels of IL-1β have been shown to be necessary for plasticity and hippocampal-dependent memory performance (Yirmiya et al., 2002). It is important to note that the literature is muddled with inconsistencies pertaining to basal levels of IL-1β in young vs. old subjects. Some laboratories report basal differences (Gemma et al., 2005; Godbout et al., 2005; Riancho et al., 1994), while others do not (Beloosesky et al., 2002; Fagiolo et al., 1993; Gayle et al., 1999; Gee et al., 2006; Kyrkanides et al., 2001). Whether these inconsistencies are due to differences in species (human, rat, mice, etc.), and/or strains of animals used or the age of the “aged” subject is unclear at this time. We have never observed a basal difference in IL-1β levels in any tissue samples between our young and old subjects. However, it is worth noting that we are using 24 mo. F344xBN rats which are considered to be a “younger” than, say, a 24 mo. old F344 rat, which is considered to be senescent at this age. We purposely chose the older group to be at an age below senescence (perhaps 36 mos. in this strain) to minimize memory impairments between young and old rats in the absence of an immune challenge.
Because a) peripheral cytokines induce the production of brain cytokines, and b) brain cytokines can interfere with hippocampal memory consolidation, individuals that have either increased peripheral inflammatory responses to immune activating agents such as bacteria, or exaggerated brain proinflammatory responses to signaling events within the brain, are likely to be more susceptible to infection-induced memory impairments. Aged individuals fall into this vulnerable category. Healthy aged animals show antigenic and morphological markers of glial activation (Frank et al., 2006; McGeer et al., 1987; Perry et al., 1993; Smith et al., 2001; Wagner et al., 1993). This is noted because glia are the predominant source of cytokines induced within the brain parenchyma (Van Dam et al., 1995), and were found to be the source of proinflammatory cytokines observed in the brains of AD patients (Lue et al., 2001). As stated earlier, basal proinflammatory cytokine levels in healthy aged subjects are not always found to be elevated (Beloosesky et al., 2002; Fagiolo et al., 1993; Kyrkanides et al., 2001). It has been suggested that glial cells with this profile may be sensitized, or primed, so that they show morphological markers of activation, but do not express elevated levels of proinflammatory cytokines unless the organism is challenged and glia therefore signaled (Perry et al., 2003). When the organism with primed glial cells is challenged, the resulting expression of proinflammatory cytokines is exaggerated (Cunningham et al., 2005), thus exacerbating local brain inflammation.
In the current study, we 1) determined the duration of brain and peripheral IL-1β protein changes after a peripheral administration of E. coli in young and aging rats. This timecourse is not known. Assessments were made 2h, 4h, 24h, 4d, 8d, and 14d after E. coli. 2) Determined whether the duration of memory impairment in aging animals after E. coli parallels the duration of hippocampal IL-1β elevation. To do this we measured long-term memory for contextual fear conditioning. The duration of any such effect is not known. 3) Examined aging rats’ ability to clear the E. coli bacteria from the peritoneal cavity, spleen, hippocampus, and blood, compared to that of young rats. This was done to determine whether the effects of aging on E. coli-induced increases in IL-1β and memory impairments could be explained by decreased clearance of E. coli.
2. Materials and Methods
2.1. Subjects
Subjects were male F344xBN F1 rats obtained from the National Institute on Aging (Bethesda, MD). Upon arrival at our facility, old rats were 24 mo. old and weighed approximately 575 g. Young rats were 3 mo. old and weighed approximately 280 g. All rats were housed 2 to a cage (52 L X 30 W X 21 H, cm), in a standard animal colony shared by several experimenters. The animal colony was maintained at 23°C on a 12-h light/dark cycle (lights on at 07:00 h). All rats were allowed free access to food and water and were given at least 1 week to acclimate to colony conditions before experimentation began.
All experiments were conducted in accordance with protocols approved by the University of Colorado Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
2.2. Immune challenge
In all experiments, animals received an intraperitoneal (i.p.) injection of either Escherichia coli (E. coli), or vehicle (PBS). Treatments were the same between cage mates, to avoid contamination of E. coli from one rat to the other. One day prior to experimentation, stock E. coli cultures (ATCC 15746; American Type Culture Collection, Manassas, VA) were thawed and cultured overnight (15–20 hr) in 40 mL of brain-heart infusion (BHI; DIFCO Laboratories, Detroit, MI) in an incubator (37°C, 95% air + 5% CO2). The number of bacteria in cultures was quantified by extrapolating from previously determined growth curves. Cultures were then centrifuged for 15 min at 4°C, 3000 RPM, supernatants discarded, and bacteria resuspended in sterile phosphate buffered saline (PBS). Bacteria were resuspended with a volume of PBS to achieve a dose of 1.0 x 1010 CFU. A volume of 250 μL was injected i.p., regardless of body weight, for a final concentration of 2.5 x 109. Thus, the older rats received a lower “dose” than did the younger subjects relative to body weight, a procedure that was adopted as a conservative measure. The amount of E. coli injected could have been made proportional to body weight, but there is no reason to believe that body weight would be the correct determining factor. This led us to use the most conservative procedure possible. We have previously shown that this concentration of E. coli induces a sickness response including fever and significant body weight loss in both young and old rats (Barrientos et al., 2006b; Campisi et al., 2003). Briefly, we found that young rats showed a robust fever 2hr after E. coli injection lasting 7 hr before returning to baseline temperatures. Old rats exhibited a fever about 6hr after E. coli injection followed by a hypothermic response during the active (dark) phase. Fever continued on days 2 and 3, during the inactive phase, but the active phase temperatures returned to baseline levels by the second day. Also, both groups lost an equivalent percentage of body weight (~4%) one day after E. coli injection. Vehicle-treated rats received an injection of sterile PBS of an equal volume (250 μL).
2.3. Tissue dissection and detection of IL-1β protein
For experiment 1, rats were euthanized at the following time points following E. coli injection: 2h, 4h, 24h, 4d, 8d, and 14d. To minimize the use of animals, we assessed vehicle controls at only two time points, 3h and 24h following vehicle injection, for each age group. However, it should be noted that, in order to minimize any contributions made by varying colony conditions, we spread out the collection of these tissues across the entire experiment, so that for example, some 3h vehicle-treated rats were euthanized on the same day as the 14 day E. coli-treated rats. Rats were anesthetized with sodium pentobarbital (50mg/kg, i.p.). Blood was collected from the heart and then rats were transcardially perfused with ice-cold 0.9% saline to minimize the contribution of peripheral blood to tissue samples. Spleens and brains were quickly removed, and hippocampus, hypothalamus, prefrontal cortex, and parietal cortex were dissected from brains. All dissections were performed on an ice-cold frosted glass plate and tissues quickly frozen in liquid nitrogen. Tissue samples were stored at −70°C until the time of sonication. To prepare the tissue for the assay, each tissue was added to 0.25–0.5 mL of Iscove’s culture medium containing 5% fetal calf serum and a cocktail enzyme inhibitor (100 mM Amino-n-caproic acid, 10 mM EDTA, 5 mM Benzamidine HCL, and 0.2 mM Phenylmethyl sulfonyl fluoride). Total protein was mechanically dissociated from tissue using an ultrasonic cell disrupter (Fisher Scientific, Pittsburgh, PA). Sonication consisted of 10 s of cell disruption at setting #10. Sonicated samples were centrifuged at 10,000 g at 4°C for 10 min. Supernatants were removed and stored at 4°C until ELISA was performed. Bradford protein assays were also performed to determine total protein concentrations in sonication samples.
Levels of IL-1β protein were determined using a commercially available rat IL-1β ELISA kit (R&D Systems, Minneapolis, MN). The assay was performed according to the manufacturer’s instructions. IL-1β protein levels were determined and normalized to total protein (pg/100 μg total protein).
2.4. Contextual fear conditioning
To determine how long after the E. coli injection aging rats would still show hippocampal-dependent memory impairments, we conducted a contextual fear conditioning experiment at several time points after the immune challenge. Rats received an i.p. injection of E. coli or vehicle either 4, 8, or 14 days prior to conditioning. Since we previously found that young rats are not impaired 4 days after E. coli (Barrientos et al., 2006a), in this experiment we included young rats in the 4 day group for replication purposes only, but we did not include them in the later time points, as there is no reason to believe they would show an impairment at 8 and/or 14 days post-infection when there was no impairment present at 4 days. The conditioning context was one of two identical Igloo ice chests (54 L x 30 W x 27 H, cm) with white interiors. A speaker and an activated 24-V DC light bulb were mounted on the ceiling of each chest. The conditioning chambers (26 L x 21 W x 24 H, cm), placed inside each chest, were made of clear plastic and had window screen tops. A 2 s, 1.5 mA shock was delivered through a removable floor of stainless steel rods 1.5 mm in diameter, spaced 1.2 cm center to center. Each rod was wired to a shock generator and scrambler (Colbourn Instruments, Allentown, PA). Chambers were cleaned with water before each animal was conditioned or tested. Rats were taken two at a time from their home cage and each was placed in a conditioning chamber. Rats were allowed to explore the chamber for 2 min before the onset of a 15 s tone (76 dB), followed immediately by a 2 s footshock (1.5 mA). Immediately after the termination of the shock, rats were removed from the chamber and returned to their home cage. Three days later, all rats were tested for fear of the conditioning context--a hippocampal-dependent task, and then for fear of the tone--a hippocampal-independent task. That is, the hippocampus is required to form a long-term memory of fear of the context, but not for fear of the tone (Kim and Fanselow, 1992). Fear of the context was assessed by placing the rat in the conditioning context for 6 min. Fear of the tone was assessed by placing the rat in an altered context for 3 min, followed by a 3 min presentation of the tone. Scoring began approximately 10 s after the animal was placed into the chamber. Every 10 s each rat was judged as either freezing or active at the instant the sample was taken. Freezing, the rat’s dominant defensive fear response, is a complete suppression of behavior that is accompanied by immobility, shallow breathing, and a variety of other autonomic changes including an increase in heart rate and pilo-erection (Fanselow and Lester, 1988). Rats exhibit the freezing response when they are in a potentially harmful situation such as those when the threat of predation is high and it is a common measure of conditioned fear (Kim and Fanselow, 1992). Rats were first tested for fear of the context and then for fear to the tone about 4h later. This order is appropriate because fear during the tone test can be the result of fear produced by the tone plus generalized contextual fear from the training context. Testing first for fear of the training context should greatly diminish generalized fear because there should be some extinction of fear to training context. Thus, this order should result in a more pure measure of conditioned fear supported just by the tone.
It may be useful for readers to know that the contextual fear conditioning paradigm requires the rat to explore his environment, sampling the many features that make up the whole context. During the test for fear of the context, it could be that the rat is sampling somewhat different features of the context than those that were sample during conditioning. Therefore, percent freezing during this test can be variable. In contrast, the tone is being imposed upon the rat both during conditioning and during test, therefore, there is little chance of the animal missing its presence. Thus, freezing to the tone is robust and less variable.
Behavior was scored by observers blind to experimental treatment and inter-rater reliability exceeded 97% for all experiments.
2.5. Bacterial clearance
To determine whether young and old rats are equally efficient at clearing live E. coli bacteria, we cultured both peripheral and central tissue samples using agar plates and counted new bacterial growth from those samples. Whole blood was collected at 4h (tail nick) and at either 24h or 48h (cardiac puncture) following the (i.p.) E. coli injection, and plated onto McConkey agar plates (0.1 mL). Either 24 or 48 hr following the challenge all rats were anesthetized with 50mg/kg of sodium pentobarbital. Their abdomens were rubbed with 70% alcohol to reduce the probability of their fur contaminating the samples. A small incision was made in the peritoneal cavity and 10mL of cold sterile PBS was pipetted in. The incision was then clamped using large hemostats, and the peritoneal cavity was roughly massaged for 30 sec. The hemostats were carefully unclamped and a sterile serological pipet inserted to collect as much fluid as possible, usually about 8 mL. Intraperitoneal lavage fluid was diluted 10E-1 and 10E-2 and 0.1 mL was plated on McConkey agar plates. Rats were then transcardially perfused with cold 0.9% saline to eliminate the contribution of peripheral blood to spleen and brain. Spleens were then dissected out, homogenized in 3 mL PBS, filtered, diluted (10E-1 and 10E-2) and 0.1 mL was plated on agar plates. To examine the possibility that E. coli had entered the brains of the aged rats, (possibly due to a leaky blood brain barrier) hippocampus was dissected out and homogenized in 3 mL cold PBS, and plated on agar plates, undiluted. All agar plates were incubated (37°C, 95% air + 5% CO2) overnight and colony forming units (CFU) were counted the next day.
3. Results
3.1. Experiment 1
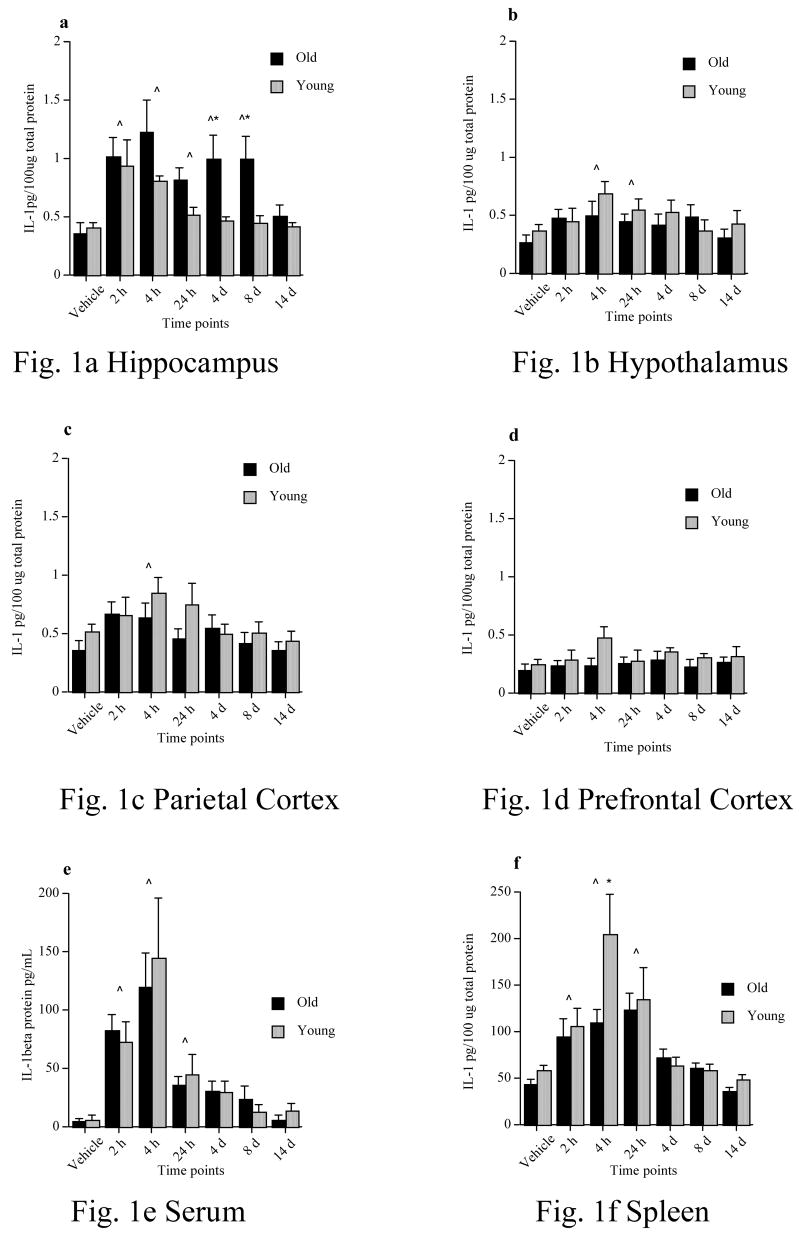
In this experiment we examined the influence of a peripheral infection on the production of the pro-inflammatory cytokine interleukin-1 beta (IL-1β) in both the periphery as well as the brain at several different time points following bacterial challenge. Among our vehicle-injected rats, there was no significant difference between the 3h and 24h time points (data not shown) for any of the tissues sampled. This lack of an effect of vehicle injection further justified omitting examination of cytokines at even more remote times from the vehicle administration. Therefore, we pooled these two vehicle control groups to form just one control group per age group. Furthermore, for each tissue, there was no significant difference between vehicle-treated young and old groups (hippocampus (F (1, 21) = .328, p > .05); hypothalamus (F (1, 23) = 1.354, p > .05); parietal cortex (F (1, 24) = 2.828, p > .05); prefrontal cortex (F (1, 23) = .747, p > .05); serum (F (1, 21) = .078, p > .05); spleen (F (1, 24) = 4.07, p > .05)). For each tissue, a 2 x 2 (challenge x age) ANOVA was conducted at each time point (data are shown in Fig. 1a–f). Because the same vehicle control group was employed for each time point analysis, we calculated a Bonferroni correction for the six analyses, making our rejection alpha level .008.
Figure 1.
IL-1β protein levels of young and old rats following a peripheral vehicle or E. coli injection at 2h, 4h, 24h, 4d, 8d, &14d in a) hippocampus, b) hypothalamus, c) parietal cortex, d) prefrontal cortex, d) serum, and f) spleen. Note that vehicle samples were collected at 3h & 24h and because they did not differ, were pooled to form just one vehicle control group. ^ = significant difference between E. coli-treated and vehicle-treated groups. * = significant difference between age groups. Error bars indicate ± SEM.
In the hippocampus (Fig. 1a) we found that both young and old E. coli-treated rats had significantly elevated levels of IL-1β compared to vehicle control rats from 2h to 24h. Furthermore, old rats continued to show elevated levels of IL-1β at 4 and 8 days post-infection, while young rats did not. Finally, at 14 days post-infection, both young and old E. coli-treated rats exhibited IL-1β levels that were no different from vehicle controls. A 2x2 ANOVA showed there was a significant main effect of the challenge at 2h (F (1, 37) = 22.54, p < .0001), 4h (F (1, 37) = 20.00, p < .0001), and 24h (F (1, 33) = 14.05 p = .0007). At these time points E. coli-treated rats had significantly higher IL-1β levels than did vehicle control rats. However, there was no main effect of age at these time points: 2h (F (1, 37) = .014, p = .91); 4h (F (1, 37) = 1.761, p = .19); 24h (F (1, 33) = 2.729, p = .11). Furthermore, there were no significant interaction effects at these time points: 2h (F (1, 37) = .28, p = .60); 4h (F (1, 37) = 2.86, p = .10); 24h (F (1, 33) = 5.48, p = .03). At 4 days, there was no main effect of age (F (1, 34) = 5.20, p = .03), but there was a significant main effect of the challenge (F (1, 34) = 10.74, p = .002), and a significant interaction (F (1, 34) = 7.68, p = .008). Fisher’s PLSD post hoc tests revealed E. coli-treated old rats had significantly higher levels of IL-1β compared to vehicle-treated old rats (p = .008). Furthermore, E. coli-treated old rats had significantly higher levels of IL-1β compared to E. coli-treated young rats (p = .03). Young E. coli-treated rats however, were not different than young vehicle-treated rats (p = .33). At 8 days, there was no main effect of age (F (1, 32) = 7.22, p = .01), but there was a significant main effect of the challenge (F (1, 32) = 13.12, p = .001), and a significant interaction (F (1, 32) = 10.53, p = .003). Similar to the 4d data, post hoc tests revealed E. coli-treated old rats had significantly higher levels of IL-1β compared to vehicle-treated old rats (p = .005), and E. coli-treated old rats had significantly higher levels of IL-1β compared to E. coli-treated young rats (p = .01). And again, young E. coli-treated rats were not different than young vehicle-treated rats (p = .58). At 14 days, there was no main effect of age (F (1, 31) = .06, p = .81), no main effect of the challenge (F (1, 31) = 1.23, p = .28), and no interaction (F (1, 31) = .92, p = .35). Thus, E coli increased IL-1β levels in the hippocampus in young and aging rats, but this increase persisted for only between 4 and 24h in young rats, while it persisted for between 8 and 14d in the aging subjects.
In the hypothalamus (Fig. 1b) and parietal cortex (Fig. 1c) we found that both young and old E. coli-treated rats had significantly elevated levels of IL-1β compared to vehicle control rats only at 4h post-infection (and 24h in hypothalamus only). Elevations at other time points were not significantly different from vehicle controls, and there were no age differences at any time point. In the prefrontal cortex (Fig. 1d) there were no significant elevations of IL-1β compared to vehicle control rats in either young or old, at any time point. 2x2 ANOVAs showed that in the hypothalamus (F (1, 36) = 12.64, p = .001) and parietal cortex (F (1, 38) = 10.87, p = .002), there was a significant main effect of challenge at the 4h time point. All other main effects were not significant in these tissues. In the prefrontal cortex, there were no significant main effects, at any time point.
In the serum (Fig. 1e), we found that both young and old E. coli-treated rats had significantly elevated levels of IL-1β compared to vehicle control rats at 2h, 4h, and 24h post-infection, but not at later time points. However, there were no differences between young and old rats at any time point. A 2x2 ANOVA showed that there was a significant main effect of the challenge at 2h (F (1, 38) = 34.19, p < .0001), 4h (F (1, 36) = 27.26, p < .0001), and 24h (F (1, 34) = 9.41, p = .004), but not at 4d (F (1, 35) = 5.19, p = .03), 8d (F (1, 31) = .65, p = .43), or 14d (F (1, 33) = .006, p = .94). There were no significant main effects of age at any time point, nor any significant interactions. Thus, aging did not potentiate the increase in plasma IL-1β produced by E. coli.
In the spleen (Fig. 1f), we found that both young and old E. coli-treated rats had significantly elevated levels of IL-1β compared to vehicle control rats at 2h, 4h, and 24h post-infection, but not at later time points. Aberrantly, 4h post-infection, young E. coli-treated rats showed a significantly higher level of IL-1β than old E. coli-treated rats, however there were no differences between young and old rats at any other time points. A 2x2 ANOVA showed that there was a significant main effect of the challenge at 2h (F (1, 40) = 16.90, p = .0002), 4h (F (1, 40) = 37.70, p < .0001), 24h (F (1, 37) = 33.53, p < .0001), but not at 4d (F (1, 37) = 6.20, p = .02), 8d (F (1, 35) = 1.87, p = .18), or 14d (F (1, 36) = 2.68, p = .11). There was a significant main effect of age only at 4h (F (1, 40) = 10.03, p = .003), with young rats exhibiting higher IL-1 levels compared to old rats. At no other time point was there a significant main effect of age. In addition, there were no significant interactions at any time point.
3.2 Experiment 2
We have previously reported (Barrientos et al., 2006a) that an E. coli injection 4 days prior to a contextual learning episode significantly impairs hippocampal memory consolidation in older rats, but not in young rats. Therefore, for replication purposes only, we included young rats in the 4 day experiment in the current study, however we did not include them in the 8 or 14 day experiments. Here we sought to determine how long this E. coli-induced deficit persists in aging rats. We conditioned rats 4, 8, or 14 days following a peripheral E. coli injection and tested them for fear of context and fear of tone 3 days later. It is worth noting that in all of our contextual fear conditioning experiments none of the rats, young or old, freeze upon presentation of the tone during conditioning (data not presented). That is, prior to the tone being paired with the shock, there is absolutely no baseline freezing to the tone alone.
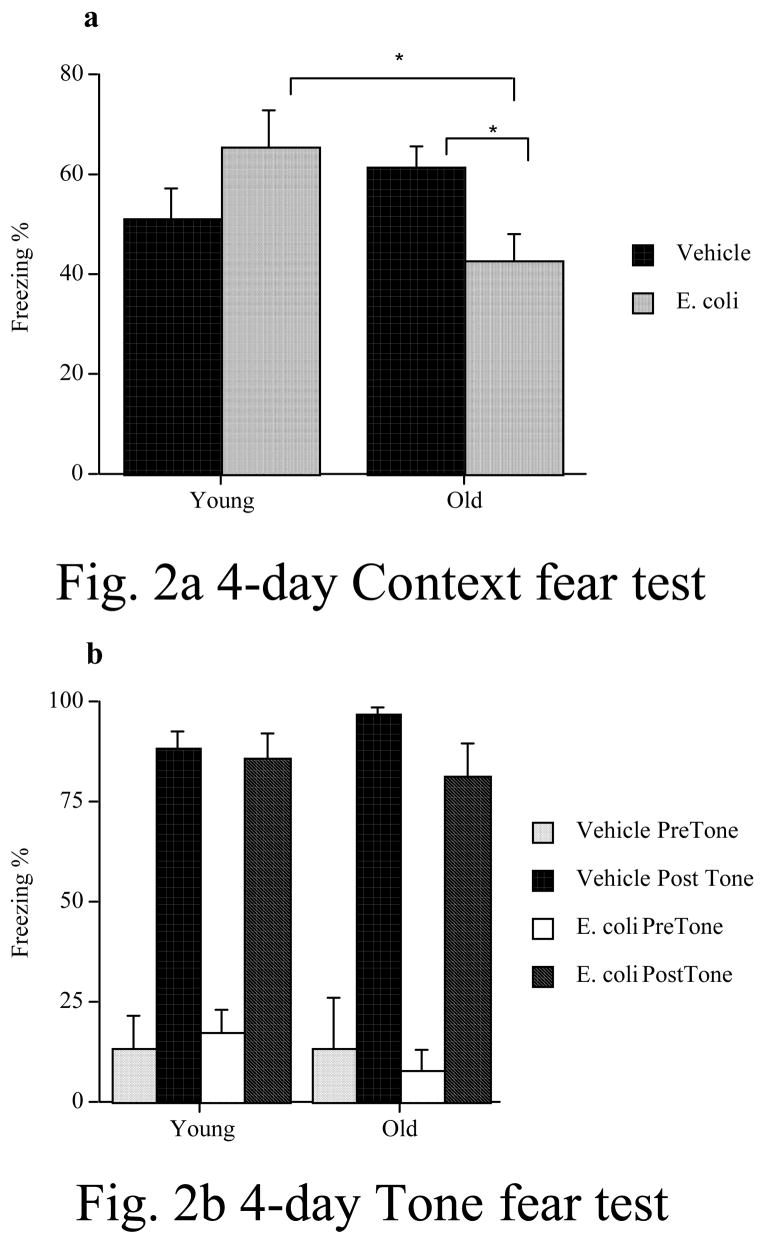
In the first of the behavioral experiments (Fig. 2a & b), we found that an E. coli infection interfered with contextul fear memory formed 4 days later in old, but not in young subjects. Moreover, this deficit was restricted to the hippocampal-dependent memory test (fear to the context), as they did not show any impairment on the hippocampal-independent memory test (fear of the tone). These data replicate our previous findings (Barrientos et al., 2006a). A 2 x 2 ANOVA (age x immune challenge) revealed no significant main effect of age (F (1, 16) = .91, p = .35), no significant main effect of challenge (F (1, 16) = .12, p = .74), but a significant age x challenge interaction (F(1, 16) = 6.608, p = .02). Fisher’s PLSD post-hoc tests revealed that young and old vehicle-treated rats did not differ (p > .05). However, old E. coli-treated rats froze significantly less than did young E. coli-treated rats (p < .05). Also, old E. coli-treated rats froze significantly less than did old vehicle-treated rats (p < .05), but young E. coli-treated rats did not differ from young vehicle-treated rats (p > .05). In the tone-cued fear test, during the pre-tone session, there was no main effect of age (F (1, 16) = .33, p = .57), or challenge (F (1, 16) = .01, p = .91), and no interaction (F (1, 16) = .33, p = .57). During the tone, there was an increase in the amount of freezing overall, compared to the pre-tone session, but there was no main effect of age (F (1, 16) = .14, p = .72), or challenge (F (1, 16) = 2.56, p = .13), and no interaction (F (1, 16) = 1.23, p = .28).
Figure 2.
An E. coli or vehicle injection was administered four days prior to contextual fear conditioning. E. coli produced a significant memory impairment in old rats but not in young rats in the test of fear of context (a), a hippocampus-dependent task. Old E. coli-treated rats exhibited significantly less freezing than did old vehicle-treated rats, and young E-coli-treated rats. Young rats showed no difference in freezing between vehicle and E. coli treatments. In the test of fear of the tone (b), a hippocampal-independent task, there were no significant differences between any of the groups during the pre-tone testing session, nor during the post-tone testing session. * = p < .05. Error bars indicate ± SEM.
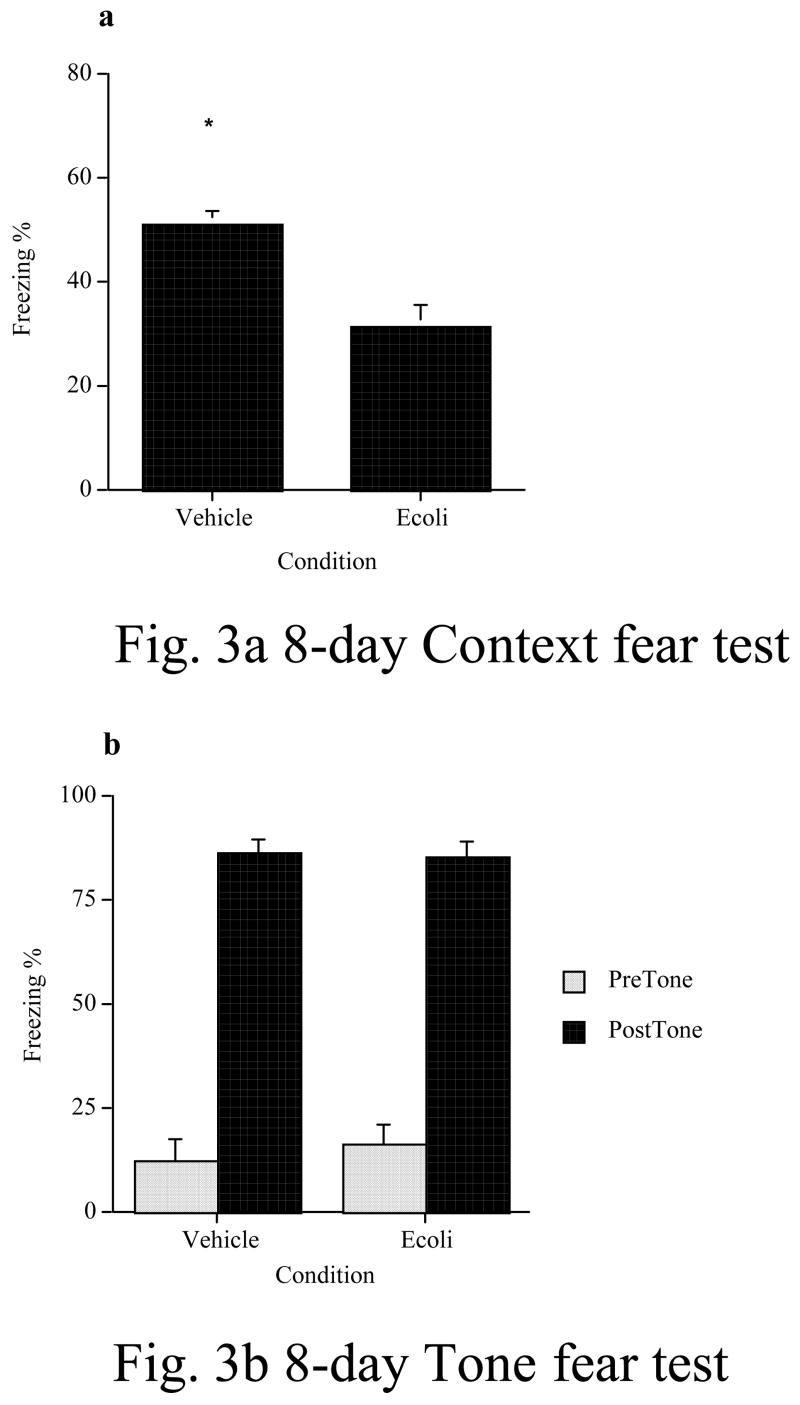
In the second behavioral experiment (Fig. 3a & b), we found that an E. coli infection continued to interfere with contextual fear memory formed 8 days later in old subjects. As in the 4-day experiment, their deficit was restricted to the hippocampal-dependent memory test (fear to the context), as they did not show any impairment on the hippocampal-independent memory test (fear of the tone). An ANOVA revealed a significant reduction in freezing during the context fear test in old E. coli-treated rats compared to vehicle-treated rats (F(1, 12) = 22.03, p < 0.0005). In the tone-cued fear test, both groups showed robust fear of the tone, relative to the pre-tone testing session, however there were no significant differences between the groups during the pre-tone (F(1, 13) = .34, p = .57) or the tone periods (F(1, 13) = .06, p = .82).
Figure 3.
An E. coli or vehicle injection was administered eight days prior to contextual fear conditioning. E. coli produced a significant memory impairment in old rats compared to those who received only a vehicle injection. Again, this impairment was limited to hippocampal-dependent memory as measured in a test for fear of the context (a). However, there were no differences between the groups in the test for fear of the tone (b), a hippocampal-independent task. * = p < .05. Error bars indicate ± SEM.
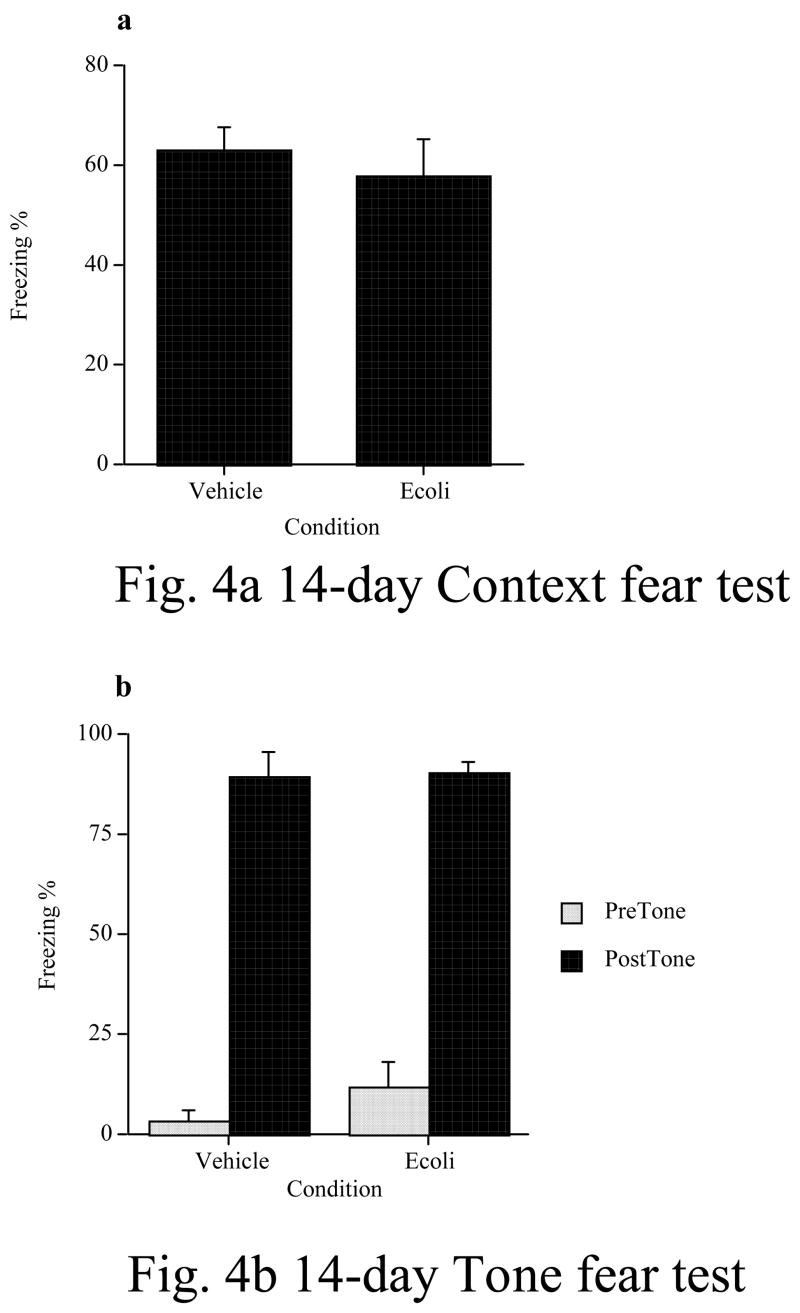
In the last of the behavioral experiments (Fig. 4a & b), we found that an E. coli infection did not interfere with contextual fear memory formed 14 days later in old subjects. Likewise, they did not show any impairment on the tone-cued memory test either. An ANOVA revealed no significant differences in freezing during the context fear test between the two groups (F(1, 10) = .37, p = .56). In the tone-cued fear test, both groups showed robust fear of the tone, relative to the pre-tone testing, however there were no significant differences between the groups during the pre-tone (F(1, 10) = 1.6, p = .23) or the tone periods (F(1, 10) = .02, p = .89).
Figure 4.
An E. coli or vehicle injection was administered 14 days prior to contextual fear conditioning. E. coli produced no memory impairment in old rats compared to those who received only a vehicle injection. This was true for both hippocampal-dependent memory as measured in a test for fear of the context (a), and hippocampal-independent memory as measured in a test for fear of the tone (b). Error bars indicate ± SEM.
3.3 Experiment 3
Bacterial clearance as assessed by number of colony forming units (CFU) in agar plates revealed that following an E. coli injection neither young nor old rats had any CFUs in blood at any time point (4, 24, or 48h). Likewise, no CFUs were detected in hippocampal homogenates of young or old rats at any time point.
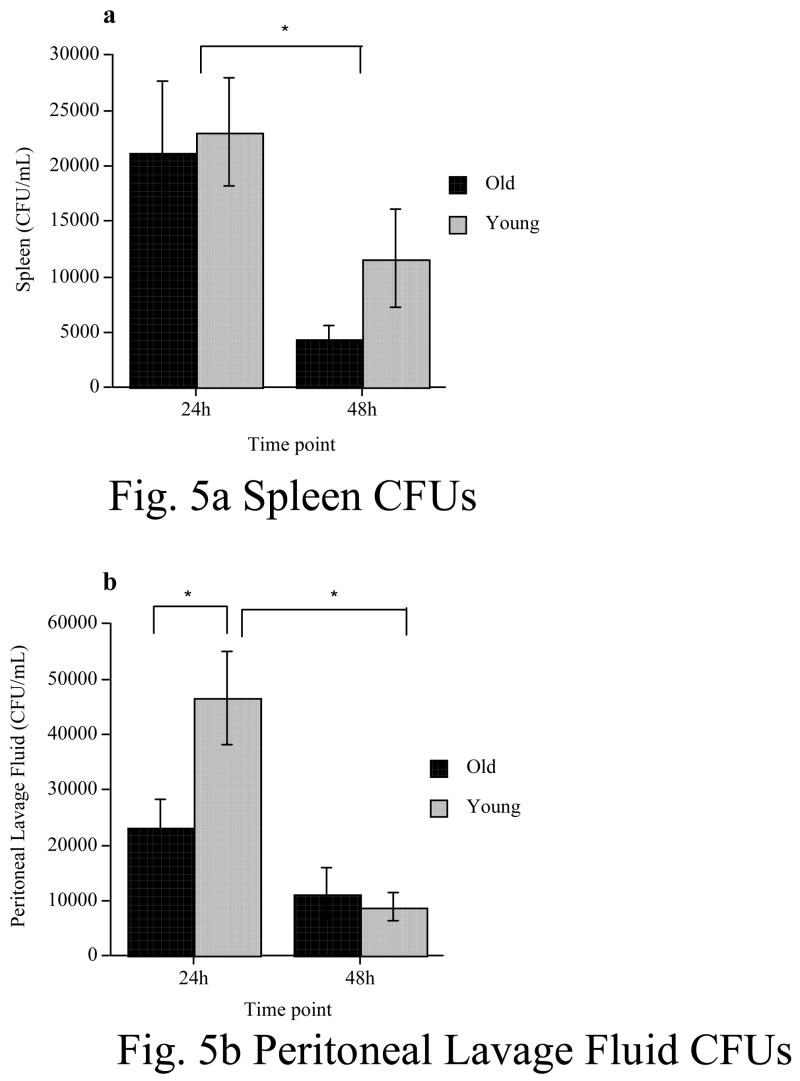
In the spleen (Fig 5a), both young and old rats had equally robust numbers of CFUs at 24h post-infection. There was a significant reduction in CFUs from 24h to 48h, but there was no difference between young and old rats at either time point. A 2 x 2 ANOVA (age x time point), revealed that there was no main effect of age (F(1, 26) = .96, p = .34), a significant main effect of time point (F(1, 26) = 9.02, p = 0.006), and no significant age x time point interaction (F(1, 26) = .33, p = .57).
Figure 5.
Colony forming units (CFUs) from cultured spleen homogenates (a) or peritoneal lavage fluid (b) either 24h or 48h following a peripheral E. coli injection in young and old rats. No differences were found between young and old rats in spleen homogenates. However, young rats showed elevated CFUs in the peritoneal lavage fluid at 24h compared to old rats. However, this difference was no longer significant at 48h. * = p < .05. Error bars indicate ± SEM.
In the PLF (Fig 5b), young rats had significantly higher CFUs than did old rats at 24h post-infection. This difference was no longer present at 48h, as there was a significant reduction in CFUs in the young subjects between the two time points. A 2 x 2 ANOVA revealed there was a significant main effect of age (F(1, 24) =4.632, p = .04), a significant main effect of time point (F(1, 24) = 26.04, p < .0001), and a significant age x time point interaction (F(1, 24) = 7.03 p = .01). Post hoc analyses revealed that young rats exhibited significantly higher CFUs at 24h than old rats at the same time point (p < .05). Young rats also showed a significant reduction in CFUs between 24h and 48h (p < .0002). CFUs in old rats however, did not differ between 24h and 48h (p > .05). Furthermore, at 48h, there was no significant difference in CFUs between young and old rats (p > .05).
4. Discussion
Results from Experiment 1 showed that following a peripheral E. coli administration, aging rats had an exaggerated and longer lasting IL-1β response, specifically in the hippocampus, relative to young rats. E. coli treatment led to increases in hippocampal IL-1β levels from 2 h to 24 h in both age groups. It was not until 4 days after E. coli that the age groups differed significantly. At 4 and 8 days, old E. coli-treated rats exhibited significantly elevated levels of IL-1β compared to E. coli-treated young rats as well as vehicle-treated control rats. Young E. coli-treated rats were not different than vehicle-treated control rats at 4 or 8 days, and by 14 days, both old and young E. coli-treated rats were no longer different from each other, or their vehicle-treated controls.
IL-1β levels in hypothalamus, parietal cortex, serum, and spleen did rise after E. coli compared to vehicle-treated controls, usually from 2h to 24h later, but these elevations were not different between the two age groups at any time point. In the prefrontal cortex, there was no significant E. coli-induced elevation in IL-1β in either age group at any time point. These data show that a peripheral bacterial infection does produce an exaggerated and persistent IL-1β response in the aging rats, and that this exaggerated response is specific to the hippocampal formation. There was no unusual or long-lasting IL-1β response in other brain tissues or in the periphery. Such an elevation in hippocampal IL-1β is important because elevated hippocampal IL-1β has been shown to impair synaptic plasticity (Hauss-Wegrzyniak et al., 2002; Vereker et al., 2000a; Vereker et al., 2000b), and severely reduce learning-produced changes in brain-derived neurotrophic factor (BDNF) (Barrientos et al., 2003; Barrientos et al., 2004; Bilbo et al., 2008), and signal transduction (Tong et al., 2007). These mechanisms are critical for hippocampal memory consolidation and, in fact, elevated IL-1β in the hippocampus has been well documented to impair hippocamapal-dependent memory (Barrientos et al., 2006a; Barrientos et al., 2002; Bilbo et al., 2008; Hauss-Wegrzyniak et al., 1998; Pugh et al., 1998; Pugh et al., 1999). While we did not measure cytokines other than IL-1β, it is likely that other proinflammatory cytokines are also elevated in hippocampus of aged rats following an E. coli injection, as several lines of research show a high correlation between IL-1β, IL-6 and TNFa elevations (Dinarello, 1991).
In Experiment 2 we found that young rats showed no memory impairments when conditioned 4 days following an E. coli injection. This replicates previous findings (Barrientos et al., 2006a). Furthermore, we found that aging rats were significantly impaired when conditioned both 4 and 8 days following E. coli, but were no longer impaired after 14 days. These data parallel the data in Experiment 1 showing that IL-1β is still elevated at 8 days, but not 14 days post-E. coli in the hippocampus in the aging animals.
Importantly, aging rats conditioned 4 and 8 days after E. coli showed significant memory impairments only on the hippocampal-dependent test (fear of context), not the hippocampal-independent test (fear of tone). This dissociation fits well with the finding that aging exaggerates E. coli-induced brain IL-1β increases only in the hippocampus. It is worth noting that we have previously shown (Barrientos et al., 2006a) that aging rats treated with E. coli either immediately after conditioning (retrograde paradigm) or 4 days prior to conditioning (anterograde paradigm) were impaired on the contextual fear test, a hippocampal-dependent task. In contrast, they were not impaired on the auditory-cued test, a hippocampal-independent task, in either the retrograde or the anterograde versions of the task. These findings showed that the memory effects are independent of generalized effects upon encoding or reductions in signal strength. Furthermore, we tested short-term memory on both contextual fear conditioning and on water maze tasks, and showed that while old E. coli-treated rats were significantly impaired on the long-term hippocampal-dependent memory tests (but not on hippocampal-independent tasks), they were unimpaired on the short-term memory tests, indicating that E. coli had not interfered with the acquisition of the task, but rather the consolidation of the memory. This is an important point because since old rats have a prolonged fever lasting 3 days, it could be that these animals are simply sick during acquisition of the tasks and thus, showing impaired memory. However, since we have shown that these animals’ short-term memory is not impaired after E. coli, this cannot explain the old animals’ long-term memory impairments.
Experiment 3 showed that bacteria clearance between young and aging rats differed in only the peritoneal lavage fluid 24h following the E. coli injection. Surprisingly, it was the young rats that had more CFUs in the PLF than did aging rats. At the present time, we have no clear explanation for this result. There is a remote possibility that some aspect of innate immunity may be working more effectively in aging rats. However, with the present data, this is only speculation. This difference between young and aging rats was no longer present at the 48h time point. Furthermore, no differences in bacterial clearance were found between young and aging rats at any time point in the spleen. No CFUs whatsoever were found in either the blood or the hippocampus of either young or aging rats. This is important because it had been suggested that the aging rats may have a compromised blood brain barrier (Chaney et al., 2003) thus possibly allowing live bacterial to enter the brain, thereby producing an inflammatory response from within the CNS. Because bacterial clearance in the old rats does not seem to be compromised, the long-lasting IL-1β response in the hippocampus is not likely to be mediated by uncleared bacteria in the periphery.
Even though aging did not retard bacterial clearance, it is possible that aging increases the peripheral innate immune response to the E. coli, thereby leading to enhanced production of the molecules that signal the brain. If this were so, then the mediator of the exaggerated IL-1β response in the hippocampus need not be in the brain, but could be in the periphery. That is, the cells in the brain that produce IL-1β would not be altered or sensitized, but rather would simply receive an enhanced signal from the periphery. IL-1β has proved to be the major molecule produced by the innate immune system that signals the brain that an infection is present (Bluthe et al., 1992; Dinarello, 1991). Therefore, blood levels of IL-1β were measured, and the serum IL-1β response to E. coli was not exaggerated by age. Furthermore, aging did not exaggerate the E. coli-induced increase of IL-1β in other peripheral tissues. Thus, it is unlikely that E. coli produced an elevated signal to the brain in the aging subjects.
The implication of the data as a whole is that the source of the exaggerated hippocampal IL-1β in the aging rats represents an age-related change within the brain. Since microglial cells are the primary source of IL-1β within the brain (Van Dam et al., 1995), an age-related change in microglia is a likely source. Since aging alone did not increase basal levels of IL-1β, the microglia in the aging rats would not be considered “activated” in the traditional sense, meaning fully pro-inflammatory. However, it has been proposed (Cunningham et al., 2005; Perry et al., 2003) that diseases and conditions which have chronic microglial activation characteristics, can have minimal cytokine expression, but then over-respond to an additional challenge. This description would appear to fit the present data, suggesting that normal aging is one such condition in which the microglia are so-called ‘primed’. In support of this conclusion, we have previously shown that normal brain aging is characterized by a shift towards a pro-inflammatory microenvironment in the brain that is largely microglia-dependent (Frank et al., 2006). Furthermore, others have shown age-related priming of microglia associated with exaggerated neuroinflammatory responses, as well as more intense sickness behaviors in aging rodents compared to young adult rodents (Abraham et al., 2008; Godbout et al., 2005; Perry et al., 2003).
The pattern of these data also supports the conclusion that peripheral infection leads to hippocampal-dependent memory impairments in aging subjects because aging potentiates the IL-1β increase that occurs in the hippocampus. This is because the duration of the hippocampal IL-1β increase paralleled the duration of the memory impairment following E. coli. Thus, it adds a converging line of support with other approaches that lead to this conclusion (Abraham et al., 2008; Barrientos et al., 2006a; Godbout et al., 2005; Godbout and Johnson, 2006; Streit et al., 2005). Finally, it can be noted that the possibilities that a) aging primes microglia in the hippocampus, and b) IL-1β secreted from microglia within the hippocampus impairs the ability of the hippocampus to produce long-term memory consolidation and perhaps other cognitive functions, may be involved in a frequently described aspect of human aging. There are numerous reports of instances in which aging individuals function quite well, but then suffer a precipitous decline in cognitive function following surgery, heart attack, or pneumonia (Bekker and Weeks, 2003; Dunn et al., 2005). Post-operative cognitive dysfunction, as it is called (Bekker and Weeks, 2003), is observed in a significant percentage of elderly patients, as is pneumonia-induced delirium (Dunn et al., 2005; George et al., 1997). Events such as surgery and pneumonia induce peripheral inflammatory responses that can provoke the enhanced synthesis of inflammatory mediators in the brain, just as occurs in the rats in the present studies given E. coli. Thus, glial priming and a consequent exaggerated neuroinflammatory response may be at the core of this sort of age-related cognitive decline.
Acknowledgments
The present work was supported by grants F32 MH064339 and RO1 AG028271.
Footnotes
Disclosure Statement: None of the authors have any actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006a;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1b administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Watkins LR, Rudy JW, Maier SF. Characterization of sickness behavior in aging rats following bacterial infection. Society for Neuroscience. 2006 Neuroscience Meeting Planner; Atlanta, GA. 2006b. [Google Scholar]
- Bekker AY, Weeks EJ. Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17:259–272. doi: 10.1016/s1521-6896(03)00005-3. [DOI] [PubMed] [Google Scholar]
- Beloosesky Y, Salman H, Bergman M, Bessler H, Djaldetti M. Cytokine levels and phagocytic activity in patients with Alzheimer’s disease. Gerontology. 2002;48:128–132. doi: 10.1159/000052830. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology (London) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Campisi J, Hansen MK, O’Connor KA, Biedenkapp JC, Watkins LR, Maier SF, Fleshner M. Circulating cytokines and endotoxin are not necessary for the activation of the sickness or corticosterone response produced by peripheral E. coli challenge. J Appl Physiol. 2003;95:1873–1882. doi: 10.1152/japplphysiol.00371.2003. [DOI] [PubMed] [Google Scholar]
- Chaney MO, Baudry J, Esh C, Childress J, Luehrs DC, Kokjohn TA, Roher AE. A beta, aging, and Alzheimer’s disease: a tale, models, and hypotheses. Neurol Res. 2003;25:581–589. doi: 10.1179/016164103101202011. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neil LAJ, Lynch MA, O’Connor JJ. Interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Innate immunity at the forefront of psychoneuroimmunology. Brain Behav Immun. 2004;18:1–6. doi: 10.1016/j.bbi.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis Assoc Disord. 2005;19:91–94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and Learning. Erlbaum; Hillside, NJ: 1988. pp. 185–211. [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Romanovitch AE, Peloso E, Satinoff E, Plata-Salaman CR. Basal and IL-1beta-stimulated cytokine and neuropeptide mRNA expression in brain regions of young and old Long-Evans rats. Brain Res Mol Brain Res. 1999;70:92–100. doi: 10.1016/s0169-328x(99)00134-5. [DOI] [PubMed] [Google Scholar]
- Gee JR, Ding Q, Keller JN. Age-related alterations of Apolipoprotein E and interleukin-1beta in the aging brain. Biogerontology. 2006;7:69–79. doi: 10.1007/s10522-005-6039-9. [DOI] [PubMed] [Google Scholar]
- Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci. 2005;22:1751–1756. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- George J, Bleasdale S, Singleton SJ. Causes and prognosis of delirium in elderly patients admitted to a district general hospital. Age Ageing. 1997;26:423–427. doi: 10.1093/ageing/26.6.423. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24:521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Griffin WST, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, Araoz C. Brain interleukin-1 and S-100 immunoreactivity are elevated Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL. Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp Neurol. 2002;176:336–341. doi: 10.1006/exnr.2002.7966. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Willard LB, Del Soldato P, Pepeu G, Wenk GL. Peripheral administration of novel anti-inflammatories can attenuate the effects of chronic inflammation within the CNS. Brain Res. 1999;815:36–43. doi: 10.1016/s0006-8993(98)01081-6. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, O’Banion MK, Whiteley PE, Daeschner JC, Olschowka JA. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J Neuroimmunol. 2001;119:269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- Lombardi VR, Garcia M, Rey L, Cacabelos R. Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer’s Disease (AD) individuals. J Neuroimmunol. 1999;97:163–171. doi: 10.1016/s0165-5728(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GM, Jr, Brachova L, Yan SD, Walker DG, Shen Y, Rogers J. Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia. 2001;35:72–79. doi: 10.1002/glia.1072. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Potential inflammatory biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2005;8:369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- Paganelli R, Di Iorio A, Patricelli L, Ripani F, Sparvieri E, Faricelli R, Iarlori C, Porreca E, Di Gioacchino M, Abate G. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer’s disease patients. Exp Gerontol. 2002;37:257–263. doi: 10.1016/s0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Riancho JA, Zarrebeitia MT, Amado JA, Olmos JM, Gonzalez-Macias J. Age-related differences in cytokine secretion. Gerontology. 1994;40:8–12. doi: 10.1159/000213568. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Smith DR, Hoyt EC, Gallagher M, Schwabe RF, Lund PK. Effect of age and cognitive status on basal level AP-1 activity in rat hippocampus. Neurobiol Aging. 2001;22:773–786. doi: 10.1016/s0197-4580(01)00240-8. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurol Res. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Liljeroth AM, Nilsson A, Minthon L, Blennow K. Decreased levels of intrathecal interleukin 1 receptor antagonist in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12:314–317. doi: 10.1159/000051276. [DOI] [PubMed] [Google Scholar]
- Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000a;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Vereker E, O’Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000b;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AP, Reck G, Platt D. Evidence that V+ fibronectin, GFAP and S100 beta mRNAs are increased in the hippocampus of aged rats. Exp Gerontol. 1993;28:135–143. doi: 10.1016/0531-5565(93)90003-v. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]