Abstract
Purpose
We used magnetic resonance imaging (MRI) to determine why lateral rectus (LR) recession has a variable effect on binocular alignment.
Design
Prospective observational and interventional case series.
Methods
Posterior LR path lengths from orbital apex to first globe contact were determined by axial plane, surface coil MRI in 8 patients with unilateral LR palsy and in four patients before and after bilateral LR recession.
Results
Posterior paths of paretic LR muscles were 2.2 to 6.0 mm longer (mean 3.4 mm, p = 0.0002) than normal contralateral paths. Each paretic LR was sharply inflected laterally at a point in the anterior orbit corresponding to the histological location of the LR pulley sleeve. Every recessed LR was 0.8 to 4.4 mm (mean 2.4 mm, p = 0.0008) longer post- than pre-operatively, with less temporal deflection.
Conclusions
The LR pulley suspension contributes to LR tension, tightening the muscle belly by stretching it temporally when LR tone is reduced. The increase in LR path length due to temporal inflection offsets recession effect by up to 4 mm. Connective tissue action explains some response variability after LR recession.
INTRODUCTION
Lateral rectus (LR) recession, the surgical weakening of the LR muscle by posteriorly shifting its scleral insertion, has long been known to have less effect on horizontal eye alignment than a corresponding amount of medial rectus (MR) recession. Published surgical tables1 routinely recommend larger LR recessions to treat exotropia (XT) than corresponding MR recessions to treat similar degrees of esotropia (ET). Many strategies have been introduced to reduce the undercorrection rate after LR recessions for XT2–4, all having the effect of increasing the distance of LR recession performed.
Recession of the LR shifts binocular alignment in a more esotropic direction in the immediate postoperative period than is ultimately attained after long-term healing, a phenomenon often resulting in reoccurrence of concomitant XT5–7. Because of this consistent clinical response to LR recession, surgical planning for concomitant XT aims to achieve an initial large overcorrection into consecutive ET, with anticipated subsequent reduction of ET over time5–7. Analogous over-correction is not advised for forms of strabismus not treated by LR recession, suggesting that some characteristic of the LR may be unique.
Orbital anatomy can have a profound effect on both strabismus and in the response of strabismus to eye muscle surgery8–10. The discovery of the rectus extraocular muscle (EOM) pulleys11, connective tissue sleeves of collagen, elastin, and smooth muscle that surround and stabilize the posterior EOM paths within the bony orbit12, 13, has increased the complexity of analysis of the biomechanical behavior of the EOMs in response to surgery14. In particular, abnormal LR path variants, including changes with aging and globe growth, have been implicated as either causes or complicating factors in certain types of strabismus15–21. We used magnetic resonance imaging (MRI) in subjects with LR palsy, and in subjects before and after LR recession, to determine what changes, if any, occurred in orbital anatomy over time that might influence the LR response to an initial weakening event.
METHODS
The study was designed as a prospective observational case series. Eight subjects were identified with unilateral LR palsy. Four subjects were identified pre-operatively with large XT for whom bilateral LR recessions, with or without MR resections, were planned. All subjects underwent complete ophthalmic examinations. Each subject then underwent high-resolution, T1-weighted MRI using a dual-phased surface coil array and, in some cases, intravenous gadodiamide contrast using techniques described in detail elsewhere22. Subjects were imaged in straight-ahead gaze fixating with the preferred eye. Subjects with XT were imaged before and at least three weeks after strabismus surgery.
Contiguous axial MRI images 3 mm thick were obtained using a 256 × 192 or 256 matrix over a 10 cm square field of view. Digital MRI images were transferred to Macintosh® computers (Apple Computer, Cupertino, CA), converted into 8-bit tagged image file format (TIFF), and quantified using the program Image J (W. Rasband, National Institutes of Health; available by ftp from zippy.nimh.nih.gov).
An observer who was masked to the clinical characteristics of each subject quantitatively analyzed only images free from degradation by motion or other artifacts. For each orbit, the posterior LR path length, defined as the length of the LR muscle belly from orbital apex to first globe contact, was measured in the axial image plane containing the longest contiguous section through the LR by tracing a curvilinear path through the center of the muscle belly. For LR palsy subjects, the posterior LR path length in the involved orbit was compared to the contralateral, uninvolved orbit. For subjects with XT, pre-operative and post-operative LR path lengths were compared.
RESULTS
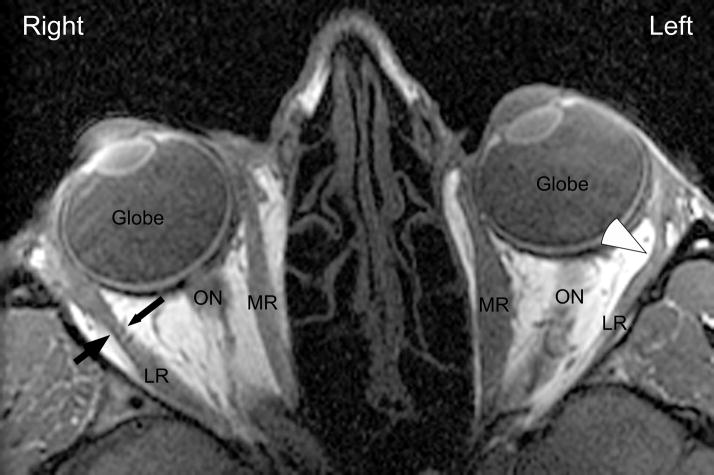
Table 1 summarizes the clinical characteristics for the 8 subjects with LR palsy. Average age at time of imaging was 49 years (range 20–76 years old), with 34 months (range 6–120 months) average duration of LR palsy. Every subject was esotropic with an average deviation of 49 Δ (range 15–90 Δ). For each subject, atrophy of the involved LR belly was confirmed by MRI23. In every case, the paretic LR posterior path length significantly exceeded the contralateral normal LR by more than 2 mm (range 2.2 to 6.0 mm, average 3.4 mm, p = 0.0002 using a paired t-test). Measured LR lengths are summarized in Table 2. The increased posterior LR path length was accompanied by a temporal and posterior bowing of the LR muscle belly in the region of the posterior part of the LR muscle pulley sleeve (Fig. 1), near the globe-optic nerve junction.
Table 1.
Clinical Characteristics of Subjects with Lateral Rectus Palsy
| Case | Sex | Age (yrs) | Laterality | Duration (months) | Cause | Esotropia (Δ) |
|---|---|---|---|---|---|---|
| Subject 1 | M | 20 | Right | 8 | Closed Head Trauma | 70 |
| Subject 2 | F | 50 | Left | 57 | Excision of Meningioma | 90 |
| Subject 3 | F | 32 | Left | 10 | Migraine | 40 |
| Subject 4 | F | 56 | Right | 6 | Meningioma, Duane Syndrome | 35 |
| Subject 5 | M | 54 | Right | 24 | Excision of Meningioma | 60 |
| Subject 6 | M | 46 | Left | 24 | Excision of Clivus Chordoma | 60 |
| Subject 7 | M | 76 | Left | 24 | Clivus Chordoma Radiotherapy | 20 |
| Subject 8 | F | 60 | Left | 120 | Idiopathic | 15 |
Laterality = affected side; Δ = prism diopters.
Table 2.
Paretic versus Normal Lateral Rectus Posterior Path Length
| LR Palsy | Paretic Length (mm) | Normal Length (mm) | Difference (mm) |
|---|---|---|---|
| Subject 1 | 39.8 | 37.4 | 2.4 |
| Subject 2 | 40.9 | 37.4 | 3.5 |
| Subject 3 | 41.8 | 35.8 | 6.0 |
| Subject 4 | 44.6 | 42.1 | 2.5 |
| Subject 5 | 39.2 | 35.2 | 4.0 |
| Subject 6 | 42.1 | 37.3 | 4.8 |
| Subject 7 | 39.0 | 36.8 | 2.1 |
| Subject 8 | 39.5 | 37.3 | 2.2 |
| Mean | 40.9 | 37.4 | 3.4 |
| SD | 1.9 | 1.9 | 1.3 |
| Paired t-test | 0.0002 | ||
Posterior Path Length is distance from orbital apex to first contact of the lateral rectus with the globe. LR = Lateral Rectus. SD – standard deviation.
Figure 1.
Normal and Contralateral Lateral Rectus Palsy Orbit - Axial Imaging. For Subject 3, the normal right lateral rectus follows a nearly straight path from orbital apex through the pulley sleeve (small white arrows) toward the globe. The path of the paretic and atrophic left lateral rectus path is bowed temporally and posteriorly in the pulley sleeve region (large white arrowhead), as the elastic and smooth muscle components of the pulley sling pull the denervated muscle against the lateral orbital wall. For this subject, the paretic lateral rectus had a posterior path length of 41.8 mm compared with the nonparetic length of 35.8 mm, a difference of 6 mm. ON = Optic Nerve; LR = Lateral Rectus; MR = Medial Rectus.
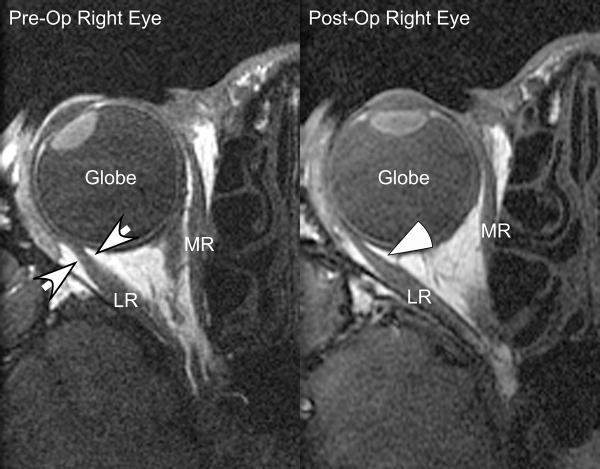
Table 3 summarizes the surgical data for the 4 subjects with XT. Each subject underwent bilateral 6 to 8 mm LR recessions, augmented in 3 cases with 5 or 6 mm MR resections for distance XT greater than or equal to 55 Δ. Each subject followed a typical course after XT surgery. Subjects 9 and 11 were initially overcorrected into ET and experienced a gradual reduction of the ET in the post-operative follow up. Subjects 10 and 12 had initial good alignment with slow recurrence of distance XT. Table 4 summarizes the pre-operative versus post-operative LR posterior path lengths. In every case, the post-operative LR posterior path length significantly exceeded pre-operative length (range 0.8 to 4.4 mm, average 2.4 mm, p = 0.0008 using a paired t-test). Once again, the increased LR posterior path length was accompanied by temporal and posterior bowing of the LR muscle belly in the region of the posterior part of the LR muscle pulley sleeve near the globe-optic nerve junction, although the effect was not as profound as seen in LR palsy (Figure 2).
Table 3.
Surgical Dosage for Exotropic Subjects
| Subject | Diagnosis | Surgery (mm) |
|---|---|---|
| 9 | Exotropia | Bilateral MR Resection 6.0, Bilateral LR Recession 7.0 |
| 10 | Exotropia | Left LR Recession 6.0, Right LR Recession 8.0 adj. |
| 11 | Exotropia | Right MR Resection 5.0, Bilateral LR Recession 7.0 |
| 12 | Exotropia | Left MR Resection 6.0, Bilateral LR Recession 8.0 |
LR = Lateral Rectus; MR = Medial Rectus; adj. = adjustable suture surgery.
Table 4.
Effect of Recession on Lateral Rectus Posterior Path Length
| LR Recession | Pre-Op Length (mm) | Post-Op Length (mm) | Difference (mm) |
|---|---|---|---|
| Subject 9 - OD | 34.5 | 36.5 | 2.0 |
| Subject 9 - OS | 31.0 | 35.4 | 4.4 |
| Subject 10 - OD | 27.5 | 31.0 | 3.5 |
| Subject 10 - OS | 30.0 | 32.9 | 2.9 |
| Subject 11 - OD | 31.8 | 32.6 | 0.8 |
| Subject 11 - OS | 30.4 | 32.7 | 2.3 |
| Subject 12 - OD | 37.8 | 38.8 | 1.0 |
| Subject 12 - OS | 36.0 | 38.3 | 2.3 |
|
| |||
| Mean | 32.4 | 34.8 | 2.4 |
| SD | 3.4 | 2.7 | 1.1 |
|
| |||
| Paired t-test | 0.0008 | ||
Posterior Path Length is distance from orbital apex to first contact of the lateral rectus onto the globe. LR = Lateral Rectus. SD = standard deviation.
Figure 2.
Pre- and Post-Op Lateral Rectus Recession - Axial Imaging. For Subject 9, the lateral rectus path after lateral rectus recession is bowed temporally and posteriorly in the anterior orbit (white arrowhead), increasing lateral rectus path length compared with pre-operatively (small white arrows). This displacement is similar in direction but smaller than observed with lateral rectus palsy. For this subject, the pre-operative LR posterior path length was 27.5 mm compared with 31.0 mm in the post-operative image. The post-operative change in lateral rectus path length reduced the effect of lateral rectus surgical recession by 3.5 mm, eliminating almost half the 8.0 mm of recession performed. LR = Lateral Rectus; MR = Medial rectus.
The net result was to offset the effective amount of surgical LR recession performed. Each millimeter increase in LR posterior path length reduces the effective slack created by LR recession by one millimeter. Comparing the change in LR path for both eyes to the surgical recession performed, the actual recession effect was reduced by 20 to 45% (average 33%) in these subjects.
DISCUSSION
Imaging reveals that the posterior LR muscle path has a clear, consistent response to an initial weakening event: temporal and posterior bowing in the region of the globe-optic nerve junction and posterior part of the LR pulley sleeve, with resulting increased posterior LR path length. In LR palsy, the path through the orbit of the denervated, slack LR muscle belly is substantially influenced by external connective tissue forces, most prominently those forces exerted by the LR pulley sleeve and its suspension23. The LR pulley, like the other rectus pulleys, is composed of focal encircling rings of collagen and elastin, embedded within more distributed connective tissue sleeves suspended elastically from the orbital walls23. These tissues drag the paralyzed LR belly temporally and posteriorly, increasing muscle path length and thus passive elastic tension. Because of the paralysis, of course, the increased path length does not increase active tension within the paralyzed muscle.
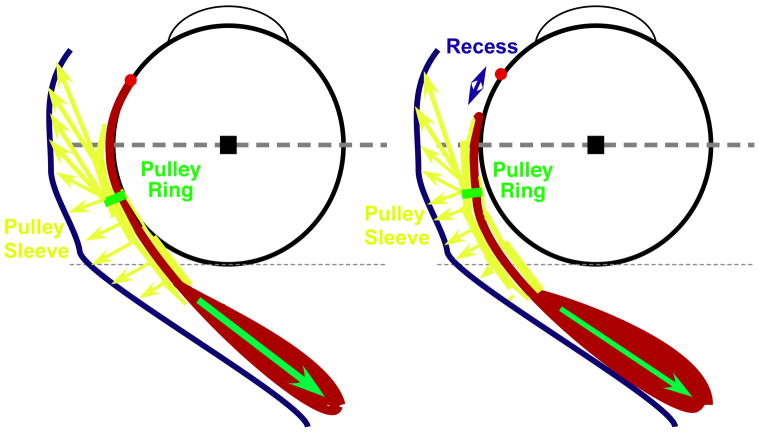
In LR recession, shortening the initial muscle length reduces passive muscle tension, but the innervated muscle still generates active force. The force exerted by the pulley suspension is still sufficient to bow the innervated muscle away from a straight-line path, increasing LR path length and offsetting some of the surgically induced slack created by recession. As shown in Figure 3, the pulley sleeve effectively “pretensions” the recessed LR muscle by dragging it laterally. The increased path length decreases recession effect by 1 – 4 mm (Table 4), with a corresponding reduction in the effective mm dosage of the recession. In the most extreme case, Subject 9 underwent planned bilateral LR recessions totaling 14 mm, but post-operatively had only a combined 7.6 mm of surgically induced slack between the two muscles, reflecting a 45% reduction in the surgically induced effect.
Figure 3.
Change in Lateral Rectus Path Length After Surgical Recession. Before recession, the lateral rectus path represents a balance between muscle tension (green arrow) and pulley tension (yellow arrows). After recession, the pulley partially restores lateral rectus tension by displacing the muscle belly temporally and posteriorly, increasing LR path length, and so negating some of the slack introduced by the surgical recession. LR = lateral rectus.
Earlier studies have considered anatomic changes resulting from surgical recession of rectus muscles, but these studies typically employed simplistic line drawings and speculation on post-operative anatomy without actually imaging and measuring the relationships within the orbit10, 24, 25. The in vivo behavior of the LR after surgery is much more complex. Orbital imaging and quantitative analysis provides a greater understanding of three widely-observed but otherwise mysterious effects of LR recession. First, these results help explain the large regression towards exotropia observed after bilateral LR recession. The average increase in LR path length following LR recession in this small group of surgical patients was 2.4 mm. A bilateral 2.4 shortening of the LR would create 10–15 Δ of XT shift, based on current surgical tables1, precisely the average amount of post-operative drift observed in many large studies of XT over many years5, 7, without postulating any other changes in orbital anatomy, number of sarcomeres, or LR innervation. In addition, the variability of the change in LR posterior path length, from 0.8 mm to 4.4 mm in our small group of subjects, also helps explain the variability of exotropic shift observed after LR recession. Simply increasing the amount of LR recession does not account for the variable effects of orbital size and connective tissue density that appears to affect the LR muscle belly.
As second issue, these results help explain why virtually every recommendation that increases the amount of LR recession performed2–4, 6 enhances the success of surgery for XT without increasing the rate of overcorrections. The increase in LR posterior path length appears to have a dose-response relationship, with greater weakness (LR palsy compared with surgical recession) creating a larger change in posterior path length. Although the small number of subjects and similar amounts of recession performed in this study prevents adequate quantification of this effect, our results would predict greater posterior path inflections for larger compared with smaller LR recessions. The greater path inflection reduces the comparative effectiveness of a larger recession, minimizing overcorrection.
A third issue is that these results help explain why the intraoperative tension of the recessed LR does not correlate its duction or with observed changes in alignment26. Modest amounts of LR recession reduce intraoperative LR tension to zero26, but postoperatively abduction remains clinically normal and alignment predictably drifts towards XT. Innervational tone in both the LR and LR pulley tissue in the alert patient appears sufficient to restore LR tension to near pre-operative levels. In fact, a recent report suggests that orbital layer force exerted on the pulley tissues is sufficient alone to permit normal or nearly normal ductions even after disinsertion of the LR from the sclera27.
There are two implicit assumptions in our analysis of this data. The first assumption is that posterior path elongation in response to weakness in the muscle belly is not shared by the antagonist medial rectus (MR). The LR has a unique path within the orbit, suspended completely within connective tissue in a curvilinear path from orbital apex to globe contact. The MR has a straighter path from apex to globe contact and is supported by a stiffer, more rigid pulley structure. Initial analysis of several subjects with MR palsy demonstrates no change in posterior path length (unpublished data). Greater numbers of subjects with this less common palsy are required to complete this study quantitatively, but if the extreme case of MR palsy does not change MR posterior path length, then neither should lesser weakening due to surgical recession. This preliminary result corresponds with clinical experience of stable eye alignment after MR recession surgery for ET.
The second assumption of this study is that change in central gaze alignment does not by itself change LR posterior path length. For example, in Figure 2, the pre-operative eye is the abducted, XT position, while the post-operative eye is in the orthotropic, straight-ahead position. In both images, however, the subject is fixating with the other, preferred eye, so the innervational tone to the LR should be similar. With a similar level of innervation in both images, an adducted position should tighten, not loosen, the LR28, resulting in less temporal and posterior bowing, not more, in the postoperative image. This tightening of the LR should only be increased by the MR resections performed in most of these patients, because tightening the MR should forcibly adduct the globe, reducing the amount of LR slack created by the recession. Instead, observed LR muscle path was increased, not diminished. The observed displacement noted in the post-operative image can only be caused by the relative balance of forces between the LR and its connective tissue pulley, and could not be an artifact of eye position.
To further support this assumption, the data was re-analyzed by gaze position before and after surgery. In each subject, the preferred eye was in the straight ahead position in both pre-operative and post-operative imaging, while the non-preferred eye moved from the pre-operative exotropic, abducted position to a more straight ahead position post-operatively. Both eyes in every subject demonstrated an increase in LR posterior path length. The magnitude of the change was similar in both groups, an average of 2.0 mm increase in the preferred eye versus 2.8 mm increase in non-preferred eye, a difference that was not statistically significant (p = 0.38). The observed change in LR path length does not appear to be dependent on gaze position.
The current study has some limitations. All imaging was performed in adults, who typically have larger orbits than observed in children. While axial length does not appear to have an effect on the response of patients to XT surgery10, 29, orbital size and the relative position of the LR with respect to the orbital wall might have an important effect. In a baby or very young child, the relatively shallow orbit and close proximity of the LR to the lateral orbital wall may minimize the effect of any temporal and posterior bowing created by surgical recession. This effect may be partially offset by the more abundant and sturdy orbital connective tissue in young patients, which diminishes over time in the elderly30.
All the LR recessions in this study were 6 to 8 mm. The results can be extrapolated somewhat toward larger recessions because of our data on LR palsy, but the effects of smaller LR recessions are unknown. A minimum threshold amount of LR recession might exist before any effect on LR muscle path can be observed. Further study quantifying the relative change in LR path with surgical dose should help improve the accuracy of the LR recession surgery.
In summary, our data demonstrates that the orbital connective tissue variably but consistently reduces LR recession effect by pulling the LR posterior belly temporally and posteriorly. The amount of LR displacement appears dependent on the degree of LR weakness, with the actual effect on path length and muscular tension presumably dependent upon both the individual stiffness of the pulley suspension and the distance between the globe and lateral orbital wall, which in turn depends on the size of the bony orbit.
Biographies
 Robert A. Clark, MD, graduated from Stanford University and completed his ophthalmology residency and pediatric ophthalmology fellowship at the Jules Stein Eye Institute, University of California, Los Angeles. He is in private practice with the Family Eye Medical Group in Long Beach, California, specializing in pediatric ophthalmology and strabismus. He is also an associate clinical professor at the Jules Stein Eye Institute, with research interests that include orbital anatomy, strabismus, and eye movement disorders.
Robert A. Clark, MD, graduated from Stanford University and completed his ophthalmology residency and pediatric ophthalmology fellowship at the Jules Stein Eye Institute, University of California, Los Angeles. He is in private practice with the Family Eye Medical Group in Long Beach, California, specializing in pediatric ophthalmology and strabismus. He is also an associate clinical professor at the Jules Stein Eye Institute, with research interests that include orbital anatomy, strabismus, and eye movement disorders.
 Joseph L. Demer, M.D., Ph.D. is Chief of Comprehensive Ophthalmology and Professor of Neurology at the University of California Los Angeles. He holds the Leonard Apt Professorship, Directs the Ocular Motility Clinical Laboratory, and chairs the EyeSTAR Training Program.
Joseph L. Demer, M.D., Ph.D. is Chief of Comprehensive Ophthalmology and Professor of Neurology at the University of California Los Angeles. He holds the Leonard Apt Professorship, Directs the Ocular Motility Clinical Laboratory, and chairs the EyeSTAR Training Program.
With a Ph.D. in Biomedical Engineering, Dr. Demer has worked for more than 30 years on neural and mechanical factors regulating ocular motility, particularly the functional anatomy of the extraocular muscles and orbital connective tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenbaum AL, Santiago AP. Clinical Strabismus Management: Principles and Surgical Techniques. Philadelphia: W.B. Saunders Company; 1999. pp. 552–5. [Google Scholar]
- 2.Lee S, Kim J, Thacker N. Augmented bilateral lateral rectus recessions in basic intermittent exotropia. J AAPOS. 2007;11:266–8. doi: 10.1016/j.jaapos.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Kushner BJ. The distance angle to target in surgery for intermittent exotropia. Arch Ophthalmol. 1998;116:189–94. doi: 10.1001/archopht.116.2.189. [DOI] [PubMed] [Google Scholar]
- 4.Kushner BJ. Selective surgery for intermittent exotropia based on distance/near differences. Arch Ophthalmol. 1998;116:324–8. doi: 10.1001/archopht.116.3.324. [DOI] [PubMed] [Google Scholar]
- 5.Raab E, Parks MM. Recession of the lateral recti: early and late postoperative alignments. Arch Ophthalmol. 1969;82:203–8. doi: 10.1001/archopht.1969.00990020205010. [DOI] [PubMed] [Google Scholar]
- 6.Keech R, Stewart S. The surgical overcorrection of intermittent exotropia. J Pediatr Ophthalmol Strabismus. 1990;27:218–20. doi: 10.3928/0191-3913-19900701-12. [DOI] [PubMed] [Google Scholar]
- 7.Ing M, Nishimura J, Okino L. Outcome study of bilateral lateral rectus recession for intermittent exotropia in children. Trans Am Ophthalmol Soc. 1997;95:433–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Demer JL, Clark RA, Miller JM. Role of orbital connective tissue in the pathogenesis of strabismus. Am Orthoptic J. 1998;48:56–64. [Google Scholar]
- 9.Kushner BJ. Factors influencing response to strabismus surgery. Arch Ophthalmol. 1993;111:75–9. doi: 10.1001/archopht.1993.01090010079030. [DOI] [PubMed] [Google Scholar]
- 10.Kushner BJ. Variation in axial length and anatomic landmarks in strabismic patients. Ophthalmology. 1990;98:400–6. doi: 10.1016/s0161-6420(91)32282-6. [DOI] [PubMed] [Google Scholar]
- 11.Miller JM, Robins D. Extraocular muscle sideslip and orbital geometry in monkeys. Vision Res. 1987;27:381–392. doi: 10.1016/0042-6989(87)90087-3. [DOI] [PubMed] [Google Scholar]
- 12.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 13.Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–1785. [PubMed] [Google Scholar]
- 14.Clark RA, Demer JL. Magnetic resonance imaging of the effect of horizontal rectus extraocular muscle surgery on pulley and globe positions and stability. Invest Ophthalmol Vis Sci. 2006;47:188–93. doi: 10.1167/iovs.05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krizok TH, Kaufmann H, Traupe H. New approach in strabismus surgery in high myopia. Br J Ophthalmol. 1997;81:625–30. doi: 10.1136/bjo.81.8.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krizok TH, Schroeder BU. Measurement of Recti Eye Muscle Paths by Magnetic Resonance Imaging in Highly Myopic and Normal Subjects. Invest Ophthalmol Vis Sci. 1999;40:2554–2560. [PubMed] [Google Scholar]
- 17.Krizok TH, Kaufmann JM. Elucidation of the restrictive motility disorders in high myopia by MRI. Arch Ophthalmol. 1997;115:1019–27. doi: 10.1001/archopht.1997.01100160189008. [DOI] [PubMed] [Google Scholar]
- 18.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 19.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Amer J Ophthalmol. 2002;134:872–8. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 20.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Amsterdam: Swets; 1999. pp. 91–4. [Google Scholar]
- 21.Demer JL, Kono R, Wright W, Oh SY, Clark RA. Gaze-related orbital pulley shift: a novel cause of incomitant strabismus. In: de Faber JT, editor. Progress in Strabismology. Lisse: Swets and Zeitlinger; 2003. pp. 207–210. [Google Scholar]
- 22.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–97. [PubMed] [Google Scholar]
- 23.Demer JL, Miller JM, Koo EY, Rosenbaum AL. Quantitative magnetic resonance morphometry of extraocular muscles: A new diagnostic tool in paralytic strabismus. J Pediatr Ophthalmol Strabismus. 1994;31:177–188. doi: 10.3928/0191-3913-19940501-10. [DOI] [PubMed] [Google Scholar]
- 24.Beisner DH. Reduction of ocular torque by medial rectus recession. Arch Ophthalmol. 1971;85:13–17. doi: 10.1001/archopht.1971.00990050015003. [DOI] [PubMed] [Google Scholar]
- 25.Miller MM, Mims JL. The influence of pulleys on the quantitative characteristics of medial rectus muscle recessions: the torque vector model. J AAPOS. 2006;10:318–23. doi: 10.1016/j.jaapos.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum AL, Egbert JE, Keogan T, Wheeler N, Wang C, Buzard K. Length-tension properties of extraocular muscles in patients with esotropia and intermittent exotropia. Am J Ophthalmol. 1994;117:791–9. doi: 10.1016/s0002-9394(14)70324-1. [DOI] [PubMed] [Google Scholar]
- 27.Hakim OM, El-Hag YG, Maher H. Persistence of eye movement following disinsertion of extraocular muscle. J AAPOS. 2008;12:62–5. doi: 10.1016/j.jaapos.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Kushner BJ, Vrabec M. Theoretical effects of surgery on length tension relationships in extraocular muscles. J Pediatr Ophthalmol Strabismus. 1987;24:126–31. doi: 10.3928/0191-3913-19870501-07. [DOI] [PubMed] [Google Scholar]
- 29.Kushner BJ, Lucchese NJ, Morton GV. The influence of axial length on the response to strabismus surgery. Arch Ophthalmol. 1989;107:1616–8. doi: 10.1001/archopht.1989.01070020694030. [DOI] [PubMed] [Google Scholar]
- 30.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–32. [PubMed] [Google Scholar]