Abstract
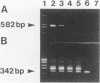
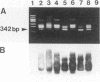
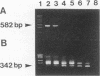
The direct detection of Bacteroides fragilis from clinical specimens was examined by using the PCR method for amplifying a specific fragment of the glutamine synthetase gene from B. fragilis. By this method, all five B. fragilis strains tested were detected, but DNAs from anaerobic bacteria of 24 other species tested, from aerobic bacteria of 12 species tested, and from human leukocytes were not amplified. Using the nested PCR method, we were able to detect as little as one bacterial cell or 100 fg of chromosomal DNA of B. fragilis. A total of 39 clinical specimens, which consisted of 19 bronchial aspirates, 10 percutaneous lung aspirates, 2 transtracheal aspirates, 6 pleural fluid specimens, and 2 pus specimens, were tested. All four culture-positive samples, of which two were bronchial aspirates, one was pleural fluid, and one was pus, were positive by PCR. Among 35 culture-negative samples, 2 bronchial aspirates were positive by PCR. One was from a patient whose two previous samples were positive by both culture and PCR. It had been submitted for culture several hours after collection, and clindamycin had been administered to the patient before collection of the specimen. The other bronchial aspirate positive by PCR was from a pneumonia patient who had also been administered clindamycin. We believe that B. fragilis was present in these two specimens but that either it was dead, it was below the level detectable by culture, or the process of anaerobic culture was unsuccessful. Thus, the PCR method may be considered useful for the sensitive and rapid detection of anaerobes in clinical specimens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G. Anaerobic bacterial infections of the lung. Chest. 1987 Jun;91(6):901–909. doi: 10.1378/chest.91.6.901. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Finegold S. M. Anaerobic infections of the lung and pleural space. Am Rev Respir Dis. 1974 Jul;110(1):56–77. doi: 10.1164/arrd.1974.110.1.56. [DOI] [PubMed] [Google Scholar]
- Hill R. T., Parker J. R., Goodman H. J., Jones D. T., Woods D. R. Molecular analysis of a novel glutamine synthetase of the anaerobe Bacteroides fragilis. J Gen Microbiol. 1989 Dec;135(12):3271–3279. doi: 10.1099/00221287-135-12-3271. [DOI] [PubMed] [Google Scholar]
- Holland R. W., Stauffer L. R., Altemeier W. A. Fluorescent antibody test kit for rapid detection and identification of members of the Bacteroides fragilis and Bacteroides melaninogenicus groups in clinical specimens. J Clin Microbiol. 1979 Aug;10(2):121–127. doi: 10.1128/jcm.10.2.121-127.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaulhac B., Nowicki M., Bornstein N., Meunier O., Prevost G., Piemont Y., Fleurette J., Monteil H. Detection of Legionella spp. in bronchoalveolar lavage fluids by DNA amplification. J Clin Microbiol. 1992 Apr;30(4):920–924. doi: 10.1128/jcm.30.4.920-924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritza A. P., Getty C. E., Shaughnessy P., Hesse R., Salyers A. A. DNA probes for identification of clinically important Bacteroides species. J Clin Microbiol. 1986 Feb;23(2):343–349. doi: 10.1128/jcm.23.2.343-349.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto C., Mitsunaga S., Oguchi T., Mitomi Y., Shimada T., Ichikawa A., Watanabe J., Nishioka K. Detection of human T-cell leukemia virus type I (HTLV-I) provirus in an infected cell line and in peripheral mononuclear cells of blood donors by the nested double polymerase chain reaction method: comparison with HTLV-I antibody tests. J Virol. 1990 Nov;64(11):5290–5294. doi: 10.1128/jvi.64.11.5290-5294.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Koga H., Kohno S., Kaku M. Nested polymerase chain reaction for detection of Mycobacterium tuberculosis in clinical samples. J Clin Microbiol. 1993 Aug;31(8):2228–2232. doi: 10.1128/jcm.31.8.2228-2232.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre C., Lecossier D., Boussougant Y., Bocart D., Joly V., Yeni P., Hance A. J. Use of a reamplification protocol improves sensitivity of detection of Mycobacterium tuberculosis in clinical samples by amplification of DNA. J Clin Microbiol. 1991 Apr;29(4):712–717. doi: 10.1128/jcm.29.4.712-717.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Moncla B., Kenny G. E. Chromosomal DNA probes for the identification of Bacteroides species. J Gen Microbiol. 1987 Jun;133(6):1423–1430. doi: 10.1099/00221287-133-6-1423. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sjöbring U., Mecklenburg M., Andersen A. B., Miörner H. Polymerase chain reaction for detection of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Oct;28(10):2200–2204. doi: 10.1128/jcm.28.10.2200-2204.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Callihan D. R. Analysis of rRNA restriction fragment length polymorphisms from Bacteroides spp. and Bacteroides fragilis isolates associated with diarrhea in humans and animals. J Clin Microbiol. 1992 Apr;30(4):806–812. doi: 10.1128/jcm.30.4.806-812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnbach M. N., Falkow S., Tompkins L. S. Species-specific detection of Legionella pneumophila in water by DNA amplification and hybridization. J Clin Microbiol. 1989 Jun;27(6):1257–1261. doi: 10.1128/jcm.27.6.1257-1261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissfeld A. S., Sonnenwirth A. C. Rapid detection and identification of Bacteroides fragilis and Bacteroides melaninogenicus by immunofluorescence. J Clin Microbiol. 1981 Apr;13(4):798–800. doi: 10.1128/jcm.13.4.798-800.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]