Abstract
The recently discovered default mode network (DMN) is a group of areas in the human brain characterized, collectively, by functions of a self-referential nature. In normal individuals, activity in the DMN is reduced during nonself-referential goal-directed tasks, in keeping with the folk-psychological notion of losing one's self in one's work. Imaging and anatomical studies in major depression have found alterations in both the structure and function in some regions that belong to the DMN, thus, suggesting a basis for the disordered self-referential thought of depression. Here, we sought to examine DMN functionality as a network in patients with major depression, asking whether the ability to regulate its activity and, hence, its role in self-referential processing, was impaired. To do so, we asked patients and controls to examine negative pictures passively and also to reappraise them actively. In widely distributed elements of the DMN [ventromedial prefrontal cortex prefrontal cortex (BA 10), anterior cingulate (BA 24/32), lateral parietal cortex (BA 39), and lateral temporal cortex (BA 21)], depressed, but not control subjects, exhibited a failure to reduce activity while both looking at negative pictures and reappraising them. Furthermore, looking at negative pictures elicited a significantly greater increase in activity in other DMN regions (amygdala, parahippocampus, and hippocampus) in depressed than in control subjects. These data suggest depression is characterized by both stimulus-induced heightened activity and a failure to normally down-regulate activity broadly within the DMN. These findings provide a brain network framework within which to consider the pathophysiology of depression.
Keywords: cognitive reappraisal, fMRI, medial prefrontal network, emotional dysregulation, activation differences
When we engage in almost any goal-directed behavior of a nonself-referential nature, certain areas of the brain decrease their activity (1) when compared with a quiet resting state (e.g., awake with eyes closed). The consistency with which certain areas of the brain do so, regardless of the nature of the goal-directed task, led to the notion of an organized default mode of brain function (2) in which some regions are most active when we are in a resting state. The areas of the brain most consistently displaying such behavior regardless of task have come to be known as the default mode network (DMN) (3, 4), which consists of areas in dorsal and ventral medial prefrontal cortices, medial and lateral parietal cortex, and parts of the medial and lateral temporal cortices.
Recently summarized data (4) indicate that the DMN is involved in the evaluation of potentially survival-salient information from the body and the world: perspective taking of the desires, beliefs, and intentions of others and in remembering the past as well as planning the future (2–4). All of these putative functions are self-referential in nature. Reduction of activity in the DMN during effortful cognitive processing (1, 5) can be interpreted as reflecting the need to attenuate the brain's self-referential activity as a means of more effectively focusing on a task. A failure to do so might well lead to interference in task performance from internal emotional states, as seen in patients with depression.
Studies in patients with major depression have identified structural and functional abnormalities in brain circuits involved in emotional processing (for reviews see refs. 6–9). These regions include the hippocampus, amygdala, anterior cingulate, ventromedial prefrontal cortex, and dorsal medial prefrontal cortex and fall within the anterior portion of the DMN. Although studies (6–9) have found depression-related abnormalities in portions of the DMN, it is not clear whether certain regions or the DMN as a whole is involved in the emotional dysregulation of depression. Therefore, in the current study, we used fMRI to measure changes in brain activity occurring within the entire DMN in 20 individuals with major depression and 21 demographically similar control subjects during an affective reappraisal task (10). Our goal was to examine the role of the DMN in emotional modulation in depression. To examine task-induced activity differences within the entire DMN rather than simply in selected regions, we used a DMN “mask” from an independent sample (see SI Text) by using resting state data to avoid the potential for influencing the results by the content of the task (11).
Results
Behavioral Data.
We used a modification of the Ochsner et al. (10) paradigm examining emotional regulation during 4 different task conditions: passively view neutral pictures (“look neutral”), passively view negative picture (“look negative”), actively make a negative picture more positive (“make positive”), and actively make a negative picture more negative (“make negative”). There were no significant differences between depressed and control subjects in the ratings of pictures either during passive viewing or explicit regulation (see SI Text for details).
Imaging Data.
To test our hypotheses, we independently identified the DMN boundaries (see Materials and Methods) to restrict regions of interest (ROIs) in our data to areas falling within the DMN. These boundaries correspond closely to published DMN maps from PET and fMRI data (1, 2, 12) (Fig. 1). We conducted 2 voxelwise ANOVAs (group X picture type X time within trial and group X regulate task X time within trial) to determine group differences. In these analyses, “group” contrasted depressed vs. control subjects, “look” (picture type) contrasted looking at neutral vs. negative pictures, and “regulate task” contrasted passively looking at negative pictures vs. consciously reframing the picture context as positive. Time within trial referred to the variation across the frame estimates (1–8) for a particular condition (see Materials and Methods for details of default region selection and image analysis).
Fig. 1.
Task-induced activity in the default mode network. The DMN is shown in light blue. The top left image shows the medial surface right hemisphere whereas the top right image shows the lateral surface left hemisphere. The bottom left shows the lateral surface right hemisphere whereas the bottom right image shows the medial surface left hemisphere. All regions are shown in Table 1 (group differences) or Table S1 (regions without group difference effects). As indicated by the legend, superimposed red colored regions show areas within the DMN where task-induced differences distinguished depressed and control subjects. The remaining regions had no group differences but had activity decreases in the regulate condition only (yellow), in the look condition only (green), in both the regulate and look conditions (brown), or had activity increases in both the regulate and look conditions (fuschia).
Regions with Task-Induced Activity Reductions.
Passive viewing of negative vs. neutral pictures (look condition).
Group effects.
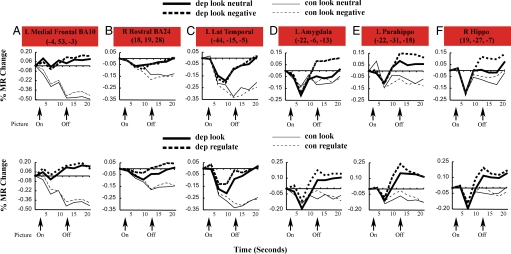
There were 14 regions within the DMN that exhibited a group difference between depressed and controls for the look condition. The typical pattern of activity for controls was decreased activity during the task, whereas depressed subjects failed to have a decrease in activity. Compared with controls, depressed individuals demonstrated less of a task-related decrease in activity (i.e., higher activity) in all 14 of these regions except L BA8 (see Table 1 and Fig. 1 regions in red). Fig. 2 shows time–activity curves for 8 representative ROIs of the 14 regions in Fig. 1 that differed between depressed and controls.
Table 1.
Regions showing significant group differences in activations in the analysis of passive viewing of neutral and negative pictures (look) and regulation vs. passive viewing of negative pictures (regulate)
| Region | (X, Y, Z) | No. of voxels | Look effect (dep > con), P value | Regulate effect (dep > con), P value |
|---|---|---|---|---|
| Decreased activity | ||||
| Anterior cingulate | ||||
| Dorsal BA32 | −18, 18, 38 | 46 | <0.001 | <0.001 |
| Rostral BA24 | 18, 19, 28 | 81 | 0.005 | <0.001 |
| Ventromedial prefrontal cortex | ||||
| Medial frontal BA10 | −4, 53, −3 | 108 | <0.0001 | <0.0001 |
| Medial frontal BA10 | 4, 54, −8 | 32 | 0.004 | <0.0001 |
| Lateral temporal cortex | ||||
| BA21 | −43, −16, −5 | 129 | <0.0001 | <0.0001 |
| BA21 | −44, −15, −5 | 132 | <0.0001 | <0.0001 |
| BA21 | −52, 1, −27 | 92 | <0.0001 | <0.0001 |
| BA21 | −50, 1, −27 | 97 | <0.0001 | <0.0001 |
| BA21 | −55, −2, −21 | 90 | <0.01 | ns |
| Lateral parietal cortex | ||||
| BA39 | 42, −71, 13 | 61 | 0.004 | <0.001 |
| Increased activity | ||||
| Amygdala | −22, −6, −13 | 55 | 0.01* | ns |
| Hippocampus | 19, −27, −7 | 73 | 0.009* | ns |
| Parahippocampal gyrus | −22, −31, −18 | 51 | 0.007 | <0.001 |
| Dorsal medial prefrontal cortex | ||||
| Medial BA8 | −11, 14, 50 | 50 | 0.005† | 0.002 |
*Time frame 5–8 s, see text.
†Dep < con.
Fig. 2.
Time–activity curves are shown for a subset of regions in Fig. 1. (A–F) Time–activity curves plot percent fMRI signal change (y axis) vs. time in seconds (x axis). Regions compare passive viewing of negative pictures to passive viewing of neutral pictures (look) (Upper), compare passive viewing of negative pictures to regulation of emotion (regulate), and time within trial that reflected modulations of task-related activation (Lower). (A–C) As indicated, there was less of a decrease in activity in depressed compared with control subjects in left ventromedial prefrontal cortex (BA10) (A), right rostral anterior cingulate (B), and left lateral temporal cortex (C). (D–F) There was a greater increase in depressed subjects in the look condition (Upper) for the left amygdala (D), left parahippocampus (E), and right hippocampus (F). The greater activity during the regulate condition (Lower) was only significant in the L parahippocampus. The regulate condition difference (Lower) was only significant in the left parahippocampus.
Picture-type effects.
In addition there were a number of regions that showed less of a decrease in activity to negative pictures than neutral pictures in both depressed and controls or a greater increase in activity for negative than neutral pictures. These regions are shown in Fig. 1, with the colors indicating whether the regions exhibited increased or decreased activity. These regions are also shown in Table S1, and the time–activity curves are shown in Fig. S1.
Regulation of emotional responses.
Group effects.
As shown in Table 1, there were 11 regions for which the depressed participants failed to show the same amount of task-related decreased activity during the make positive condition as controls, including 2 rostral anterior cingulate regions (Fig. 2 and Fig. S2), dorsal anterior cingulate (Fig. S2), 2 ventromedial prefrontal cortex (BA 10) regions (Fig. 2 and Fig. S2), left medial prefrontal cortex BA8 (Fig. S2), lateral parietal cortex (BA 39) (Fig. S2), and 5 lateral temporal cortex (BA 21) regions (Fig. 2 and Fig. S2). Regions not shown in Fig. 2 because of space limitations had very similar patterns of activity, and their time courses are shown in Fig. S2.
In addition there were a number of regions that showed decreased activity in both depressed and controls during the regulate condition (see Fig. 1, Fig. S1, and Table S1).
Regions with Task-Induced Activity Increases.
Passive viewing of negative vs. neutral pictures (look condition).
Group effects.
Three regions showed more increase in activity in depressed subjects than in controls, as shown in Table 1 and Fig. 2. In the left parahippocampus, depressed subjects had significantly greater activity than controls (across the entire time course) in response to negative as compared with neutral pictures. Although there were no group differences in the overall time courses (frames 1–8) in amygdala and hippocampus, examination of the time courses suggested that depressed patients may have shown enhanced activity in the later part of the trial, after onset of the picture. Therefore, we conducted post hoc analyses in these regions focusing on the frames covering 10–17.5 s.
The left amygdala [F (1, 43) = 6.47, P = 0.01] and right hippocampus [F (1, 43) = 7.42, P = 0.009] showed significantly different patterns of responses to picture types in depressed vs. control subjects (i.e., picture type X group interaction). As can be seen in Fig. 2, controls did not show a significant difference in responses to neutral vs. negative pictures, but the depressed individuals showed significantly greater activity to negative compared with neutral pictures. In one region (left dorsal medial prefrontal cortex BA8), controls had significantly greater activity in response to negative pictures than depressed (Table 1 and Fig. S2).
Picture-type effects.
Several regions showed increased activity for negative pictures compared with neutral pictures in both depressed and controls. These regions are shown in Fig. 1 and Table S1.
Regulation of emotional responses.
We used regulate task (look-negative vs. make positive) and time within trial as within-subject factors and diagnostic group as a between subject factor.
Group effects.
As shown in Table 1, 2 regions showed group differences that varied as a function of the task. In the left parahippocampus and left dorsal medial prefrontal cortex, depressed had greater activity than controls during the emotional regulation (make positive) condition compared with passively looking at negative pictures.
Picture-type effects.
Several regions showed more increased activity in the make positive condition compared with passive viewing of negative pictures in both depressed and controls. By using the regulate task (look-negative vs. make positive) and time (within trial) as within-subject factors, these regions showed picture task X time interactions that did not further interact with group. (Fig. 1, Fig. S1, and Table S1).
Exploratory effects.
To look for nonpredicted effects in regions outside the a priori DMN ROI, we conducted a whole-brain ANOVA to determine group main effects or interaction with group. We identified 4 regions, all in the cerebellum, that met threshold criteria. See SI Text for discussion.
Discussion
The DMN (Fig. 1) is a constellation of brain areas defined functionally on the basis of their coordinated behavior in the human brain (2, 5). This behavior manifests itself in several ways. First, the areas together typically exhibit activity decreases during the performance of a wide range of goal-directed tasks (1–5). Exceptions to this widespread decrease in activity, as in the present study, relate to the task-relevance of the functionality associated with specific components of the DMN, such as that attributed to the ventral medial prefrontal cortex.
Second, the areas comprising the DMN exhibit striking temporal coherence in the resting state (resting quietly with eyes closed). This resting state temporal coherence emerges from the spontaneous fluctuations in the fMRI BOLD signal, a phenomenon first noted by Biswal and colleagues (13) in the somatomotor system. It has since been extended to virtually all cortical systems including the DMN (for recent review see ref. 11) and subcortical structures (14).
Finally, the DMN follows a developmental trajectory in humans in which interhemispheric coherence within the DMN appears strong by age 6, but anterior–posterior coherence between parietal regions and medial prefrontal cortex is weak (15). This longitudinal development of DMN coherence suggests an important experiential component in sculpting the DMN. As such, this sculpting may also be affected by early life stressors and trauma that have been shown to cause a predisposition to the development of depression (16), for example, through changes in neurotrophic factors or other factors that could affect neuroplasticity and DMN connectivity (17). Aberrant regulation of neuronal plasticity may result in maladaptive changes in neural networks that underlie the development of major depressive disorder.
The results of the current study provide information about the role of DMN in emotional activity and regulation in major depression. We found very consistent evidence widely distributed within the DMN that depressed individuals differed from controls during the performance of emotional tasks. Specifically, there were group differences (differences in both the look and regulate tasks) in multiple DMN regions. Furthermore, we found clear evidence in controls, as well as depressed subjects, that manipulation of negative emotional content of pictures and the need to regulate emotion modulated activity in many DMN (Fig. 1 and Table S1).
Our results confirm and extend the results of other studies examining DMN in depression. One study (18) found increased activity in some DMN, including amygdala and anteromedial prefrontal cortex (BA 9/8) but found decreases in anterior cingulate (BA 32). Grimm et al. (19) reported alterations in 2 midline DMN regions and found that these activity differences correlated with depression severity and feelings of hopelessness. Greicius et al. (20) found abnormally increased resting state connectivity of subgenual anterior cingulate by using functional connectivity MRI. Whereas other studies have examined cognition or affect related processing in some DMN regions, our study is unique in determining DMN differences from a focused theoretical perspective, independently determining the DMN from a separate resting state dataset and identifying any activation differences within the network as a whole. We found the failure to decrease activity in the DMN in depression was not specific to voluntary regulation of affect but appears instead to be a more general pattern of response, suggesting that DMN abnormalities might contribute to deficits in “automatic” and controlled processing of affective stimuli. Automatic processing occurred in the current experiment when subjects saw emotional stimuli and had a subliminal or automatic brain response. We suggest that dysregulation of automatic emotional processing indicates the fundamental importance of the DMN in depression.
Our working hypothesis to explain the origin of increased activity in DMN regions during emotional modulation tasks is their extensive anatomical connections to regions involved in emotion, internal inspection, and endocrine regulation (e.g., hypothalamus, amygdala, and periaquaductal gray of the brainstem) (Fig. 3). DMN regions have been thought to be involved in self-inspection and monitoring of the internal and external milieu (2, 4–5), which are activities that may also be overactive in depression, especially in the form of ruminations (21).
Fig. 3.
Anatomical connections of the medial prefrontal network in the macaque adapted from Saleem et al. (23). The superimposed ROI in red are the areas in the current study (also shown in Fig. 1) in which depressed subjects differed from controls. The medial prefrontal cortex and its connected regions are part of the DMN and have extensive interconnections with each other and visceral control areas.
An emerging point is that the DMN is composed of a group of relatively large but widely separated areas that each have their own unique anatomy, connections, and functionality. One of the best understood areas resides in the medial prefrontal cortex, which, along with its connections, as defined in monkeys, has been dubbed the “medial prefrontal network” by Price and colleagues (22). This anterior portion of the DMN, including the anterior cingulate and ventromedial prefrontal cortex (BA 10), is extensively interconnected and intimately tied to limbic and other structures, such as the hypothalamus, that provide internal visceral surveillance. Although the recognition of the DMN arose from studies that identified common activity during functional tasks, it is interesting that many of these regions are also part of the medial prefrontal cortex or its connections. In the current study, we found many DMN regions that differed between depressed and controls that are anatomically connected with the medial and dorsal prefrontal cortex network in macaques (23). These anatomically connected regions include left lateral temporal cortex (23) and limbic regions (24) (Fig. 3).
The parahippocampal area seen in this study theoretically corresponds to the entorhinal and posterior parahippocampal cortex in monkeys. The macaque rostral superior temporal gyrus region may be homologous with rostral middle temporal gyrus regions that have shifted ventrally because of differential enlargement of the midportion of the temporal lobe in humans. The same shift is seen in the movement of the visual association areas from the ventrolateral to the ventral surface of the temporal lobe (25).
Although we did not find evidence of differences between depressed and control subjects in the posterior cingulate and precuneus, which are part of the posterior DMN, these regions did, nonetheless, show significant effects of both looking at negative pictures and regulating emotion (picture type and regulate task effects) in both depressed and control subjects (Fig. 1 and Table S1) and thus are important in emotional modulation. However, the lateral parietal cortex is a region that has not been found to be anatomically connected to the medial and prefrontal cortex, but it is an important part of the DMN and differed significantly in emotion-mediated activity between depressed and controls in the current study.
In depressed patients there was increased activity, relative to controls, in response to negative pictures in the hippocampus, parahippocampal cortex, and amygdala, consistent with other studies (26–29). Although these structures are not as frequently described as components of the DMN, there have been a number of studies that clearly implicate them as having decreased activity during cognitive tasks (1). Studies have shown that decreases in activity during focused attention reflect a dynamic interaction between cognitive demands and the person's emotional state (30–34). In depression, it has been hypothesized that heightened limbic responses to negative affect-eliciting stimuli may provide a bottom up source of input that can serve to dysregulate cognitive control systems that might normally suppress such affective responses (30, 35, 36). This hypothesis is supported by studies of cognitive task performance in depression that have revealed a failure to reduce activity in medial prefrontal regions in response to increased cognitive demand (37) or during an emotional conflict task (38). As such, the failure of depressed patients to appropriately decrease activity in medial prefrontal regions suggests an impaired ability to suppress attention to internal emotional states.
Although, like other studies (33, 39), we found increased prefrontal activity in the dorsal and rostral cingulate (BA10 and BA8, respectively) in depressed subjects, relative to controls, during emotional regulation, we did not find clear evidence, unlike other studies (10, 33, 39), that either controls or depressed patients were able to modulate amygdala activity in response to demands to down-regulate responses to negative stimuli. Nonetheless, we did see clear evidence for a left amygdala response to negative pictures with left amygdala hyperactivity in depression.
Another somewhat surprising finding in the current study was that depressed participants did not differ from controls in their ratings of the picture stimuli. Such ratings are very susceptible to demand characteristics, however, and it is possible that participants responded in a way that they knew was expected of them (e.g., less negative ratings of pictures in the regulation condition), highlighting the importance of objective measures of brain function that may be less susceptible to such effects. Importantly, the absence of behavioral differences between groups allowed us to interpret the fMRI differences in depressed subjects without correcting for behavioral differences. However, additional studies would be needed to show the importance of correlating fMRI activity with clinical characteristics, such as rumination, to further explore the functional significance of the neural findings.
In summary, we found differences between depressed and control subjects in important regions within the DMN. Regions in the DMN are part of a core system that is critical in self-referential properties. In the face of emotional stimuli, the DMN is overactive for both implicit and explicit emotional modulation. We hypothesize that whether interrogating visceral reactions, emotions, potential threats, or remembering the past, to name a few functions of the DMN, there is an increase in the degree of self-referential focus in depression. Thus, depression can be thought of as an illness involving a pathological inability of the DMN to regulate self-referential activity in a situationally appropriate manner.
Materials and Methods
Participants.
Participants were screened by the same criteria as described in ref. 38 resulting in 24 individuals with major depression [M/F: 12/12, mean age: 34 years (SD = 9.4), education: 15 years (SD 2)], and 21 demographically similar controls [M/F: 6/15, age: 35 (SD = 7.3), education: 16 (SD 2)]. There were no significant group differences in age (of 43 subjects, t = .25, P = 0.81), gender (Chi-Square = 2.14, df = 1, P = 0.14), or education (of 43 subjects, t = 1.10, P = 0.79). Given the gender imbalance between depressed and control subjects in the current study, we reanalyzed our data excluding 4 males from the depressed group to result in a group of 20 depressed subjects. Our results continued to reveal significant differences between the groups in all of the same regions.
Depressed participants met criteria for a current episode of unipolar recurrent major depression by the Diagnostic and Statistical Manual of Mental Disorders-IV criteria (40). All participants were free of psychotropic medication for a minimum of 4 weeks, were administered a 17-item Hamilton Depression Rating Scale (HDRS) (41), and were excluded for acute physical illness, history of trauma resulting in loss of consciousness, and lifetime psychiatric disorders. Depressed participants were included with HDRS scores of ≥18 (mean 21 ± 3.5). Control participants had scores ≤8 (mean 0 ± 0.4). All participants provided written informed consent in accordance with Washington University Human Subjects Committee criteria and were paid $25.00 per hour for their participation.
Procedure.
We used a modification of the Ochsner et al. (10) paradigm examining emotional regulation. We used 4 different task conditions: passively look at neutral pictures (look neutral), passively look at negative picture (look negative), actively make a negative picture more positive (make positive), and actively make a negative picture more negative (make negative). The latter was included to ensure that subjects would reliably regulate their emotions (i.e., had they been asked to only make pictures positive or look neutral, they might regulate everything in a make positive direction, including the neutral pictures). Because we had no a priori hypothesis about the make negative condition, it was not included in the data analysis.
In the make positive condition, participants were instructed to depersonalize the image such that it did not pertain to them, that the image was not real, and that the outcome of the scene portrayed was positive. In the make negative condition, participants were instructed to imagine the pictures were pertinent to themselves or a loved one and that the outcome was negative. Before entering the scanner, participants received instruction on how to attenuate emotional response and spent ≈15 min practicing (with 10 additonal minutes of practice in the scanner).
Stimulus Presentation.
Stimuli from the International Affective Picture Series (42) were counterbalanced for picture type (see SI Text).
fMRI Image Acquisition, Processing, and Analysis.
All fMRI data were obtained on the same Siemens 3T Allegra MRI scanner and processed as described (30) (see SI Text).
DMN for ROI Identification.
To test our hypotheses, we identified a priori the DMN boundaries to determine ROIs in our data falling within the DMN by using standard methods (12) (see SI Text).
Statistical Analysis.
To test our hypotheses, we used a priori defined ROIs, consisting of the DMN as defined above and in the SI Text. Voxel clusters within the a priori defined default connectivity map showing effects of interest were identified by using a 3-stage process. (i) We required voxels to show significant effects at P < 0.001 and to belong to clusters of at least 14 contiguous voxels. (ii) We required that the activity peak of a given voxel cluster fall within the DMN in order for a cluster to be counted as included. (iii) We then conducted analyses by using the clusters identified in the previous step and (to protect against Type I error) required results to show post hoc effects at P < 0.01.
Exploratory Effects.
To look for nonpredicted effects in regions outside the a priori DMN ROI, we conducted a whole-brain ANOVA to determine group main effects or interaction with the group. We identified 4 regions, all in the cerebellum, that met threshold criteria. See SI Text for further discussion.
Supplementary Material
Acknowledgments.
We thank Tony Durbin for his assistance in subject recruitment and fMRI scanning. This work was supported by National Institutes of Health Grants R01 MH64821, K24 MHO79510 (to Y.I.S.), and P50NS06833 (to M.E.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812686106/DCSupplemental.
References
- 1.Shulman G, et al. Common blood flow changes across visual tasks II: Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Raichle M, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1099. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 4.Buckner R, Andrews-Hanna J, Schacter D. The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 5.Gusnard D, Akbudak E, Shulman G, Raichle M. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ressler K, Mayberg H. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheline Y. Neuroimaging studies of mood disorder effects on the brain. Biological Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 8.Davidson R, Pizzagalli D, Nitschke J, Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 9.Drevets W. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 10.Ochsner K, Bunge S, Gross J, Gabrieli J. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J Cog Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 11.Fox M, Raichle M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 12.Fox M, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, et al. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fair D, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 2008;41:45–57. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Uys J, et al. Developmental trauma is associated with behavioral hyperarousal, altered HPA axis activity and decreased hippocampal neurotrophin expression in the adult rat. Ann NY Acad Sci. 2006;1071:542–546. doi: 10.1196/annals.1364.060. [DOI] [PubMed] [Google Scholar]
- 18.Anand A, et al. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol Psych. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Grimm S, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- 20.Greicius M, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray R, et al. Individual differences in trait rumination modulate neural systems supporting the cognitive regulation of emotion. Cog Affect Behav Neurosc. 2005;5:156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- 22.Ongür D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 23.Saleem K, Kondo H, Price J. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- 24.Carmichael S, Price J. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Sheline Y, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psych. 2001;9:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 27.Davidson R, Irwin W, Anderle M, Kalin N. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psych. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 28.Fu C, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event related functional magnetic resonance imaging study. Arch Gen Psych. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 29.Siegle G, Thompson W, Carter C, Steinhauser S, Thase M. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psych. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 30.Drevets W, Raichle M. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implication for interactions between emotion and cognition. Cognit Emot. 1998;12:353–385. [Google Scholar]
- 31.Simpson J, et al. The emotional modulation of cognitive processing An fMRI study. J Cognit Neurosci. 2000;12:157–170. doi: 10.1162/089892900564019. [DOI] [PubMed] [Google Scholar]
- 32.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cognit Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 33.Ochsner K, Gross J. The cognitive control of emotion. Trends Cognit Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayberg H. Limbic-cortical dysregulation: A proposed model of depression. J Neuropsychiatr and Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 36.Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Wagner G, et al. Cortical inefficiency in patients with unipolar depression: An event related MRI study with the Stroop task, 126. Biol Psych. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Fales C, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psych. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phan K, et al. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psych. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. DSM-IV-R: Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 41.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang P, Bradley M, Cuthbert B. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainsville, FL: NIMH CSEA; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.