Abstract
Background
Cerebral aging is a complex and heterogeneous process that is associated with a high degree of inter-individual variability. Structural magnetic resonance imaging (MRI) can be used to identify and quantify non-disease-related aging of the cerebral white matter.
Methods
The present article reviews the findings from several MRI techniques, including morphometric approaches, study of white matter hyperintensities, diffusion tensor imaging, and magnetization transfer imaging that have been used to examine aging of the cerebral white matter. Furthermore, the relationship of MRI indices of white matter integrity to age-related cognitive declines is reported.
Results
A general pattern of age-related preservation and decline emerges indicating that the prefrontal white matter is most susceptible to the influence of age. Studies that combine MRI with cognitive measures suggest that such age-related reductions in white matter integrity may produce a disconnection state that underlies some of the age-related performance declines in age-sensitive cognitive domains.
Conclusions
White matter aging may contribute to a disconnection state that is associated with declines in episodic memory, executive functions, and information processing speed.
Keywords: Aging, White Matter, MRI
Cerebral aging is a complex and heterogeneous process that is associated with a high degree of inter-individual variability and characterized by a pattern of selective loss and preservation. Magnetic resonance imaging (MRI) makes in vivo characterization of brain alterations occurring with advancing age possible. The use of MRI allows thorough characterization of non-disease-related brain aging and facilitates the study of anatomical alterations that contribute to the cognitive and affective symptoms of late-life psychiatric illnesses. This review will focus on the results of MRI studies of white matter changes that occur with normal aging and the relationship of age-associated changes in white matter to age-related declines in cognitive abilities.
Histopathological Characteristics of Cerebral White Matter Aging
Histopathological studies reveal that brain aging is marked by degradation of white matter including myelin pallor (Kemper, 1994), loss of myelinated fibers (Bartzokis, 2004; Marner et al., 2003; Pakkenberg and Gundersen, 1997; Meier-Ruge et al., 1992), and malformation of myelin sheaths (Peters, 2002). In elderly non-human primates, localized splitting of myelin lamellae and spherical cytoplasmic cavities or “balloons” within the myelin sheath or in the axoglial junction are commonly observed (Peters, 2002). Concomitantly, however, continued myelin production termed “redundant myelin” has been observed in older monkeys (Peters et al., 2001), perhaps as a compensatory mechanism for myelin degeneration. This redundant, splitting myelin is associated with decreased conduction velocity observed in aging (Peters et al., 1996; Xi et al., 1999; Aston-Jones et al., 1985; Peters, 2002; Peters et al., 2000; Peters and Sethares, 2002). Furthermore, evidence suggests that myelination from late-differentiating oligodendrocytes is less effective and more vulnerable than myelination from oligodendrocytes present earlier in development (reviewed in (Bartzokis et al., 2004).
Macrostructural Aging of the Cerebral White Matter
On MRI, there is little doubt that the brain shrinks with age. The rate of shrinkage accelerates with age and is most precipitous after about age 50 (Raz and Rodrigue, 2006). Bulk volume loss of white matter is in line with the idea that the aging brain is characterized by declining neuronal connectivity (Albert, 1993) and the question of the degree of global grey versus white matter aging has received particular attention in recent years (Peters and Rosene, 2003). Several studies have shown a relatively greater age-associated decline in white matter volume or the presence of white matter volume loss in the absence of grey matter loss (Allen et al., 2005; Bartzokis et al., 2003; Guttmann et al., 1998; Jernigan et al., 2001; Resnick et al., 2003). For example, in individuals ranging from 30 to 90 years of age, Jernigan and colleagues (2001) observed a 26% reduction in white matter tissue volume, relative to a 14% reduction in gray matter tissue volume (Greenwood, 2007). However, several other studies have shown the opposite effect, with greater age-associated grey matter loss (Blatter et al., 1995; Sullivan et al., 2004; Thompson et al., 2003). A number of factors could account for these discrepancies. First, white matter volume loss may be a feature of more advanced age (Salat et al., 1999); that is grey matter loss may begin earlier and progress gradually, whereas white matter loss may start later and progress more precipitously (Raz et al., 2005). Second, grey/white matter segmentation algorithms and intensity thresholds may differ across laboratories, producing variable results.
Most MRI studies of the regional distribution of age-related gray matter volumetric reductions, have shown the greatest effects in frontal lobe (Allen et al., 2005; DeCarli et al., 1994; Raz et al., 1997; Raz et al., 2004; Resnick et al., 2003; Salat et al., 1999; Tisserand et al., 2002), followed by the temporal lobes (Cowell et al., 1994; Sullivan et al., 1995), with relative sparing of primary sensory areas and the occipital lobes (Bartzokis et al., 2001; Good et al., 2001; Raz et al., 1997; Raz et al., 2005). Our recent cross-sectional analysis of grey and white matter prefrontal volumes in 70 individuals across the adult lifespan indicated that grey matter volume in the lateral prefrontal cortex showed the greatest age-associated effect and appeared to decline linearly across ages, with about a 3% volume loss per decade (Brickman et al., 2005). Importantly, white matter volumes appeared relatively stable, except in the oldest participants (i.e., ages 70s and 80s) who had reduced white matter volumes in dorsolateral and orbital regions, which is consistent with the idea that white matter volume changes may be most prominent among the very old (Raz et al., 2005; Salat et al., 1999).
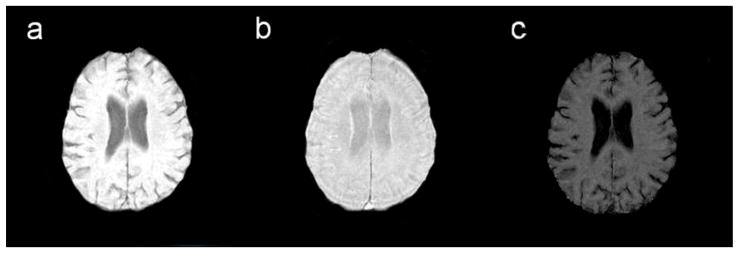
While the presence and degree of age-related volumetric loss in cerebral white matter remains somewhat inconsistent, the frequent presence of foci of increased signal on MRI scans of older adults is a well-established phenomenon. White matter hyperintensities (WMH) (Figure 1) are areas of increased intensity appearing on T2-weighted images and are taken to indicate white matter damage. They can be discrete, or punctate, or may appear more confluent with the lateral ventricles. Until recently, WMH were considered clinically irrelevant, but a culmination of sample- and population-based research has demonstrated their functional significance (Gunning-Dixon and Raz, 2000; Malloy et al., 2007). The presence of WMH is common among normal elderly adults and chronological age appears to be the strongest predictor of severity (de Leeuw et al., 2001; Jernigan et al., 1991; Brickman et al., In press). Vascular risk factors, such as hypertension, also account for much variability in severity of WMH (de Leeuw et al., 2001; Firbank et al., 2007; Manolio et al., 1994; Spilt et al., 2005). With the exception of WMH that appear as smooth “rims” or “caps” along the surface of the lateral ventricles, WMH are thought to be ischemic in nature and often reflect rarefaction of myelin, breakdown of vessel endothelium, and microvascular disease (Fazekas et al., 1995; Fernando et al., 2004; Oppenheimer et al., 1995; Smith et al., 2000). Regarding the progression of WMH in normal aging, a study of 51 older adults (mean age 71) revealed a 43.8 and 29.7% rate increase in WMH burden for deep and perilventricular WMH, respectively. Furthermore, rate of WMH progression is greatest in anterior white matter with WMH in occipital white matter being relatively stable (Sachdev et al., 2007).
Figure 1.
White matter hyperintensities on a FLAIR image.
The WMH studies are associated with some limitations. First, there are numerous WMH rating scales and these scales vary widely in scope, sensitivity, and reliability. Second, post-mortem studies of the histology of WMH suggest that white matter abnormalities reflect a number of pathological processes; however, the methodology prevents reliable discrimination among mechanisms. Third, analysis of WMH does not allow accurate identification of specific affected white matter tracts. Thus, one hopes the introduction of new MRI approaches (e.g., diffusion tensor, magnetization transfer) will complement the information gleaned from studies of WMH.
Aging of the White Matter Microstructure
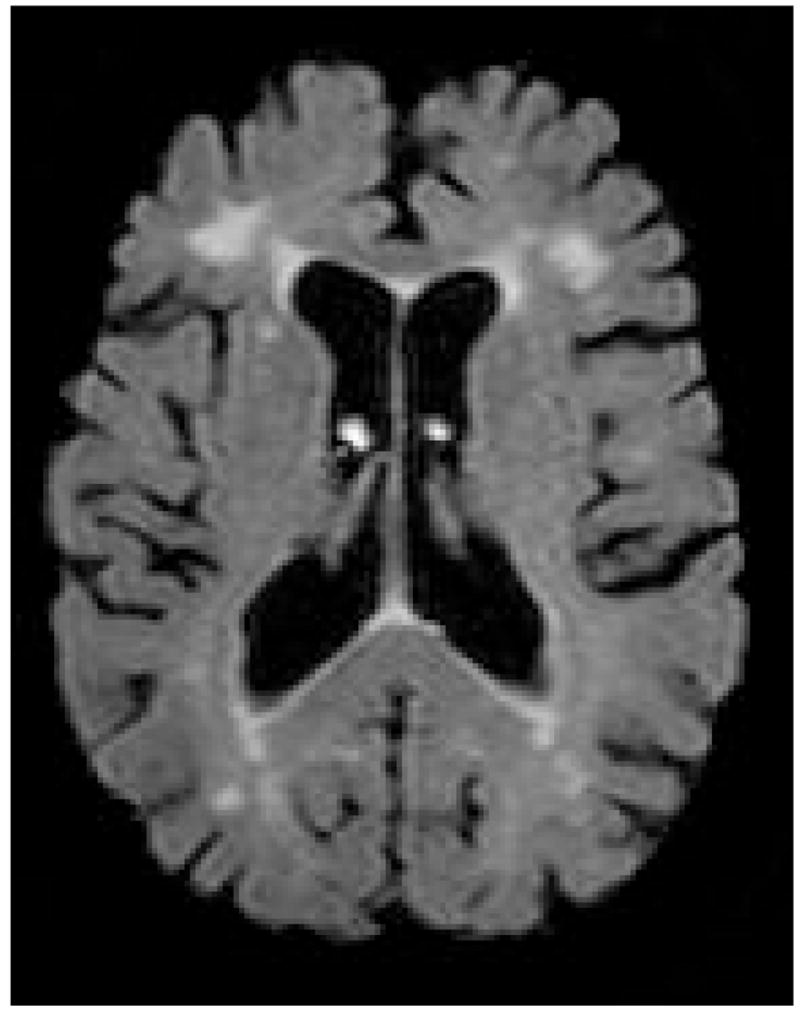
Diffusion tensor imaging (DTI) offers a promising new technique for the identification of cerebral networks most vulnerable to the aging process. This method measures the magnitude and orientation of self-diffusion of water. When no barriers to such diffusion are present, diffusion occurs equally in all directions (i.e., it is isotropic). However, when barriers are present, diffusion tends to follow the long axis of those barriers (i.e., diffusion is anisotropic). Barriers to diffusion in the brain include cell membranes, myelin sheaths, and white matter fiber tracts. Diffusion anisotropy can be quantified by a number of different metrics, including fractional anisotropy (FA) (Figure 2), which is a measure of the strength of the directional dependence of diffusion and tissue disruption. Removal or degradation of structural barriers to the molecular motion of water (e.g., cell membrane) typically decreases FA values.
Figure 2.
Fractional anisotropy image from a diffusion tensor scan.
The most consistent finding from DTI studies of advancing age is a vulnerability of the prefrontal white matter to aging. Following the initial reports of greater age-related FA reductions in frontal regions of interest (ROIs) relative to more posterior ROIs (O’Sullivan et al., 2001; Sullivan et al., 2001), several studies provided converging support for an anterior-posterior gradient of age-associated decreases in FA (Ardekani et al., 2007; Grieve et al., 2007; Head et al., 2004; Pfefferbaum and Sullivan, 2003; Salat et al., 2004) as well as some evidence for age-related reductions in select striatal regions (Ardekani et al., 2007; Salat et al., 2004; Abe et al., 2006). Furthermore, there may be differential aging effects on FA within the frontal regions, with specific white matter areas including the ventromedial prefrontal and deep frontal white matter exhibiting the most robust relationship with age (Salat et al., 2005). Voxelwise analysis of the relationship of atrophy to age-related reductions in FA suggests that FA is a sensitive marker of aging that may precede atrophy in many regions of the brain (Hugenschmidt et al., 2007). Furthermore, FA and brain volume may be complementary indices of brain aging (Abe et al., 2006).
Abnormalities of the corpus callosum, the major commissure connecting the cerebral hemispheres, can interfere with the efficiency of interhemispheric transfer in older adults and likely contribute to specific patterns of cognitive aging (Janowsky et al., 1996; Jeeves and Moes, 1996). Post mortem studies reveal subtle aging effects in the corpus callosum, with the less myelinated fibers of the genu being particularly susceptible to the deleterious effects of aging (Kemper, 1994; Aboitiz et al., 1992). Studies of regional FA of the corpus callosum also follow an anterior posterior gradient with the genu and rostral body exhibiting the strongest relationship with advancing age, whereas FA in the splenium is relatively stable (Ota et al., 2006; Pfefferbaum et al., 2005; Salat et al., 2005). Furthermore, application of a quantitative fiber tracking approach revealed that within the corpus callosum, there was disproportionately lower FA in anterior fiber bundles relative to posterior bundles in the older group compared to younger group (Sullivan et al., 2006).
Diffusion tensor imaging indices also distinguish normal from pathological aging. For example, Head and colleagues (2004) used diffusion tensor imaging to characterize the influence of both normal and pathological (e.g., Alzheimer’s disease) aging on FA. Aging effects in nondemented adults were greater in anterior compared to the posterior corpus callosum as well as greater in frontal relative to parietal, temporal, and occipital regions. In addition, demented subjects exhibited only minimal differences in anterior regions relative to their age-matched counterparts, but did show significantly reduced FA in posterior regions (Head et al., 2004).
Overall, results from studies of FA suggest that microstructural white matter integrity differentiates normal from pathological aging because normal aging is associated with microstructural deterioration that generally occurs in an anterior to posterior gradient whereas dementia is associated with deterioration of more posterior regions. Some controversy exists over interpretation of DTI results. Specifically, DTI has been described as a measure of white matter integrity, but it is simply a measure of anisotropic diffusion. Furthermore, some have argued that DTI primarily measures axonal membrane integrity, with myelin playing a modulatory role (Beaulieu, 2002).
Macromolecular White Matter Changes
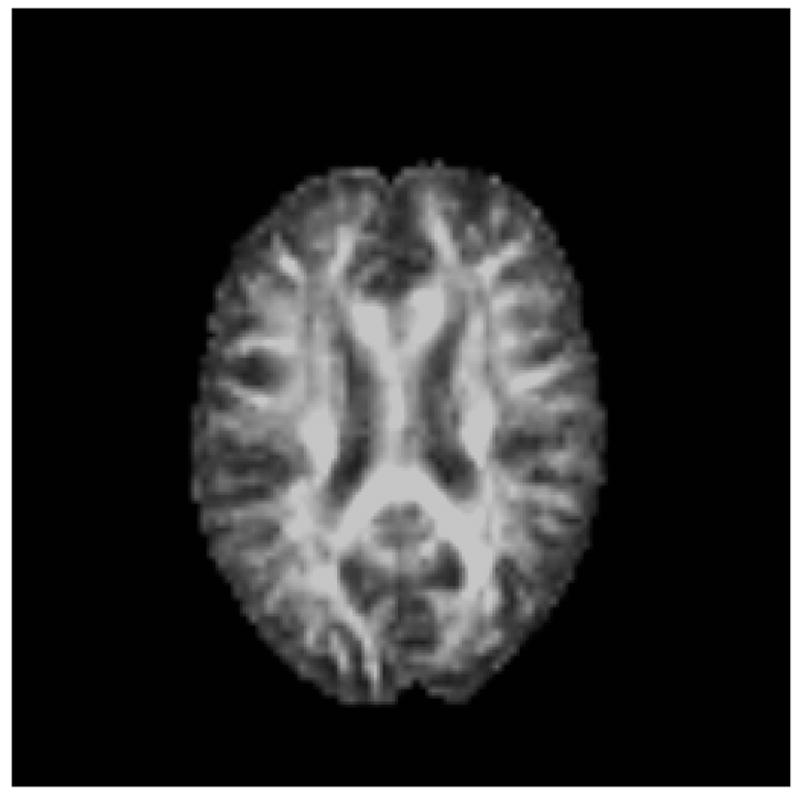
Magnetization transfer ratio (MTR) imaging provides information about the macromolecular structure of cerebral white matter based on the interaction of the normally observed tissue water signal with protons contained in large macromolecules (including myelin). Macromolecular semisolid structures in the brain, such as myelinated axons, are ordinarily not detectable with MRI because of their extremely short transverse relaxation times. However, protons bound to them can be selectively excited using off-resonance radio-frequency pulses. To achieve MTR contrast, two MR sequences are used. The first is a proton density (PD) weighted sequence, which reflects the total water signal. The second employs an additional pulse prior to the basic proton density sequence and serves to null the signal from water molecules that are associated with macromolecules. Thus, the second sequence reflects the signal from free water. The percent contrast difference between the two image sets is usually expressed as the magnetization transfer ratio: MTR = (M0 – MSAT)/M0 (Figure 3). Studies conducted in early development and multiple sclerosis suggest that DTI and MTR may provide complementary information about white matter integrity with DTI primarily reflecting organization of fiber tracts and MTR being particularly sensitive to myelin integrity (Wozniak and Lim, 2006).
Figure 3.
a. MT0 =Standard proton density sequence (measures bound + free protons)
b. MTSat =Saturation pulse that nulls signal due to protons bound to macromolecules including myelin (measures free protons).
c. MTR = Magnetization transfer ratio.
MTR in early development shows an increase during the initial years of life that coincides with brain myelination and follows the posterior to anterior temporal pattern that occurs in neurodevelopment (Wozniak and Lim, 2006). However, the relationship of MTR to age in adulthood is more controversial. For example, in 52 healthy older adults between the ages of 20 and 86 years, MTR histograms were significantly lower in the group older than 50 than those in the younger group (Ge et al., 2002). The MTRs started to decline around 40 years of age. Some have suggested that if WMH are excluded, MTR remains relatively stable throughout adulthood with a trend for MTR in some regions to increase with age perhaps due to redundant myelin (Armstrong et al., 2004). However, the bulk of the evidence does not support this assertion. Studies have shown that MTR values are 8 to 10% lower in WMH than in normal appearing white matter (Fazekas et al., 2005; Tanabe et al., 1997); but MTR is moderately negatively correlated with age both in areas of WMH and normal appearing WMH (Tanabe et al., 1997). MTR of normal appearing white matter is lower in elderly subjects related to young subjects (Fazekas et al., 2005; Spilt et al., 2005), but does not differ between individuals with minimal versus extensive WMH (Fazekas et al., 2005). Thus, these studies support the existence of age-related reductions of MTR, which are exacerbated in areas of WMH but also occur in normal appearing white matter.
Cognition and White Matter Aging
Cognitive aging is a selective process that is marked by significant declines on tasks for which successful performance demands substantial mental effort, rely heavily on processing speed, and are characterized by complexity and novelty of the stimuli. On the other hand, performance on tasks that depend on semantic knowledge and/or overlearned skills is relatively preserved (McArdle et al., 2002; Horn, 1986).
Correlations between regional brain volumes and cognitive abilities tend to be modest; however, the strength of these relationships increases with advancing age (Greenwood, 2007; Zimmerman et al., 2006) suggesting that variability in neuromorphometry among older adults at least partially accounts for age-associated variability in cognition. For example, we recently showed that relative frontal lobe white matter volume mediated the association between age and performance on tasks of memory and executive functions (Brickman et al., 2006). Furthermore, using a multivariate approach, the degree to which older adults manifested a pattern of age-associated density loss was associated with poorer performance on tasks of memory and executive abilities (Brickman et al., 2007a; Brickman et al., 2007b).
In addition to frontal white matter volumes, the severity of WMH is associated with poorer performance in age-sensitive domains, including executive functions, episodic memory, and slowed processing speed, among older adults (Gunning-Dixon and Raz, 2000). The contribution of WMH to age-related declines in executive skills may be independent from that of the prefrontal volume (Gunning-Dixon and Raz, 2003). Longitudinal studies also support a role of WMH in age-related declines in executive skills (Kramer et al., 2007; Cook et al., 2004), working memory (Raz et al., 2007), and fluid intelligence (Raz et al., 2007).
The relationship of microstructural aging to age-related cognitive decline
O’Sullivan and colleagues (2001) reported early evidence that DTI indices are related to cognitive performance in healthy older adults. In particular, lower attentional set shifting scores correlated with greater diffusivity in a frontal ROI, whereas lower verbal fluency scores correlated with lower FA in a middle white matter ROI. The authors interpreted these relationships between DTI and attention and executive performance as evidence for cortical “disconnection” contributing to age-related cognitive declines.
Subsequent studies have provided additional evidence for the contribution of microstructural white matter reductions to select deficits in working memory and executive skills. Voxelwise analysis of FA and two measures of attention/executive skills in a sample of adults ranging in age from 20 to 73 years, detected a relationship between FA and performance on a task reliant on planning and response speed in extensive frontal, parietal, and thalamic regions, whereas no relationship was detected between performance on the attention switching task and FA (Grieve et al., 2007). In an examination of the relationship between FA in specific ROIs (anterior, middle, and posterior white matter of centrum semiovale) and executive skills, working memory, and processing speed, FA was correlated with the working memory domain only, irrespective of ROI (Charlton et al., 2006). Furthermore, in a subsample of individuals for whom MRS data were available, N-acetly-aspartate correlated with FA suggesting age-related FA reductions may be mediated by axonal loss (Charlton et al., 2006).
Regarding information processing speed, Madden and colleagues (2004) observed that higher FA in the anterior limb of the internal capsule was associated with faster response times in the older adults, whereas higher FA in the splenium of the corpus callosum was associated with faster reaction times in the younger adults only. Furthermore, results from another study suggested that age-related reductions in FA in the pericallosal frontal region and in the genu of the corpus callosum, but not in other regions, mediate the relationship between processing speed and episodic retrieval (Bucur et al., 2007). Thus, these findings provide preliminary evidence that the relationship between FA and visual attention/information processing speed may differ between young and older adults and that white matter integrity in prefrontal regions may be one mechanism underlying the relationship between age-associated individual differences in perceptual speed and episodic memory retrieval.
Overall, results from studies examining the relationship between DTI measures and cognitive performance provide preliminary support for the idea that loss of microstuctural white matter integrity may contribute to poorer performance in age sensitive domains including executive skills, working memory, processing speed, and episodic memory.
Conclusions and Future Directions
As with most features of normal aging, observations from structural MRI aging studies are notable for increased variability and individual differences with advancing age. However, a general pattern of age-related preservation and decline has emerged with evidence that the prefrontal white matter is most susceptible to the influence of age. Studies that combine MRI with cognitive measures suggest that such age-related reductions in white matter integrity may produce a disconnection state that underlies some of the age-related performance declines on tasks of information processing speed, episodic memory, and executive functions. Furthermore, a disconnection state likely predisposes some individuals to the presentation and/or exacerbation of psychiatric illnesses (e.g., geriatric depression) that either appear or are exacerbated in late life.
We are in the early stages of understanding how genes contribute to the individual differences in white matter aging. For example, in a family-based, healthy sample from the Framingham Heart Study, heritability of WMH increased with age (Atwood et al., 2004), and there is evidence showing that a gene influencing WMH is linked to chromosome 4 (DeStefano et al., 2006; Seshadri et al., 2007). Regarding the microstructure, DTI maps of genu FA and splenium FA show higher concordance in elderly MZ twins compared to elderly DZ twins, suggesting that there are regulated genetic effects on white matter microstructure that are quantifiable in late life (Pfefferbaum et al., 2001).
While emerging evidence that use of antihypertensive medications and hormone replacement therapy appear to slow select aspects of gray matter aging, little data exist of interventions that protect against white matter aging (Erickson et al, 2005; Raz et al., 2004; Raz et al., 2006; Raz et al., 2007). Preliminary findings suggest that cardiovascular fitness may have a significant influence on cerebral aging. Cardiovascular fitness has been associated with increased density in areas susceptible to aging, with the most significant effect in the anterior white matter tracts and the transverse tracts running between the frontal and posterior parietal lobes (Colcombe et al., 2003). Additionally, a 6-month randomized clinical trial of older community-dwelling adults showed that significant increases in both gray matter and white matter volumes were associated with the aerobic fitness training group but not with the nonaerobic control group (Colcombe et al., 2006). Future research should identify interventions that influence the course of white matter aging.
Acknowledgments
Supported by grants K23 MH074818 (FGD) K23 AG029949 (AMB) R01 NIRG-05-14586 (AMB), MH65653 (GSA), P30 MH68638 (GSA)
Footnotes
Conflict of Interest: None
Disclosures: Dr. Alexopoulos has received research grants by Forest Pharmaceuticals, Inc. and Cephalon and consults for Forest Pharmaceuticals, Sanofi-Aventis, and Novartis. He has given lectures supported by Forest, Cephalon, Bristol Meyers, Janssen, Glaxo, Pfizer and Lilly. Dr. Brickman has received compensation from ePharmaSolutions and ProPhase Training Group. Drs. Gunning-Dixon and Ms. Cheng have nothing to disclose.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Zaidel E. Morphometry of the Sylvian fissure and the corpus callosum, with emphasis on sex differences. Brain. 1992;115:1521–41. doi: 10.1093/brain/115.5.1521. [DOI] [PubMed] [Google Scholar]
- Albert M. Neuropsychological and neurophysiological changes in healthy adult humans across the age range. Neurobiol Aging. 1993;14:623–5. doi: 10.1016/0197-4580(93)90049-h. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–60. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magn Reson Imaging. 2007;25:154–67. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Armstrong CL, Traipe E, Hunter JV, Haselgrove JC, Ledakis GE, Tallent EM, Shera D, van Buchem MA. Age-related, regional, hemispheric, and medial-lateral differences in myelin integrity in vivo in the normal adult brain. Ajnr: American Journal of Neuroradiology. 2004;25:977–84. [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rogers J, Shaver RD, Dinan TG, Moss DE. Age-impaired impulse flow from nucleus basalis to cortex. Nature. 1985;318:462–4. doi: 10.1038/318462a0. [DOI] [PubMed] [Google Scholar]
- Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D’Agostino RB, DeCarli C. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35:1609–13. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25:5, 18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–5. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60:393–8. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25:843–51. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Ramos MA, Scarmeas N, Stern Y. Multivariate-defined spatial networks of age-associated atrophy. Poster Presentation at the annual meeting of the International Society of Magnetic Resonance in Medicine; Berlin. April.2007a. [Google Scholar]
- Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging. 2007b;28:284–95. doi: 10.1016/j.neurobiolaging.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Paul RH, Cohen RA, Williams LM, MacGregor KL, Jefferson AL, Tate DF, Gunstad J, Gordon E. Category and letter verbal fluency across the adult lifespan: relationship to EEG theta power. Arch Clin Neuropsychol. 2005;20:561–73. doi: 10.1016/j.acn.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain Morphology in elderly African Americans, Caribbean Hispanics, and Caucasians from northern Manhattan. Archives of Neurology. doi: 10.1001/archneur.65.8.1053. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Williams LM, Clark CR, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60:444–53. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2003;58:176–80. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2006;61:1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF, Morgan ML, Dunkin JJ, Witte E, David S, Mickes L, O’Hara R, Simon S, Lufkin R, Abrams M, Rosenberg S. Longitudinal progression of subclinical structural brain disease in normal aging. American Journal of Geriatric Psychiatry. 2004;12:190–200. [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994;14:4748–55. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, van Gijn J. [Cerebral white matter lesions in the elderly: vascular risk factors and cognitive consequences].[see comment] Nederlands Tijdschrift voor Geneeskunde. 2001;145:2067–71. [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Gillette JA, Haxby JV, Teichberg D, Schapiro MB, Horwitz B. Lack of age-related differences in temporal lobe volume of very healthy adults. AJNR Am J Neuroradiol. 1994;15:689–96. [PMC free article] [PubMed] [Google Scholar]
- DeStefano AL, Atwood LD, Massaro JM, Heard-Costa N, Beiser A, Au R, Wolf PA, DeCarli C. Genome-wide scan for white matter hyperintensity: the Framingham Heart Study. Stroke. 2006;37:77–81. doi: 10.1161/01.STR.0000196987.68770.b3. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Raz N, Korol DL, Scalf P, Webb A, et al. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiology of Aging. 2005;26:1205–1213. doi: 10.1016/j.neurobiolaging.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Ropele S, Enzinger C, Gorani F, Seewann A, Petrovic K, Schmidt R. MTI of white matter hyperintensities. Brain. 2005;128:2926–32. doi: 10.1093/brain/awh567. [DOI] [PubMed] [Google Scholar]
- Fernando MS, O’Brien JT, Perry RH, English P, Forster G, McMeekin W, Slade JY, Golkhar A, Matthews FE, Barber R, Kalaria RN, Ince PG. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol Appl Neurobiol. 2004;30:385–95. doi: 10.1111/j.1365-2990.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol. 2007;254:713–21. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part II: quantitative magnetization transfer ratio histogram analysis. Ajnr: American Journal of Neuroradiology. 2002;23:1334–41. [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology. 2007;21:657–73. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. Ajnr: American Journal of Neuroradiology. 2007;28:226–35. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–32. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–41. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–8. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential Vulnerability of Anterior White Matter in Nondemented Aging with Minimal Acceleration in Dementia of the Alzheimer Type: Evidence from Diffusion Tensor Imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Horn JL. Intellectual ability concepts. In: Sternberg RJ, editor. Advances in psychology of human intelligence. Erlbaum: Hillsdale, NJ; 1986. [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating Imaging Indices of White Matter Integrity and Volume in Healthy Older Adults. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Kaye JA, Carper RA. Atrophy of the corpus callosum in Alzheimer’s disease versus healthy aging.[see comment] Journal of the American Geriatrics Society. 1996;44:798–803. doi: 10.1111/j.1532-5415.1996.tb03736.x. [DOI] [PubMed] [Google Scholar]
- Jeeves MA, Moes P. Interhemispheric transfer time differences related to aging and gender. Neuropsychologia. 1996;34:627–36. doi: 10.1016/0028-3932(95)00157-3. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biol Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–94. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuroanatomical and neuropathological changes during aging and in dementia. In: Albert ML, Knoepfel EJE, editors. Clinical neurology of aging. Oxford University Press; New York: 1994. pp. 3–67. [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–8. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–81. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Malloy P, Correia S, Stebbins G, Laidlaw DH. Neuroimaging of white matter in aging and dementia. Clinical Neuropsychologist. 2007;21:73–109. doi: 10.1080/13854040500263583. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Gardin JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–27. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age.[see comment] Journal of Comparative Neurology. 2003;462:144–52. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Dev Psychol. 2002;38:115–42. [PubMed] [Google Scholar]
- Meier-Ruge W, Bruder A, Theodore D. Histochemical and morphometric investigation of the pathogenesis of acute brain infarction in primates. Acta Histochem Suppl. 1992;42:59–70. [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–8. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Bryan RN, Conturo TE, Soher BJ, Preziosi TJ, Barker PB. Proton magnetic resonance spectroscopy and gadolinium-DTPA perfusion imaging of asymptomatic MRI white matter lesions. Magn Reson Med. 1995;33:61–8. doi: 10.1002/mrm.1910330109. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–52. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. Journal of Comparative Neurology. 1997;384:312–20. [PubMed] [Google Scholar]
- Peters A. Structural changes in the normally aging cerebral cortex of primates. Prog Brain Res. 2002;136:455–65. doi: 10.1016/s0079-6123(02)36038-2. [DOI] [PubMed] [Google Scholar]
- Peters A, Moss MB, Sethares C. Effects of aging on myelinated nerve fibers in monkey primary visual cortex. J Comp Neurol. 2000;419:364–76. doi: 10.1002/(sici)1096-9861(20000410)419:3<364::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL. In aging, is it gray or white? J Comp Neurol. 2003;462:139–43. doi: 10.1002/cne.10715. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861–74. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–91. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J Comp Neurol. 2001;435:241–8. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–9. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine. 2003;49:953–61. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Genetic regulation of regional microstructure of the corpus callosum in late life. Neuroreport. 2001;12:1677–81. doi: 10.1097/00001756-200106130-00032. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–48. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–8. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–57. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68:214–22. doi: 10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56:338–44. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Seshadri S, DeStefano AL, Au R, Massaro JM, Beiser AS, Kelly-Hayes M, Kase CS, D’Agostino RB, Sr, Decarli C, Atwood LD, Wolf PA. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Snowdon D, Markesbery WR. Periventricular white matter hyperintensities on MRI: correlation with neuropathologic findings. J Neuroimaging. 2000;10:13–6. doi: 10.1111/jon200010113. [DOI] [PubMed] [Google Scholar]
- Spilt A, Geeraedts T, de Craen AJ, Westendorp RG, Blauw GJ, van Buchem MA. Age-related changes in normal-appearing brain tissue and white matter hyperintensities: more of the same or something else? AJNR Am J Neuroradiol. 2005;26:725–9. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16:1030–9. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25:185–92. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Tanabe JL, Ezekiel F, Jagust WJ, Schuff N, Fein G. Volumetric method for evaluating magnetization transfer ratio of tissue categories: application to areas of white matter signal hyperintensity in the elderly. Radiology. 1997;204:570–5. doi: 10.1148/radiology.204.2.9240555. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–69. [PubMed] [Google Scholar]
- Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience & Biobehavioral Reviews. 2006;30:762–74. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi MC, Liu RH, Engelhardt JK, Morales FR, Chase MH. Changes in the axonal conduction velocity of pyramidal tract neurons in the aged cat. Neuroscience. 1999;92:219–25. doi: 10.1016/s0306-4522(98)00754-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry. 2006;14:823–33. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]