Abstract
OBJECTIVES:
To determine whether, in well-functioning older adults, a lower score on the Digit Symbol Substitution Test (DSST) and slower gait are associated with greater risk of mortality and of developing incident disability independent of other risk factors, including brain structural abnormalities (white matter hyperintensities, brain infarcts, ventricular enlargement) and whether the combination of varying levels of DSST score and gait speed are associated with a greater risk of mortality and disability than low DSST or slow gait alone.
DESIGN:
Observational cohort study.
SETTING:
Community.
PARTICIPANTS:
Three thousand one hundred fifty-six (43% men, 29% black, mean age 70.4) participants in the Cardiovascular Health Study (CHS), free from stroke and physical disability and with a modified Mini-Mental State Examination (3MS) score of 80 or higher.
MEASUREMENTS:
Total mortality and incident disability (self-report of any difficulty performing one or more of the six activities of daily living) ascertained over a median follow-up time of 8.4 years.
RESULTS:
By the end of follow-up, 704 participants had died and 1,096 had incident disability. In Cox proportional hazards models adjusted for age, sex, race, education, cardiovascular disease, and brain magnetic resonance imaging abnormalities, lower DSST score and slower gait remained significantly associated with greater risk of mortality and of incident disability. Mortality rates were higher in those who had both low DSST score (<27 points) and slow gait (speed <1.0 m/s) than in those who had only low DSST score, only slow gait, or neither (rates per 1,000 person years (p-y): 61.2, 42.8, 20.8, and 16.3, respectively). A similar risk gradient was observed for incident disability (82.0, 57.9, 47.9, and 36.0/1,000 p-y, respectively).
CONCLUSION:
In well-functioning older adults, low DSST score and slow gait, alone or in combination, could be risk factors for mortality and for developing disability, independent of other risk factors, including measures of brain integrity.
Keywords: digit symbol substitution test score, gait speed, mortality and disability
It has been consistently shown that clinical measures of cognitive1-6 and physical function,7-9 as well as imaging markers of underlying structural disease in the brain,10-12 are predictors of incident disability and mortality in older adults. In particular, lower score on a simple cognitive test, the Digit Symbol Substitution Test (DSST), and slower gait10,13-16 are associated with greater risk of mortality and physical disability.1,4,9,17-20 However, previous studies had relatively short follow-up times or examined patient populations who already had some degree of disability at baseline and did not examine whether the inclusion of lower DSST and slower gait improve the prediction for disability and mortality or whether the combined effect of these two measures affects risk of mortality and of incident disability more than impairment in either of these two tests alone. Furthermore, previous studies did not ascertain whether these relationships remain independent of underlying brain structural disease, such as white matter disease, ventricular enlargement, and brain infarcts. Because of their association with cognitive21-24 and physical impairment,12,25,26 as well as with mortality and disability, white matter disease, ventricular enlargement, and brain infarcts could attenuate the associations between DSST score and slower gait and mortality and disability. These are important questions because of the sheer increase in the number of older adults who remain free from disability, stroke, or dementia even late in life. Answering these questions could increase understanding of whether lower DSST score and slower gait could be risk factors for mortality and future disability in older adults who may not have manifest clinical diseases at the time of examination.
This study examined more than 8 years of longitudinal data on total mortality and disability for 3,156 participants of the Cardiovascular Health Study (CHS) cohort who were free from stroke and physical disability and had a modified Mini-Mental State Examination (3MS) score of 80 or higher at baseline.27 DSST score, gait speed, and prevalence of risk factors for mortality and physical disability, including brain magnetic resonance imaging (MRI) data, were obtained at baseline. The hypothesis that baseline lower DSST score and slower gait would be associated with greater rate of mortality and of incident disability, independent of other risk factors, including white matter disease, ventricular enlargement, and brain infarcts, and that the combination of lower DSST scores and slower gait contributed to a greater risk of mortality and disability than DSST score alone was tested.
METHODS
The CHS is a population-based study of risk factors for cardiovascular disease in older adults sponsored by the National Heart, Lung, and Blood Institute.28 The original cohort of 5,201 participants was enrolled in 1989/90, and a second cohort of 687 predominantly black participants was enrolled in 1992/93. The combined cohort of 5,888 individuals was 58.0% female and 16.0% black. The average age at enrollment was 72.8 ± 5.6. The baseline for this analysis was the 1992/93 visit. By this time, 335 participants had died, yielding a sample of 5,553 available for analysis. From this sample, the participants who, at the time of the 1992/93 clinic visit, had a history of clinically evident stroke (n=377), self-reported physical functional disability (any difficulty with ≥1 of 6 activities of daily living (ADLs), n=517 or difficulty walking half a mile, n=652), and 3MS score lower than 80 (n=320) were excluded. Those missing the clinic visit in 1992/93 accounted for the exclusion of another 531, yielding a final sample size of 3,156 participants for this analysis. Of these, 2,436 also received a brain MRI during 1992 to 1994. All subjects provided written informed consent. The University of Pittsburgh institutional review board approved the protocol.
The DSST
The DSST is a pencil and paper test of psychomotor performance29 in which the subject is given a key grid of numbers and matching symbols and a test section with numbers and empty boxes. The test consists of filling as many empty boxes as possible with a symbol matching each number. Time is 90 seconds, and the score is the number of correct number–symbol matches. The strategy to solve the DSST consists of sequential encoding and retrieval of numbers and matching symbols. First, the number in the test section is encoded in short-term memory and temporarily stored. Then, while the subject visually scans the key grid to search for the number–symbol match, the number in the short-term memory is repeatedly retrieved and compared with the numbers of the key grid. Once the number is recognized, the matching symbol is encoded in short-term memory. Finally, the attention is turned toward the test section again, and the symbol is retrieved from short-term memory and copied below the number. Incidental memory, perceptual organization, visuomotor coordination, and selective attention are important factors that determine the final score.29 The ability to filter out irrelevant information (e.g., symbols that may look alike) also influences performance. This test has high test–retest reliability.30 The DSST was used as a continuous variable and as quintiles computed from the parent population of 5,888. Quintiles were computed from the parent population to obtain cutoff values more representative of a population of this age, and because there is not an agreed-upon threshold for normality for the DSST score.
Gait Speed
Gait speed is a reliable and valid measure of motor performance,31 and it was measured as the time needed to walk a 15-foot course at usual pace after beginning from a standstill position recorded using a stopwatch (timed to 0.1 seconds). Gait speed14,32 was used as a continuous variable, as quartiles (<0.76 m/s, 0.76–0.90 m/s, 0.91–1.13 m/s, and ≥1.14 m/s) and as dichotomous (<1.0 m/s vs >1.0 m/s). Results are shown for gait speed as dichotomous for simplicity, and because gait speed less than 1.0 m/s identifies persons who are at high risk of developing health-related adverse events13-15 and older adults who are more likely to have underlying brain MRI abnormalities.
Outcomes
Individuals were seen annually in the clinic or at home through 1998/99 and received semiannual telephone calls. Participants were queried twice per year for new diagnoses, hospitalizations, and procedures. After 1998/99, only telephone follow-up was continued. Mortality outcome included all-cause mortality ascertained according to adjudicated events occurring through June 30, 2002. To enhance the complete ascertainment of events, hospitalization data from Health Care Finance Administration records were also obtained for missing hospitalizations.33 A modified version of the Health Interview Survey Supplement on Aging Questionnaire34 was used at the clinic visit and at 6-month intervals through 1998/99 and through 6-month phone follow-up afterward, to ask “Do you have difficulty or are you unable to … ?” Incident disability was defined as the self-reported difficulty or inability to perform at least one of the six ADLs without assistance: bathing, dressing, eating, using the toilet, walking around the home, and getting out of a bed or chair. The self-report of any difficulty performing ADLs without assistance was chosen as an indicator of disability, because it is a simple, valid, and reliable measure.14,35-37
Brain MRI Markers
Standardized sagittal T1-weighted spin-echo images, axial spin density/T2-weighted images, and T1-weighted images were acquired using a 1.5 T Signa scanner (General Electric Medical Systems, Milwaukee, WI) with high-performance gradients (4 G/cm and 150 T/m-s). A volumetric spoiled gradient recalled acquisition sequence with parameters optimized for maximal contrast in gray matter, white matter, and cerebrospinal fluid was acquired in the coronal plane (TE/TR=5/25, flip angle=40°, NEX=1, slice thickness=5 mm/0 mm interslice). A neuroradiologist trained in a standardized protocol38 interpreted scanned data at a central MRI reading center according to an atlas of predefined visual standards,23,39 which included white matter disease (presence of periventricular and subcortical signal abnormalities, graded from 0 to 9), brain atrophy (ventricular size, graded from 0 to 9), and infarct-like lesions (any lesion >3 mm). Because the distributions of MRI variables were highly skewed, modeling approaches using the continuous variables showed that there were too few cases with high measures of either score to consider the linear model reliable. Cutpoints of white matter hyperintensities of 3 and of ventricular grade of 4 were chosen based on previous studies conducted on the CHS cohort.23
Covariates and Potential Confounders
In addition to age, race, sex, and education, other potential confounders measured at the 1992/93 visit included risk factors and conditions that were associated with the predictors (DSST and gait speed) and the outcomes (death and incident disability) but were not an intermediate step in the causal pathway. The models controlled for prevalence of cardiovascular disease, hypertension, and diabetes mellitus (see definitions below) because of their known association with brain structural changes, mortality, and disability. A composite measure of subclinical cardiovascular disease was any one of the following: major electrocardiogram abnormalities based on the Minnesota Code, a ratio of ankle–arm systolic blood pressure of 0.9 or less, more than 25% stenosis of the internal carotid artery based on ultrasound, intimal medial thickness of the internal or common carotid artery greater than the 80th percentile of the CHS distribution, positive Rose angina pectoris, or claudication without clinical history of angina pectoris. Clinical cardiovascular disease included baseline history of myocardial infarction, congestive heart failure, or atrial fibrillation; presence of a pacemaker; history of coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty; history of angina pectoris or use of nitroglycerine; or history of claudication, peripheral vascular surgery, stroke, transient ischemic attack, or carotid artery surgery. Hypertension was defined as a previous diagnosis of hypertension, taking hypertensive medication, or having a current systolic blood pressure of 140 mmHg or higher or a diastolic blood pressure of 90 mmHg or higher. Persons were considered to have diabetes mallitus if they had a validated medical diagnosis or a fasting glucose level of 126 mg/dL or higher. Because of its associations with mortality and disability, grip strength (the average strength in kg from two handheld dynamometer trials using the dominant hand) was also controlled for.40,41 Difficulties with at least one of the instrumental activities of daily living (IADLs), smoking, and physical activity were also considered as potential confounders.
Statistical Analysis
Analysis of variance and partial correlation coefficients for continuous variables and chi-square tests for categorical variables were used to compare differences in population characteristics of those excluded with those included and across the quintiles of DSST. For the analysis of mortality risk, the occurrence of mortality was the end point. Participants who were lost to follow-up were censored at the time of death. For the analysis of disability risk, the first occurrence of self-report of any difficulty with at least one ADL was the endpoint. Participants who died or were lost to follow-up were censored at the time of death or date of last information. Kaplan-Meier survival risk assessments were used to plot cumulative survival function. Crude incidence rates of events were computed per 1,000 person years (p-y) by dividing the number of events by the total p-y at risk.
The predictive value of DSST and gait speed was compared with the predictive value of the demographic variables using c-statistics (which are equivalent to areas under the receiver operating characteristic curves) of the models including these variables. The c-statistic represents the probability that a randomly selected participant with lower DSST has a greater predicted risk of dying (or of developing disability) than a randomly selected participant with a higher DSST. The Hosmer-Lemeshow statistic was used to examine the models' goodness of fit. The assumption of proportional hazards was tested using the log minus log test of proportionality, in which the cumulative survival estimate after the ln(-ln) transformation is applied to the estimate. Cox proportional models were generated in stages. First, the primary hypothesis (DSST and gait speed are associated with the outcomes independently of other risk factors and of brain MRI variables) were tested as follows: (A) DSST plus demographic factors; (B) DSST and gait speed plus demographic factors; (C) models from B further adjusted for subclinical disease, myocardial infarction, congestive heart failure, hypertension, diabetes mellitus, physical activity and 3MS score; and (D) a final model evaluating risk factors from the above plus brain MRI variables. The statistical approach to addressing potential modification of the association between DSST (or gait speed) and the outcomes in steps C and D was to examine the size of the change in hazard ratio of the main independent variable after forcing in the potential confounders. A significant effect modification was defined as a change (or attenuation) in regression coefficients of more than 10%.42 The analyses were conducted on 3,156 participants for all analyses except for the analysis to test the modifying effect of brain MRIs, which was restricted to 2,436 participants who had received a brain MRI. Models were run using DSST and gait speed as continuous measures. The fully adjusted model was then repeated using quintiled DSST to examine whether the relationship between DSST and disability and mortality was linear and to see whether this association would be stronger for specific DSST score values. Second, to examine the hypothesis of combined effect of lower DSST score and slower gait on mortality and disability risk, the associations between quintiled DSST and mortality and disability were examined after stratifying for gait speed less than 1.0 m/s. Thus, the worst level of function would be for those in the lowest quintile of DSST and with gait speed less than 1.0 m/s, whereas the best would be for those in the highest quintile of DSST and with gait speed of 1.0 m/s or greater. In sensitivity analyses, these models were repeated using gait speed as quartiles, in the whole cohort, and using quintiles from the population of 3,156. All analyses were done using SPSS version 13 (SPSS Inc., Chicago, IL).
RESULTS
The subjects who were included were younger and had a lower prevalence of IADL difficulty and of cardiovascular disease and conditions and higher cognitive and mobility function than those who were excluded from the analysis (Table 1). Of subjects who were included, those who had a higher DSST score were younger, were more likely to have more years of education and a lower prevalence of cardiovascular risk factors and diseases, were more physically active, had a greater 3MS score, had a faster gait speed, and had a lower prevalence of markers of brain structural changes as seen on brain MRI. Results from Spearman correlation coefficients between DSST score and the characteristics shown in Table 1 were consistent with the results from analysis of variance and chi-square tests. Age-adjusted associations between DSST and population characteristics (Table 1) had P-values <.001, except physical activity (P=.07) and white matter hyperintensities (P=.02). The scores measured at two consecutive visits were high for DSST (Pearson correlation coefficient (r)=0.82, P<.001) and gait speed (r=0.77, P<.001). Gait speed and DSST were also significantly correlated with each other (r=0.21, P<.001).
Table 1.
General Characteristics of the Population at Baseline (N=3,156)
| Characteristic | Excluded (n=2,397) |
Included (n=3,156) |
|---|---|---|
| Age, mean ± SD | 76.8 ± 6.2 | 74.0 ± 4.6 |
| Women, n (%) | 1,472 (61.4) | 1,794 (56.8) |
| Black, n (%) | 413 (21.4) | 393 (12.4) |
| Education, mean ± SD | 12.5 ± 4.8 | 14.7 ± 4.4 |
| Subclinical cardiovascular diseases, n (%) |
1,424 (81.6) | 2,018 (63.9) |
| Congestive heart disease, n (%) | 701 (29.2) | 565 (17.9) |
| Myocardial infarction, n (%) | 340 (14.2) | 262 (8.3) |
| Hypertension, n (%) | 1,259 (66.7) | 1,657 (52.6) |
| Diabetes, n (%) | 332 (16.5) | 293 (9.3) |
| Physical activity, kcal, median ± SD |
490.0 ± 13,140.0 | 1,095.0 ± 13,965.0 |
| Modified Mini-Mental State Examination score, mean ± SD |
86.8 ± 12.4 | 93.4 ± 5.3 |
| Digit Symbol Substitution Test score, mean ± SD |
31.4 ± 14.0 | 42.1 ± 12.3 |
| Gait speed, second, mean ± SD* | 0.75 ± 0.2 | 0.96 ± 0.2 |
| Difficulty with ≥1 instrumental activity of daily living, n (%) |
954 (34.9) | 399 (12.6) |
| Grip strength, kg, mean ± SD | 25.6 ± 9.6 | 28.9 ± 10.0 |
| Markers of brain structural changes, n (%)† | ||
| Subclinical brain infarcts | 184 (33.6) | 619 (25.5) |
| Ventricular enlargement ≥Grade 4 | 512 (42.0) | 370 (15.3) |
| White matter hyperintensities ≥Grade 3 |
167 (30.3) | 283 (11.6) |
Note: All comparisons between included and excluded had P-values <.001, except sex (P=.10).
Twenty-six participants were missing values for gait speed.
Computed for those who received a brain magnetic resonance imaging scan in 1992/93 (n=3,660), for those included (n=2,436), and those excluded (n=1,224).
SD=standard deviation.
By the end of follow-up (median of 8.4 years), 704 participants had died (mortality rate of 31.8/1,000 p-y), with an overall cumulative mortality rate of 22.3%. This was similar to the mortality patterns in the whole CHS cohort (46.3/1,000 p-y and a 31.2% rate) and consistent with previous mortality rates obtained in this cohort.16 Incident disability occurred in 1,096 (35%) of participants, which was also consistent with a previous study.12
DSST score and gait speed were inversely associated with risk of mortality and incident disability (Table 2), and the risk of mortality and of developing disabilities was lower for higher DSST scores and faster gait. The point estimates for DSST and gait speed predicting mortality remained significant when they were both present in the model (Table 2, Model 2 vs Model 1). Point estimates for DSST and gait speed remained significant and minimally changed after further adjustment for baseline cardiovascular risk factors and diseases, physical activity, and 3MS score (Table 2, Model 3 vs 2). The adjustment for brain MRI variables did not substantially change these results (Table 2, Model 4 vs Model 3). Results were similar when each of the three MRI markers entered the model separately. In the fully adjusted model, other significant and independent predictors of mortality were older age, male sex, presence of at least one subclinical disease, prevalence of myocardial infarction, prevalence of diabetes mellitus, and presence of subclinical brain infarcts. After adjusting for all confounders, the other significant and independent predictors of incident disability were older age, female sex, prevalence of diabetes mellitus, grip strength, white matter hyperintensities, and ventricular enlargement. The interaction terms of DSST score by these risk factors were not significant (P>.50). Further adjustment for IADLs did not change the results.
Table 2.
Risk Estimates Associated with Digit Symbol Substitution Test (DSST) Score and Gait Speed (m/s)
| Standardized Hazard Ratio (95% Confidence Interval) |
|||||||
|---|---|---|---|---|---|---|---|
| Event | Events n |
Events per 1,000 Person-Years |
Predictor | Model 1:(DSST+Age, Sex, Race, Education) |
Model 2: (Model 1+Gait Speed) |
Model 3: (Model 2+Confounders†) |
Model 4: (Model 3+Brain Magnetic Resonance Imaging Variables‡) |
| Mortality | 704 | 31.8 | DSST | 0.77(0.68–0.87)** | 0.79(0.69–0.89)** | 0.78(0.69–0.89)* | 0.79(0.69–0.90)* |
| Gait speed | 0.87 (0.78–0.98)** | 0.89 (0.80–0.99)* | 0.90(0.80–1.00)* | ||||
| Incident disability | 1,096 | 35.0 | DSST | 0.75 (0.68–0.82)*** | 0.77 (0.70–0.85)*** | 0.76 (0.68–0.84)*** | 0.76 (0.69–0.84)*** |
| Gait speed | 0.88 (0.80–0.96)*** | 0.88 (0.80–0.96)* | 0.88 (0.81–0.97)** | ||||
Higher score and speed indicate better performance. Follow-up time from 1992 to 2002.
Confounders: subclinical disease, congestive heart failure, hypertension, diabetes mellitus, myocardial infarction, physical activity, modified Mini-Mental State Examination score, and grip strength.
Includes presence of at least one subclinical brain infarct, ventricular enlargement ≥Grade 4 and white matter hyperintensities ≥Grade 3.
P< .001
.005
.03.
Models with DSSTand demographics had a significantly greater predictive value than models with demographics only for mortality (c-statistic=0.68, 95% confidence interval (CI)=0.65–0.71; c-statistic=0.70, 95% CI=0.68–0.72, difference significant at P=.002). Similar results were observed for the predictive value of models with demographics only (c-statistic=0.61, 95% CI=0.59–0.64) and demographics plus DSST (c-statistic=0.62, 95% CI=0.59–0.65, difference significant at P=.002). Similar results were observed for gait speed (not shown).
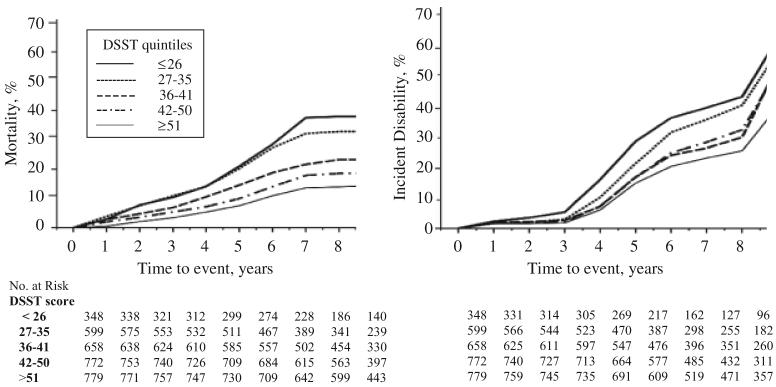
To better appreciate the increasing gradient of event risk for decreasing DSST score, unadjusted Kaplan-Meier estimates of death and of incident disability were obtained after stratifying according to DSST quintile (Figure 1). Trends over time of event rates were similar for each quint-ile of DSST, with a steep increase in mortality and disability rates toward the last years of follow-up due to the smaller number of participants at risk and their increasing age. Crude incident rates of disability for those in the highest quintile of DSST score were almost half as high as the incidence rates of those in the first quintile of DSST scores. Results were similar when using quintiles from the analytical sample.
Figure 1.
Kaplan-Meier plots of mortality and of incident disability event rates according to quintiles of Digit Symbol Substitution Test (DSST) score. The first quintile of DSST (<26 points) indicates worse performance, and the fifth quintile (>51 points) indicates best performance.
In analyses to examine the association between varying levels of DSST score and gait speed and mortality and disability risk (Table 3), lower DSST score remained associated with higher mortality rates after stratifying for gait speed of 1.0 m/s or greater (test of trend: P<.001 in both strata). Similar results were obtained for incident disability (test of trend: P<.001). Additionally, within each quintile of DSST score, participants with slower gait had higher mortality rates than those with faster gait, and this was more evident for the lowest quintile (61.2/1,000 p-y vs 42.8/1,000 p-y) than for the highest quintiles of DSST (20.8/1,000 p-y vs 16.3/1,000 p-y). The unadjusted event rates were consistent with the point estimates from multivariate Cox models adjusted for demographics, health-related risk factors, and brain MRI variables (Table 3). Subjects in the lowest quint-ile of DSST who also had a gait speed of 1.0 m/s or greater had a 48% greater risk of mortality than those in the first quintile (reference group). In contrast, for subjects who were in the lowest DSST quintile and had gait speed less than 1.0 m/s, the risk of mortality was up to 113% greater than in the reference group. Similar gradients were observed for incident disability risks. Those in the lowest quintile of DSST had a 55% greater risk of mortality than those in the first quintile (reference group) if they had a gait speed of 1.0 m/s or greater, whereas the risk was 151% greater than in the referent group if they had gait speed less than 1.0 m/s. Similar trends were obtained when gait speed was used as quartiles. The interaction term of DSST score by gait speed was not significant in models predicting mortality or in models predicting incident disability (P>.2).
Table 3.
Risk Estimates According to Quintiles of Digit Symbol Substitution Test (DSST) Score Stratified According to Gait Speed
| Gait Speed, m/s |
||||||||
|---|---|---|---|---|---|---|---|---|
| ≥1.0 |
<1.0 |
|||||||
| DSST Score Quintile |
Included (n=1,179) |
Events n |
Events per 1,000 Person-Years |
Hazard Ratio* (95% CI) |
Included (n=1,950) |
Events n |
Events per 1,000 Person-Years |
Hazard Ratio* (95% CI) |
| Mortality | ||||||||
| 1 (<27) | 80 | 23 | 42.8 | 1.48 (0.88–2.48) | 264 | 103 | 61.2 | 2.13 (1.00–4.51) |
| 2 (27–35) | 177 | 43 | 35.9 | 1.54 (1.00–2.30) | 419 | 144 | 54.4 | 1.75 (1.00–3.17) |
| 3 (36–41) | 225 | 48 | 31.2 | 1.04 (0.67–1.60) | 425 | 98 | 33.0 | 1.38 (0.82–2.33) |
| 4 (42–50) | 299 | 47 | 21.7 | 0.95 (0.62–1.46) | 468 | 89 | 27.1 | 1.13 (0.67–1.90) |
| 5 (>50) | 398 | 48 | 16.3 | 1.00 | 374 | 56 | 20.8 | 1.00 |
| Incident disability | ||||||||
| 1 (<27) | 80 | 29 | 57.9 | 1.55 (0.84–2.84) | 264 | 120 | 82.0 | 2.51 (1.65–3.80) |
| 2 (27–35) | 177 | 53 | 47.9 | 1.45 (0.91–2.31) | 419 | 175 | 73.6 | 2.08 (1.46–2.97) |
| 3 (36–41) | 225 | 59 | 41.3 | 1.24 (0.82–1.86) | 425 | 162 | 60.8 | 1.70 (1.22–2.37) |
| 4 (42–50) | 299 | 86 | 43.0 | 1.11 (0.77–1.60) | 468 | 186 | 63.3 | 1.65 (1.20–2.27) |
| 5 (>50) | 398 | 99 | 36.0 | 1.00 | 374 | 115 | 47.9 | 1.00 |
Note: Follow-up time from 1992 to 2002. Event rates and point estimates are shown after stratification for gait speed ≥1.0 m/s. The first quintile of DSST (worst performance) was used as the reference group.
Adjusted for age, sex, race, education, gait speed, subclinical disease, congestive heart failure, hypertension, diabetes mellitus, myocardial infarction, physical activity, Modified Mini-Mental State Examination score, presence of at least one subclinical brain infarct, ventricular enlargement ≥Grade 4, and white matter hyperintensities ≥Grade 3.
CI = confidence interval.
DISCUSSION
In this population of well-functioning community-dwelling elderly subjects free from disability and stroke and with a 3MS score of 80 or higher, DSST score and gait speed were associated with risk of mortality and incident disability over a median follow-up of 8 years, independent of each other and of other risk factors, including markers of subclinical and clinical vascular disease and also independent of white matter disease, ventricular enlargement, and brain infarcts. Adding DSST and gait speed resulted in better prediction for both outcomes than models with demographics only. Although the interaction between DSST and gait speed was not significant, these two simple tests in combination distinguished a wide range of mortality and incident disability risks. The co-occurrence of lower DSST and slower gait appear to be a marker for general physiological deterioration that is associated with adverse outcomes in addition to traditional measures of health status such as comorbid conditions and also after adjusting for brain MRI variables. Lower DSST score had a smaller effect on the outcomes when gait speed was 1.0 m/s or greater and a greater effect when gait speed was less than 1.0 m/s (Table 3), although this discrepancy was more evident for the lower than for the higher values of DSST, which may explain why the interaction term was not significant. Lower DSST score and slower gait may be useful longitudinal risk factors for greater mortality and disability risk for older adults without manifest clinical diseases. This is important, because the prevalence of low DSST (first quintile) was high (11%) even in this population of well-functioning older adults who were free from stroke and disability and had a 3MS score of 80 or higher.
The DSST score is a measure of executive cognitive function, working memory, processing speed, and visuo-spatial attention. In older adults, these domains all play a crucial role in the execution of motor tasks, including walking,12,43 and is has previously been shown25,44 that DSST score and gait speed are significantly associated with each other. Performing the DSST and walking may rely on similar or shared domains of brain functioning and underlying brain networks. Accordingly, impairment in these two tests may reflect similar brain structural changes, and it had been hypothesized that brain MRI markers of underlying disease would attenuate the relationships between lower DSST and slower gait and mortality and disability. White matter hyperintensities, ventricular enlargement, and brain infarcts modified these associations only minimally. Perhaps lower DSST score and slower gait reflect early focal changes affecting the white matter and the gray matter of specific executive function-related networks, whereas the diffuse markers used in this study, white matter hyperintensities, ventricular enlargement, and brain infarcts, are manifestations of nonspecific, age-related changes. Because of the low resolution of this imaging technique, particularly small lesions may have remained undetected. Future studies will need to address whether localization of damage in executive control function networks could affect walking and the relationship between such brain structural abnormalities and mortality and disability. Another question is whether these findings are specific for DSST or are more-generalized effects of attention and control processes. Recent studies2,3 on the relationship between other tests of executive control function and disability seem to suggest the latter.
Although this study focused on well-functioning community-dwelling elderly people, results were similar when subjects with stroke and with physical functional limitations were included in the cohort. Limits on the generalizability of these results to the common population of older adults remain. Strengths of this study included the length of follow-up, the sample size, the rich characterization of the health status of the participants and of many important risk factors for mortality and disability, and that DSST score and gait speed are reliable measures of function. Furthermore, analyses were repeated using continuous measures, to ensure adequate statistical power, and also using categorical classifications of DSST score and gait speed. Given the skewed distribution of the gait speed measures, a categorical classification provides a good approach for clinical classifications of individuals at risk for mortality and disability.
Further studies on the use of DSST, in combination with gait speed or alone, as a prognostic risk factor for mortality and physical disability in community-dwelling older adults who may be free from severe clinical diseases would be important because of the rapidly growing proportion of older adults who are at risk of developing these outcomes. Lower DSST score and slower gait speed may indicate early brain structural and functional changes that are amenable to treatment. Clinical trials have shown that prevention of cardiovascular risk factors contribute to delay the progression of white matter hyperintensities45 and that cardiovascular conditioning, environmental enrichment, and physical activity can also affect DSST score,46,47 as well as brain atrophy48 and the efficiency of neuronal activation and brain metabolism in areas that are involved in executive control function.49,50 Future studies on disability and mortality that include trajectories of DSST score and gait speed changes, as well as other measures of cognition and physical function, are warranted.
ACKNOWLEDGMENTS
Sponsor's Role: The organizations funding this study had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: CR: National Institute on Aging Grants 1 P30 AG024827, R03 AG025076-01A1, 1K23 AG028966-01, and R01 AG029232. The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this manuscript.
REFERENCES
- 1.Pavlik VN, de Moraes SA, Szklo M, et al. Relation between cognitive function and mortality in middle-aged adults: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;157:327–334. doi: 10.1093/aje/kwf209. [DOI] [PubMed] [Google Scholar]
- 2.Royall DR, Chiodo L, Polk M. An empiric approach to level of care determinations: The importance of executive measures. J Gerontol A Biol Sci Med Sci. 2005;60A:1059–1064. doi: 10.1093/gerona/60.8.1059. [DOI] [PubMed] [Google Scholar]
- 3.Royall DR, Palmer R, Chiodo L, et al. Declining executive control in normal aging predicts change in functional status: The Freedom House Study. J Am Geriatr Soc. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 4.Schupf N, Tang MX, Albert SM, et al. Decline in cognitive and functional skills increases mortality risk in nondemented elderly. Neurology. 2005;65:1218–1226. doi: 10.1212/01.wnl.0000180970.07386.cb. [DOI] [PubMed] [Google Scholar]
- 5.Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–761. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
- 6.Sauvaget C, Yamada M, Fujiwara S, et al. Dementia as a predictor of functional disability: A four-year follow-up study. Gerontology. 2002;48:226–233. doi: 10.1159/000058355. [DOI] [PubMed] [Google Scholar]
- 7.Newman A, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 9.Grigsby J, Kaye K, Baxter J, et al. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley health and aging study. J Am Geriatr Soc. 1998;46:590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 10.Kerber KA, Whitman GT, Brown DL, et al. Increased risk of death in community-dwelling older people with white matter hyperintensities on MRI. J Neurol Sci. 2006;250:33–38. doi: 10.1016/j.jns.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the Cardiovascular Health Study. Neurobiol Aging. 2006;29:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Rosano C, Brach J, Longstreth WT, Jr, et al. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 13.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60A:1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 17.Anstey KJ, Luszcz MA, Giles LC, et al. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging. 2001;16:3–11. doi: 10.1037/0882-7974.16.1.3. [DOI] [PubMed] [Google Scholar]
- 18.Swan GE, Carmelli D, LaRue A. Performance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol. 1995;141:32–40. doi: 10.1093/oxfordjournals.aje.a117342. [DOI] [PubMed] [Google Scholar]
- 19.Kuo HK, Leveille SG, Yu YH, et al. Cognitive function, habitual gait speed, and late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. Gerontology. 2006;53:102–110. doi: 10.1159/000096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perera S, Studenski S, Chandler JM, et al. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60A:894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 21.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 22.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 23.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29:388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 24.Glodzik-Sobanska L, Rusinek H, Mosconi L, et al. The role of quantitative structural imaging in the early diagnosis of Alzheimer's disease. Neuroimag Clin North Am. 2005;15:803–826. doi: 10.1016/j.nic.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Rosano C, Simonsick EM, Harris T, et al. Association between motor and cognitive performance in well-functioning elderly. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- 26.Camicioli R, Moore MM, Sexton G, et al. Age-related brain changes associated with motor function in healthy older people. J Am Geriatr Soc. 1999;47:330–334. doi: 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- 27.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 28.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler Adult Intelligence Scale—Revised. Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- 30.Matarazzo JD, Herman DO. Base rate data for the WAIS-R: Test-retest stability and VIQ-PIQ differences. J Clin Neuropsychol. 1984;6:351–366. doi: 10.1080/01688638408401227. [DOI] [PubMed] [Google Scholar]
- 31.Bohannon R. Comfortable and maximum walking speed of adults aged 20–79 years: Reference values and determinants. Age Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 33.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 34.Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital Health Stat. 1987;1:1–115. [PubMed] [Google Scholar]
- 35.Kane RA, Kane RL. Assessing the Elderly: A Practical Guide to Measurement. Lexington Books; Lexington, MA: 1981. [Google Scholar]
- 36.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonsick EM, Kasper JD, Guralnik JM, et al. Severity of upper and lower extremity functional limitation: Scale development and validation with self-report and performance-based measures of physical function. WHAS Research Group. Women's Health and Aging Study. J Gerontol B Psychol Sci Soc Sci. 2001;56:S10–S19. doi: 10.1093/geronb/56.1.s10. [DOI] [PubMed] [Google Scholar]
- 38.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 39.Bryan RN, Wells SW, Miller TJ, et al. Infarctlike lesions in the brain: Prevalence and anatomic characteristics at MR imaging of the elderly—data from the Cardiovascular Health Study. Radiology. 1997;202:47–54. doi: 10.1148/radiology.202.1.8988191. [DOI] [PubMed] [Google Scholar]
- 40.Gale CR, Martyn C, Cooper C, et al. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 41.Al Snih S, Markides KS, Ottenbacher KJ, et al. Hand grip strength and incident ALD disability in elderly Mexican Americans over a seven-year period Aging. Clin Exp Res. 2004;16:481–486. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 42.MacKinnon DP, Warsi G. A simulation study of mediated effect measures. Multivar Behav Res. 1995;30:41–62. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maylor EA, Allison S, Wing AM. Effects of spatial and nonspatial cognitive activity on postural stability. Br J Psychol. 2001;92(Part 2):319–338. [PubMed] [Google Scholar]
- 44.Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: The Health Aging and Body Composition Study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: The PROGRESS (PeRindopril prOtection aGainst REcurrent Stroke Study) magnetic resonance imaging substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 46.Miller RE, Shapiro AP, King HE, et al. Effect of antihypertensive treatment on the behavioral consequences of elevated blood pressure. Hypertension. 1984:202–208. [PubMed] [Google Scholar]
- 47.Croog SH, Kong BW, Levine S, et al. Links hypertensive black men and women. Quality of life and effects of antihypertensive medications. Black Hypertension Quality of Life Multicenter Trial Group. Arch Intern Med. 1990;150:1733–1741. doi: 10.1001/archinte.150.8.1733. [DOI] [PubMed] [Google Scholar]
- 48.Colcombe SJ, Erikson KL, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 49.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 50.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61A:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]