Abstract
Age-associated changes in gait are exacerbated when another task is performed simultaneously. We quantified the converse, i.e., the effects of walking on cognitive abilities, and determined the role of aging and executive function (EF) in any observed changes. 276 healthy older adults and 52 healthy young adults performed three cognitive tasks, i.e., serial 7 and 3 subtractions and phoneme-monitoring, while sitting and again while walking. Among the elderly, walking decreased performance on serial 3 and 7 subtractions and the number of phonemes counted (p<0.0001), but enhanced content recall. In contrast, for the young adults, walking did not alter serial 3 subtractions, phoneme-monitoring or content recall, while serial 7 subtraction performance decreased during walking (p=0.047). Measures of EF explained the age-associated changes in performance of the cognitive task during walking. Findings in both young and old subjects underscore the idea that gait is attention-demanding and is not a purely motor task. Even young, healthy adults demonstrate decreased cognitive performance while walking, when the cognitive task is sufficiently difficult. Age-associated declines in EF apparently contribute to the difference in dual tasking abilities during walking between young and older adults.
Keywords: executive function, aging, dual task, gait
1. Introduction
A growing number of recent studies have demonstrated that cognitive factors can influence the regulation of gait and have shown that gait is not solely a motor task, especially among older adults (Chen et al., 1996; Snijders et al., 2007; Van lersel et al., 2007; Woollacott and Shumway-Cook, 2002; Yogev et al., 2005; Holtzer et al., 2008). Examination of the changes in gait that occur when older adults simultaneously perform a cognitive task suggests that there is a cognitive component to generating and maintaining a normal and consistent gait pattern. Otherwise, dual tasking should not affect gait. While there is an abundance of studies examining the effects of the simultaneous performance of a second task (i.e., dual tasking) on gait and the implications regarding disability and fall risk (Faulkner et al., 2007; Verghese et al., 2002; Springer et al., 2006; Holtzer et al., 2007; Bootsma-van der Wiel et al., 2003), relatively few studies have examined the effects of walking on performance of the cognitive “dual task” in healthy individuals.
Recent reports have demonstrated that when compared to other cognitive domains, executive function1 is differentially associated with gait performance under dual tasking conditions (Coppin et al., 2006; Springer et al., 2006). EF refers to several different cognitive sub-domains involved in processing tasks such as divided attention and planning. Several studies have identified the importance of EF in mediating dual task situations involving gait and another task by examining the deterioration of gait performance in response to the cognitive challenge of dual tasking (Yogev-Seligmann et al., 2008; Coppin et al., 2006). In general, when walking and performing another task, healthy subjects will prioritize the maintenance of gait and posture over the performance of the second task (Bloem et al., 2006), but changes in the gait pattern have been associated with EF (Brown et al., 1999; Lezak, 1995; Yogev-Seligmann et al., 2008; Holtzer et al., 2004; Holtzer et al., 2005). EF tends to decline as a part of normal aging, even in healthy individuals (Lezak, 1995; Yogev-Seligmann et al., 2008; Holtzer et al., 2004; Holtzer et al., 2005) and these changes might also play a role in the alterations in the performance of the cognitive task.
Specific dual tasks (DT) differ in terms of the attention they demand and the cognitive load that they place on the subject. The primary aim of this study was to examine the effects of walking on the performance of three dual tasks: phoneme monitoring, serial 3 subtractions, and serial 7 subtractions. Phoneme monitoring tests working memory and content recall using a passive listening paradigm, while serial subtractions involves articulation and working memory, as well as basic mathematical abilities. The change in performance in these three tasks between sitting and walking in young healthy adults and healthy older adults was used to evaluate the effect of gait on the cognitive tasks. The second purpose of this study was to investigate the changes in performance mediated by age and the impact that age-associated changes in EF might have on cognitive performance while walking.
2. Results
2.1 Subject characteristics
As summarized in Table 1, all of the participants were generally healthy, without significant cognitive impairment (e.g., mean MMSE near 30) and the groups were homogeneous with respect to gender (p=0.87). Among the older adult subjects, scores on the SF-36 and PASE were consistent with activity levels typical of healthy elderly individuals who are functionally independent. Small, but significant differences were found in years of education, MMSE and average gait speed between the young and older adults.
Table 1.
Subject Characteristics
| Subject Characteristics (Mean ± Standard Deviation or %) | ||||
|---|---|---|---|---|
| Older Adults
(N=276) |
Young Adults
(N=52) |
p-value | ||
| Background Measures | Age (years) | 76.4 ± 4.5 | 24.1 ± 2.7 | |
| Gender (% female) | 58.9% | 58.7% | 0.87 | |
| Education (years) | 13.6 ± 3.7 | 14.8 ± 1.8 | p<0.0001 | |
| MMSE (30) | 28.7 ± 1.3 | 29.7 ± 0.5 | p<0.0001 | |
| Baseline gait speed (m/s) | 1.3 ± 0.2 | 1.4 ± 0.2 | p<0.0001 | |
| Timed Up & Go (seconds) | 9.5 ± 1.7 | 7.3 ± 1.1 | p<0.0001 | |
| SF-36 | 69.1 ± 17 | NA | ||
| PASE | 113 ± 66.4 | NA | ||
| Measures of Affect | GDS (0-30) | 5.2 ± 4.6 | 3.5 ± 3.7 | p= 0.005 |
| Spielberger Anxiety State | 32.1 ± 10.1 | 27.0 ± 8.1 | p= 0.286 | |
| Spielberger Anxiety Trait | 33.9 ± 8.7 | 32.6 ± 7.8 | p<0.0001 | |
| Executive Function | Verbal fluency (# of words) | 33.5 ± 12.3 | 39.8 ±10.5 | p<0.0001 |
| Digit span forward (# of words) | 9.2 ± 2.4 | 11.8 ± 2.2 | p<0.0001 | |
| Digit span backward (# of words) | 6.1 ± 2.4 | 8.6 ± 2.4 | p<0.0001 | |
| Digit span total (# of words) | 15.2 ± 4.1 | 20.4 ± 4.1 | p<0.0001 | |
| TMT A time (sec) | 81.0 ± 36.8 | 35.0 ± 12.5 | p<0.0001 | |
| TMT B time (sec) | 151.2 ± 62.2 | 66.1 ± 21.2 | p<0.0001 | |
| TMT B-A time (sec) | 70.4±51.3 | 31.1±15.1 | p<0.0001 | |
PASE = Physical Activity Scale for the Elderly, MMSE = Mini Mental State Exam, GDS = Geriatric Depression Scale, TMT = Trails Making Test
2.2 Effects of walking on dual task performance
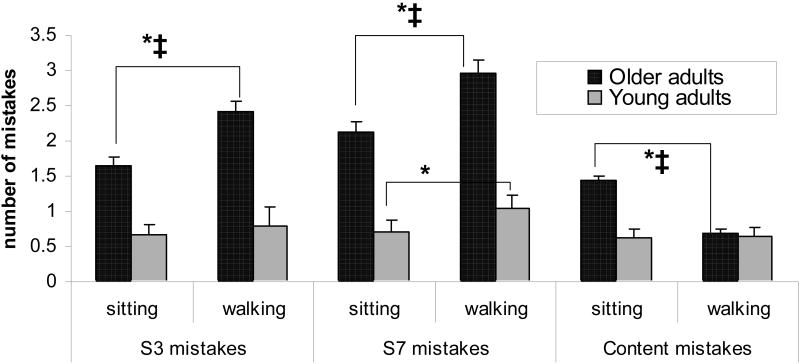
As shown in Table 2, young adults had similar performance while sitting and walking on the total number of serial 3 subtractions, serial 3 subtraction mistakes, number of phonemes counted and in number of mistakes while answering questions about the content of the audio passage. They did, however, have an increased number of mistakes while walking when compared to sitting in the serial 7 subtraction task (p=0.047). In contrast, the older adults performed significantly poorer on some of the cognitive tasks while walking. This included increased mistakes in serial 3 and serial 7 subtraction tasks and fewer number of phonemes counted (p<0.0001 for all measures). Interestingly, there was no change in total number of serial 3 subtractions (p=0.519) and an increase in the number of serial 7 subtractions (p<0.0001). Furthermore, the elderly subjects displayed improved performance in answering questions about the passage heard while walking, answering fewer questions incorrectly (p<0.0001) (please see Figure 1).
Table 2.
Effect of gait on cognitive performance and comparison of dual task performance between young and older adults
| Task | Within Group Effects | Between Groups
F, p-value** |
||
|---|---|---|---|---|
| Older Adults
Mean ± SD (p-value)* |
Young Adults
Mean ± SD (p-value)* |
|||
| S3 mistakes | Sitting
Walking |
1.65 ± 2.06
2.41 ± 2.56 (0.0001) |
0.67 ± 1.04
0.80 ± 1.73 (0.558) |
19.44, 0.0001 |
| S3 total subtractions | Sitting
Walking |
45.08 ± 17.87
44.96 ± 16.86 (0.519) |
54.81 ± 14.53
55.17 ± 17.68 (0.610) |
9.21, 0.003 |
| S7 mistakes | Sitting
Walking |
2.13 ± 2.28
2.95 ± 3.06 (0.0001) |
0.71 ± 1.24
1.04 ± 1.22 (0.048) |
25.66, 0.0001 |
| S7 total subtractions | Sitting
Walking |
24.68 ± 11.10
27.33 ± 12.57 (0.0001) |
30.62 ± 10.20
32.82 ± 13.62 (0.112) |
45.72, 0.0001 |
| Phonemes counted | Sitting
Walking |
7.12 ± 1.61
6.60 ± 1.35 (0.0001) |
7.06 ± 1.11
6.88 ± 0.615 (0.297) |
0.390, 0.533 |
| Content mistakes | Sitting
Walking |
1.43 ± 1.09
0.69 ± 0.868 (0.0001) |
0.62 ± 0.889
0.65 ± 0.905 (0.776) |
18.35, 0.0001 |
p-value testing for a within-group effect of gait on the cognitive task
p-value testing for a between-groups effect of gait on the cognitive task
Figure 1.
The number of mistakes performed on the three cognitive tests during sitting and walking. Within group differences, i.e., sitting vs. walking, are indicated by *. Across group differences, which were present for all tasks, are indicated by ‡. The older adults made more subtraction mistakes during walking, for both serial 3's and serial 7's, while only serial 7 mistakes increased in the young adults. Content recall was similar in sitting and walking for the young adults, while it improved in the older adults during walking. Bars reflect the standard errors.
Both the young and elderly groups had a significant reduction in gait speed (p<0.0001) when simultaneously performing each of the dual tasks, when compared with the condition of walking without any additional DT. For example, young adults slowed down from 1.41 ± 0.17 m/sec during usual-walking to 1.32 ± 18 m/sec during serial 3's and the older adults slowed down from 1.24 ± 0.21 m/sec to 1.11 ± 0.22 during serial 3 subtractions. Elderly people tended to walk slower while subtracting serial 7s than while subtracting serial 3s (p=0.082), while young adults showed no difference in gait speed between the two cognitive tasks (p=0.392).
2.3 Potential covariates
The elderly cohort performed significantly worse than the young group on verbal fluency, digit span and trail making test (p<0.0001; see Table 1). There were also significant differences between the groups in the GDS and Spielberger Inventory (which measures anxiety state), with the older group reporting increased depressive and anxious symptoms (p<0.0001 for both tests), though neither group had scores indicating suspicion for a psychiatric disorder.
Serial 3 subtractions presented the greatest difference in performance between the young and older groups (Figure 2a); therefore, we further examined this task to gain insight into potential correlations to performance with EFs and covariates. None of the background measures (gender, years of education, Timed Up and Go time, and gait speed) correlated with serial 3 subtraction performance. Of all cognitive and affective measures, MMSE, digit span, verbal fluency, trail making test (parts A and B), and anxiety state were found to correlate with performance on the number of mistakes in the serial 3 subtraction task while walking (Table 3). The correlated variables were then analyzed to assess their influence as potential covariates. Forward digit span, and trails making test (parts A and B) were found to be covariates significantly affecting performance, with TMT A time accounting for the highest influence on performance. When the ANOVA model was run after adjusting for the covariates (e.g., taking into account Digit forward and TMTs), the difference between the groups in the number of serial 3 mistakes was no longer significant (Figure 2b).
Figure 2.
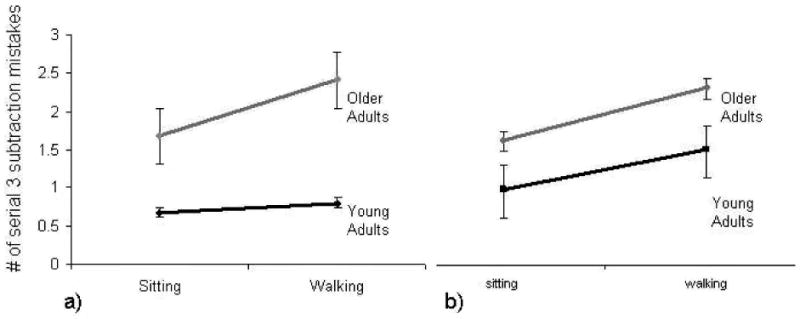
a) Difference between young and older adults in dual task performance of serial 3 subtractions (age-group X condition interaction effect p=0.019). b) Estimated difference between young and older adults in dual task performance after adjusting for executive function covariate (TMT-B see the text for further details). The age-group X condition interaction effect is no longer significant (p=0.533) when executive function is taken into account.
Table 3.
Factors associated with the number of Serial 3 mistakes during walking
| Older Adults Pearson's r
(p-value) * |
Young Adults Pearson's r
(p-value) * |
|
|---|---|---|
| Verbal Fluency | -0.173
(0.005) |
-0.115
(0.446) |
| Forward Digit Span | -0.136
(0.028) |
-0.076
(0.616) |
| Backward Digit Span | -0.319
(0.0001) |
-0.085
(0.572) |
| TMT A time | 0.264
(0.0001) |
-0.05
(0.741) |
| TMT B time | 0.197
(0.001) |
0.032
(0.834) |
| Anxiety State | 0.118
(0.068) |
0.327
(0.027) |
| MMSE | -0.206
(0.001) |
-0.026
(0.863) |
TMT = Trails Making Test, MMSE = Mini Mental State Exam
Significant correlations between cognitive and affective measures are shown in bold.
For the task of phoneme monitoring, a different picture emerged. For both cohorts, none of the parameters tested, including those measuring EF and affect, correlated with either the total number of phonemes counted or the accuracy of answering questions about the content of the auditory selection.
3. Discussion
The primary findings of this study are: 1) Cognitive performance during walking not only affects spatio-temporal gait features. The converse is also true: even among healthy young adults, walking alters cognitive performance. 2) The effect of walking on cognitive performance is much larger among elderly people and is dependent on the task being performed. 3) Age-associated changes in EF can explain the difficulties in performance of cognitive tasks while walking among elderly people.
It is somewhat surprising that even the young cohort demonstrated a decline in performance of the cognitive task, or a dual task decrement (DTD), while walking. Previous work involving a memorization task showed no difference in performance of the cognitive task in young subjects between sitting, standing, and walking (Lindenberger et al., 2000). In our study, the DTD in young subjects was apparent during serial 7 subtractions, but not in the other tasks tested. One explanation of dual tasking, the capacity-sharing theory, proposes that the attempt to simultaneously perform two attention-demanding tasks will cause the performance of one or both of the tasks to suffer due to limited information processing (Tombu and Jolicoeur, 2003). Based on the results in the group of young subjects, it seems that even in young, healthy subjects, the limit of attention capacity falls between the serial 3 subtraction task and serial 7 subtraction task. In other words, our data suggest that for healthy young adults there seems to be a fine line demarcating the border of attention capacity and this border is likely somewhere between the serial 3 and serial 7 subtraction task. Further, this finding supports the idea that gait is attention-demanding, even in healthy young adults.
It has been previously postulated that when challenged with simultaneous gait or complex postural activity and a cognitive task, healthy subjects will prioritize the performance of the postural or gait task (Bloem et al., 2001). Using gait speed as a measure of the postural task performance, this “posture first” strategy appears not to have been utilized by either the healthy young or healthy elderly cohort that we studied, as gait speed significantly declined in both groups with the performance of all of the different tasks. Though performance in subtraction and phoneme-monitoring tasks declined in the elderly group, the young group maintained performance of phoneme monitoring as well as serial 3 subtractions, seemingly at the expense of gait speed. It may be that the decrease in gait speed in the young (about 0.1 m/sec), while statistically significant, is not a clinically significant change in performance and that other measures of gait performance (e.g., gait variability) are preserved in response to dual task challenge, consistent with the “posture first” hypothesis (Bloem et al., 2001; Springer et al., 2006).
In our study, subjects were given no instructions as to which task to prioritize. This presents a potential limitation in that individual subjects could have consciously decided to prioritize either the walking or cognitive task. By not assigning priority to either task, we hoped to learn something about how subjects unconsciously manage two given tasks. Another limitation of the study is the potential for subjects to improve performance on the cognitive tasks with practice. Since all subjects performed the cognitive task while sitting first, it is possible that their performance on the task a second time, while walking, benefited from a practice effect and that the DTD would have been even larger if the subjects did not have prior exposure to the cognitive task. Despite this possible order effect, only phoneme monitoring in the older adults tended to improve during walking, compared to sitting (recall Figure 1). Thus, it's likely that the effects of walking on the performance of the cognitive task generally outweighed any effects of test order.
In comparison with the young group, the elderly group displayed marked DTD?? with almost every task, with the exception of answering comprehension questions about the content of the phoneme-monitoring selection. In examining the factors that accounted for the difference in performance between the elderly and young groups, three measures of EF emerged: forward digit span, total digit span, and both parts A and B of the trails making test. Importantly, not every tested measure of EF could explain the differences in dual task performance as verbal fluency and backward digit span were not found to be significant covariates.
The slight but significant improvement in performance by the elderly group during phoneme monitoring is surprising. The factors accounting for the improved performance remain unclear, but it is possible that the group used the rhythm of gait to aid in concentration during the auditory listening task, while abandoning this strategy during the serial subtractions. It's also possible that the test order influenced phoneme monitoring performance, but if so, one needs to explain why this occurred only in the older adults and only for this task.
EF is complex, with multiple components that can breakdown at any phase of planned or intentional activity (e.g., Lezak, 1995; Yogev-Seligmann et al., 2008; Holtzer et al., 2004; Holtzer et al., 2005). The results of the different examinations of EF (i.e., verbal fluency, digit span, and TMT) were highly correlated with each other in the older cohort, but not in the younger group. Each of the EF tests administered in this study measures a slightly different component of EF. For example, forward and backward digit span measure different aspects of EF, with the former measuring simple immediate attention and memory for auditory stimuli (i.e., the amount of information that can be grasped at once), and the latter measuring complex attentional tasks (i.e., responding to multiple elements or operations within a task) and working memory (Lezak, 1995; Yogev-Seligmann et al., 2008; Holtzer et al., 2004; Holtzer et al., 2005). Recent fMRI data of elderly and young subjects has shown anatomic differences in the way these two tasks are processed and that functional changes occur with normal aging in the localization of the two digit span tasks (Sun et al., 2005). Based on the results from the young and old cohorts in our study, it is possible that the ability to maintain simple attention, rather than complex attention and working memory, may be more important in the performance of the cognitive task under dual task conditions.
Aspects of EF tested by both parts of the trails making test (TMT) were also significant covariates in DT performance, with TMT part A having more of an effect than part B. While parts A and B are similar, part A of the TMT specifically tests visual scanning, numeric sequencing, and visuomotor speed, whereas part B examines the integration of 2 independent sequences, cognitive flexibility and shifting, serial retention and integration, as well as planning (Corrigan and Hinkeldey, 1987). Many of these tasks appear to be important in DT performance. In this study, performance on each part of the TMT was highly correlated with DT performance in both the young and older groups (p<0.0001 for both groups), even though it is important to keep in mind that the TMT taxes sequencing and shifting, a skill set that is slightly different than simultaneous performance of two dual tasks. The difference between TMT B and TMT A was increased in the older adults, compared to the young, as expected, however, somewhat surprisingly, this difference was not related to DT performance.
In contrast to other measures of EF, verbal fluency as measured by speech is normally maintained by healthy individuals well into the eighth decade (Lezak, 1995). As such, it is not surprising that it was not found to significantly explain the DT differences between healthy young and older adults. It is also noteworthy that although there was a significant difference between groups in MMSE scores, MMSE performance was not a significant covariate effecting DT performance. This suggests that not all cognitive changes associated with aging mediate the age-related dual task performance difference.
All of the subjects in this study were healthy without clinically significant cognitive deficits. The elderly subjects all lived independently in the community and were without significant co-morbidities. However, EF has been shown to decline even as a part of physiologic aging and it seems that this decline in certain aspects of EF contributes to the differences in DT performance between the young and elderly groups. In addition, walking has been shown to involve increased cognitive load as a part of aging (Chen et al., 1996). The increased cognitive demand of gait combined with decreased reserve of such cognitive resources as EF, leads to worsened DT performance when subjects are challenged with a second cognitive task while walking.
As this study was carried out in an active, healthy population, one could speculate that the deterioration of DT performance would be even worse in certain patient populations, such as those with a history of stroke, traumatic brain injury, or neurodegenerative diseases. Indeed, previous work has shown that declining EF contributes to changes in gait that are known markers of fall risk in patients with Parkinson's disease (Yogev et al., 2005), elderly fallers (Holtzer et al., 2004; Springer et al., 2006), Alzheimer's Disease (Sheridan and Hausdorff, 2007) and subtle changes in gait have been noted in patient populations with other forms of dementia (Scherder et al., 2007; Verghese et al., 2007). While further testing of performance of the cognitive task while walking is warranted in these populations, the results of the current study may have some clinical ramifications as well. If healthy elderly subjects are more likely to make errors in the cognitive task while walking, they may be advised to conduct important cognitive activities while sitting. Further, if the task is particularly demanding, such that it exceeds even the attention capacity of young subjects, “to stop walking while talking” may be good advice for both young and old adults. In addition, testing patients' ability to perform simultaneous cognitive and motor tasks may reveal subtle deficits in EF that cannot be seen with conventional bedside measures, such as the MMSE. DT performance testing might provide a method of early identification for patients at risk for developing deficits in EF.
4. Experimental Procedures
4.1 Subjects
Two hundred seventy six older adult men and women (age range 70-90 years) and 52 young adults (age 20-30 years) participated in this study. Subjects were recruited from local senior centers, via flyers, advertising, and word of mouth. After an initial phone screening consisting of general health history, eligible subjects who were independent, community-dwelling ambulators, free from disease likely to directly impact gait (e.g., vestibular, orthopedic, neurological disease) were invited to participate. Subjects were excluded if they had severe pain during walking, acute illness, brain surgery, major depression, history of stroke, or if they scored less then 25 on the Mini Mental State Exam (MMSE) (Folstein et al., 1975). Young subjects were excluded if they were taking any prescription medication or were identified as having Attention Deficit Disorder or dyslexia. The study was approved by the human studies committee of the Tel-Aviv Sourasky Medical Center and informed written consent was obtained.
4.2 Assessments
Demographic information and medical history and status were obtained using a structured interview, clinical exam, and questionnaires. The older adults completed the Physical Activity Scale for the Elderly (PASE) (Washburn et al., 1999) and the relative components of the SF-36 (McHorney et al., 1993) to characterize physical activity levels and self-report of general health, respectively. Higher scores on these tests reflect better health and functional abilities.
Subjects completed several traditional tests of cognitive ability and EF, including the Trails Making Test, verbal fluency, and digit span (Lezak, 1995). Because affect has also been shown to influence gait performance (Hausdorff et al., 2004), we also used the Geriatric Depression Scale (GDS) to measure depressive symptoms and emotional well-being (Yesavage et al., 1982) and the Spielberger State-Trait Anxiety Inventory (STAI) to quantify anxiety (Spielberger and et al., 1983). The Timed Up and Go test (Podsiadlo and Richardson, 1991) (TUG) assessed functional mobility and fall risk (AGS Guidelines, 2001).
4.3 Dual task performance
Cognitive function was evaluated during sitting and walking and under three dual task conditions: 1) serial 3 subtractions, 2) serial 7 subtractions, and 3) phoneme monitoring. During the serial subtraction tasks, subjects recited serial subtractions of 7 or 3 out loud while seating and while walking up and down a 25 meter-long, 2-meter wide hallway at their self-selected, usual walking speed for 2 minutes while wearing force-sensitive insoles. During phoneme-monitoring, subjects listened to a story (via headphones) while walking (knowing that they would be questioned about its contents) and counted the number of times two pre-specified words appeared. Average gait speed was determined using a GaitRite mat (placed in the middle of the walkway). The GaitRite system is an electronic walkway that connects to a computer, and automates the measurement of temporal and spatial gait parameters, such as cadence, step length and velocity. The walkway contains approximately 13,000 sensors encapsulated in a roll up carpet to produce an active area of 4 meters long and sampled at 80Hz.
Before performing the task while walking, each of these tasks was conducted while sitting (with a different text for phoneme-monitoring and different starting 3-digit numbers for the serial subtractions). Previous work has shown that even healthy young adults decrease gait speed when they perform these tasks (Springer et al., 2006; Yogev et al., 2005).
Subjects were instructed to walk at their self-selected, comfortable pace under each of the four conditions (baseline, serial 3 subtraction, serial 7 subtraction and phoneme monitoring). No instruction for prioritization of one of the tasks (walking vs. cognitive task) was given. After a practice walk, the order of the tasks was randomized. Performance on the phoneme-monitoring task was evaluated using the total number of words counted correctly and the number of multiple choice questions correctly answered about the story. Evaluation of performance on the serial subtractions included the total number of subtractions made correctly and the number of mistakes. The effects of the dual tasks on gait in the older adults are described elsewhere (Hausdorff et al., 2008).
4.4 Data analysis
Histograms and frequency distributions were constructed to evaluate normality and homogeneity of the distribution and corrections were made using the Greenhouse Geisser filter if needed. Data was analyzed using a two (groups) by two (conditions) factorial analysis of variance (ANOVA) for each dependent variable. The analysis determined the main effects of the task within the groups as well as the between group effects. Pearson correlation was used to assess relationships between variables, and the repeated measures analysis of covariance (ANCOVA) was used to determine covariate influence on performance. Pairwise comparisons were used to assess the weight of each variable on the independent variable. All data were analyzed using the SPSS, version 15.0. A significant level of 0.05 was set for the primary analyses and corrections were made for post-hoc analyses.
Acknowledgments
This work was supported in part by NIH (AG-14100), by the Israel Ministry of Absorption and by the European Union Sixth Framework Program (FET 018474-2, Dynamic Analysis of Physiological Networks, DAPHNet, and STREP 045622 SENSing and ACTION to support mobility in Ambient Assisted Living, SENSACTION-AAL). The authors are indebted to the participants and staff of the “Holchim Rachok” project for their invaluable contribution to this project especially Marina Brozgol, Noit Inbar-Borovsky, Leon Maryasin, Aner Weiss, Galit Yogev, and Leor Gruendlinger.
Footnotes
Executive Function: EF
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AGS Guidelines Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson's disease. J Neurol Sci. 2006;248:196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Valkenburg VV, Slabbekoorn M, Willemsen MD. The Multiple Tasks Test: development and normal strategies. Gait Posture. 2001;14:191–202. doi: 10.1016/s0966-6362(01)00141-2. [DOI] [PubMed] [Google Scholar]
- Bootsma-van der Wiel A, Gussekloo J, de Craen AJ, van Exel E, Bloem BR, Westendorp RG. Walking and talking as predictors of falls in the general population: the Leiden 85-Plus Study. J Am Geriatr Soc. 2003;51:1466–1471. doi: 10.1046/j.1532-5415.2003.51468.x. [DOI] [PubMed] [Google Scholar]
- Brown LA, Shumway-Cook A, Woollacott MH. Attentional demands and postural recovery: the effects of aging. J Gerontol A Biol Sci Med Sci. 1999;54:M165–M171. doi: 10.1093/gerona/54.4.m165. [DOI] [PubMed] [Google Scholar]
- Chen HC, Schultz AB, Ashton-Miller JA, Giordani B, Alexander NB, Guire KE. Stepping over obstacles: dividing attention impairs performance of old more than young adults. J Gerontol A Biol Sci Med Sci. 1996;51:M116–M122. doi: 10.1093/gerona/51a.3.m116. [DOI] [PubMed] [Google Scholar]
- Coppin AK, Shumway-Cook A, Saczynski JS, Patel KV, Ble A, Ferrucci L, Guralnik JM. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol. 1987;43:402–409. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Faulkner KA, Redfern MS, Cauley JA, Landsittel DP, Studenski SA, Rosano C, Simonsick EM, Harris TB, Shorr RI, Ayonayon HN, Newman AB. Multitasking: association between poorer performance and a history of recurrent falls. J Am Geriatr Soc. 2007;55:570–576. doi: 10.1111/j.1532-5415.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Peng CK, Goldberger AL, Stoll AL. Gait unsteadiness and fall risk in two affective disorders: a preliminary study. BMC Psychiatry. 2004;4:39. doi: 10.1186/1471-244X-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Schweiger AS, Herman T, Yogev-Seligmann G, Giladi N. Mediators of the dual task decrements in gait among healthy older adults: the role of cognitive and motor reserve. J Gerontol: Med Sci. 2008;63A doi: 10.1093/gerona/63.12.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21:540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Stern Y, Rakitin BC. Age-related differences in executive control of working memory. Mem Cognit. 2004;32:1333–1345. doi: 10.3758/bf03206324. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Stern Y, Rakitin BC. Predicting age-related dual-task effects with individual differences on neuropsychological tests. Neuropsychology. 2005;19:18–27. doi: 10.1037/0894-4105.19.1.18. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. New York: Oxford University Press, Inc.; 1995. [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Scherder E, Eggermont L, Swaab D, van HM, Kamsma Y, de GM, van WR, Mulder T. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31:485–497. doi: 10.1016/j.neubiorev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders AH, Verstappen CC, Munneke M, Bloem BR. Assessing the interplay between cognition and gait in the clinical setting. J Neural Transm. 2007;114:1315–21. doi: 10.1007/s00702-007-0781-x. [DOI] [PubMed] [Google Scholar]
- Spielberger C, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: 1983. State-Trait Anxiety Inventory. Self-evaluation questionnaire (form Y) [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang X, Chen X, Zhang P, Bao M, Zhang D, Chen J, He S, Hu X. Age-dependent brain activation during forward and backward digit recall revealed by fMRI. Neuroimage. 2005;26:36–47. doi: 10.1016/j.neuroimage.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- Van lersel B, Kessels R, Bloem B, Verbeek A, Olde Rikkert M. Executive function influences gait and balance in community-living elderly people. J Gerontol Med Sci. doi: 10.1093/gerona/63.12.1344. in press. [DOI] [PubMed] [Google Scholar]
- Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G, Lipton R. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50:1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Holtzer R, Lipton R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–35. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: Which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]