Abstract
Eph receptors have been implicated in regulating a diverse array of cellular functions in the developing nervous system. Recently, Eph receptors have been shown to promote cell death in adult germinal zones; however, their mechanisms of action remain ill-defined. In this study, we demonstrate that EphA4 is a new member of the dependence receptors family, which can initiate cell death in the absence of its ligand ephrinB3. Upon removal of its ligand, EphA4 triggers cell death that is dependent on caspase activation as caspase inhibitors prevent cell death. EphA4 itself is cleaved by caspase-3-like caspase in the intracellular domain at position D773/774, which is necessary for cell death initiation as mutation of the cleavage site abolishes apoptosis. In the adult subventricular zone, abolishing ephrinB3 results in increased cell death, while the absence of EphA4 results in excessive numbers of neuroblasts. Furthermore, infusion of soluble ephrinB3 into the lateral ventricle reduced cell death, and together these results support a dependence role for EphA4 in adult neurogenesis.
Keywords: Ephrins, Eph receptors, Dependence Receptor, Apoptosis
INTRODUCTION
Eph receptors constitute the largest family of receptor tyrosine kinases in mammalians and are subdivided into two A and B classes, and bind either A-class or B-class ligands. Receptor-ligand interactions occur upon cell-cell contact as both receptors and ligands are membrane-bound, and control various mechanisms such as axon pathfinding and branching, dendritic spine modeling, angiogenesis, cell migration and positioning [1]. Recently, they were shown to be expressed in the subventricular zone (SVZ), and control stem/progenitor cells proliferation and fate [2–5]. More surprisingly, some ephrins and Eph receptors were found to affect cell death in neurogenic regions. Stimulation of EphA7 through ectopic over-expression of ephrinA5 in the embryonic cortex induced a wave of neural progenitors apoptosis, thus influencing the development of the brain [6]. In contrast, we observed that the absence of ephrinB3 was associated with an increase in TUNEL-positive cells in the SVZ of adult mice [4]. The fact that the lack of ephrinB3 may increase cell death led us to question whether its receptor(s) may function as a dependence receptor.
Dependence receptors can transduce two different types of signals. When bound to their ligand, they transduce a positive signal that elicits a positive response such as survival, differentiation or migration. However, when deprived of their ligand, they trigger apoptosis. Dependence receptors include p75NTR, the netrin receptors DCC and Unc5H1-3, Ret, the androgen receptor, Patched, the αVβ3 integrin, APP [7], as well as Neogenin, Met, and TrkC [8–10]. Dependence receptors are cleaved by specific caspases, leading to the release/exposure of an addiction/dependence domain (ADD), which can amplify caspase activity [11]. Mutation of the caspase cleavage site abolishes the pro-apoptotic activity of the receptors. Furthermore, computational analyses unveiled a conserved motif in the transmembrane region of the known dependence receptors named the DART (Dependence-Associated Receptor Transmembrane) motif [12]. Here we show that EphA4 is a new dependence receptor, where it induces apoptotic cell death, is cleaved by caspase, and that its pro-apoptotic effect can be blocked by ephrinB3. In the adult germinal zone, ephrinB3 and EphA4 function to regulate cell numbers, where EphA4 functions as a dependence receptor in the absence of ephrinB3.
MATERIALS AND METHODS
Plasmids
EphA4 was subcloned into pcDNA 3.1 His-V5 (InVitrogen). EphA4 intracellular domain was subcloned into pcDNA3.1 (InVitrogen) by PCR using the following primers: 5′-caccatgggcgatggagcca-3′ and 5′-tcagacaggaaccatcctgc-3′. The construct for the kinase dead mutant pcDNA3-EphA4-K653M was obtained from Dr Joachim Egea (Martinsried, Germany). EphA4 mutants were obtained by Quick change site directed mutagenesis (Stratagene) using the following primers: EphA4-D773/774N: ggtgctggagaataaccccgaagc and gcttcggggttattctccagcacc; EphA4-D806N: ctcagccagtaatgtctggag and ctccagacattactggctgag; EphA4-Y596/602F: agttctaacaccttgattcaaatgtttctc and caaggtgttagaacttttgtggatccc, tgtaaagggatccacataagttctaacacc and gtggatccctttacattcgaagaccccaac.
Cell culture and death analysis
Cell death was analyzed as described previously [13]. Briefly, 100,000 HEK 293T cells were transfected with 1.5 μg DNA using Lipofectamine (InVitrogen) for 6 hrs. The medium was replaced with DMEM without serum and cell death was analyzed 48 hrs later using Trypan blue exclusion. Pre-clustered ephrinB3-Fc (R&D) was added at the time of medium change and again 24 hours later at a final concentration of 1 μg/ml. Clustering was performed with goat anti-human Fc antibodies (1:10 anti-Fc:ligand) for 1 hour at 37°C. Apoptosis was quantified by TUNEL assay (In Situ Cell Death kit, Roche) following the manufacturer’s instructions. Apoptosis was also analyzed using the caspase substrate caspACE FITC-VAD-fmk (Promega). Briefly, 48 hr after transfection, the cells were washed and resuspended in PBS and incubated 20 min at 37°C with 10 μM caspACE-FITC-VAD-fmk (Promega). They were counted using a FACS Calibur (Becton Dickinson) and cellQuest analysis software (excitation and emission: 488 nm and 525–550 nm). Apoptosis was also quantified with the Caspase-3 Fluorometric Assay Kit (Gentaur). Caspase inhibitors z-VAD-fmk and z-DEVD-fmk were used at 10 μM.
Caspase cleavage reactions
Purified caspases were a generous gift from Guy Salvesen (The Burnham Institute, La Jolla, CA). The intracellular domain of EphA4 (amino acids 571–987) was cloned into pcDNA3.1 (InVitrogen) and was transcribed and translated in vitro using the TNT system (Promega). To analyze receptor cleavage in 293T cells, cells were washed once in PBS and lysed in 20 mM Hepes-KOH, 10 mM KCl, 1.5 M MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 0.1 mM PMSF buffer containing DTT, pepstatin, leupeptin and aprotinin. The lysates were then incubated for 30 minutes at 37°C. Immunoblots were then performed using an anti-V5 antibody (InVitrogen). In vitro transcription/translation and incubation with caspase 3 or 8 were performed as described previously [13].
Protein analysis
Expressed proteins were immunoprecipitated with an anti-V5 (Invitrogen) or anti-EphA4 antibody (Santa Cruz) (in the case of EphA4-K653M) using a ratio of 1 μg Ab/100 μg protein extract overnight at 4ºC. 25 μl of protein G-agarose beads (Calbiochem) were added for 30 min. Washes were performed in 10 mM Tris-HCl, pH 7.0, 1 mM EDTA, 0.5% Triton X-100 with decreasing salt concentrations (1 M NaCl, then 0.2 M NaCl, then no NaCl). Immunoprecipitates were boiled and run on SDS-PAGE gels, transferred and the membranes were probed with either anti-V5 or anti-EphA4 antibodies, and anti-phosphotyrosine antibodies (BD Pharmingen).
Animals and tissue analysis
Two-month old male CD1 mice were used following the University of Miami Institutional Animal Care and Use Committee guidelines. Animals were perfused intracardially with 4% paraformaldehyde and the brains were harvested, frozen in OCT (Tissue-Tek) and sectioned using a cryostat. Anti-PSA-NCAM (Chemicon) was used at 1:1000 for 1 hr at room temperature. Labeling of proliferating cells was performed by injecting BrdU (50 μg/g body weight) intraperitoneally for one hour. Mice were perfused with paraformaldehyde and cryostat sections were stained with anti-BrdU antibodies after treatment of the tissue with 2N HCl for 30 min at 37ºC followed by neutralization with 0.1 M sodium borate pH 8.5. Pre-clustered ephrinB3-Fc (140 μg/ml in PBS) and z-VAD-fmk (50 μM) were infused into the lateral ventricle at the following coordinates from bregma (P: −0.5 mm; L: 0.7 mm; 3 mm below dura) using Alzet osmotic pumps (model 1007D) connected to a brain infusion device placed on the skull. Animals were perfused after 7 days of infusion. TUNEL staining was performed using the ApopTag kit (Chemicon) following the manufacturer’s instructions. Stereological counts were performed using the StereoInvestigator software (MicroBrightfield) on 16 μm sections every fifteenth section.
RESULTS
EphA4 functions as a pro-apoptotic receptor in the absence of ephrinB3
EphA4 was transiently over-expressed in human embryonic kidney (HEK) 293T cells, and cell death was monitored 2 days after transfection using trypan blue exclusion. Over-expression of EphA4 was able to increase the level of dead cells as compared to cells transfected with a mock vector (Fig 1a, b; Suppl. Fig 1). A similar effect was observed in NIH3T3 fibroblasts and immortalized neuronal 13.S.24 cells (not shown). The enhancement of cell death induced by EphA4 could be reversed by adding its ligand ephrinB3 (Fig. 1a–c) in a dose-dependent manner (Fig. 1d), whereas little to no effects were observed with soluble ephrinA1 or ephrinA4 (Suppl. Fig. 2) or in control 293T cells treated with ephrinB3 (not shown). EphA4-induced cell death was concluded to be apoptotic for two reasons: (i) EphA4 expression induced an increase in DNA degradation as visualized by TUNEL staining (Fig. 1c, d) and (ii) it triggered caspase activity as determined by measuring the cleavage of the FITC-VAD-fmk substrate (Fig. 2a). Our observations suggest that EphA4 functions as a dependence receptor as it induces apoptosis when dissociated from its ligand.
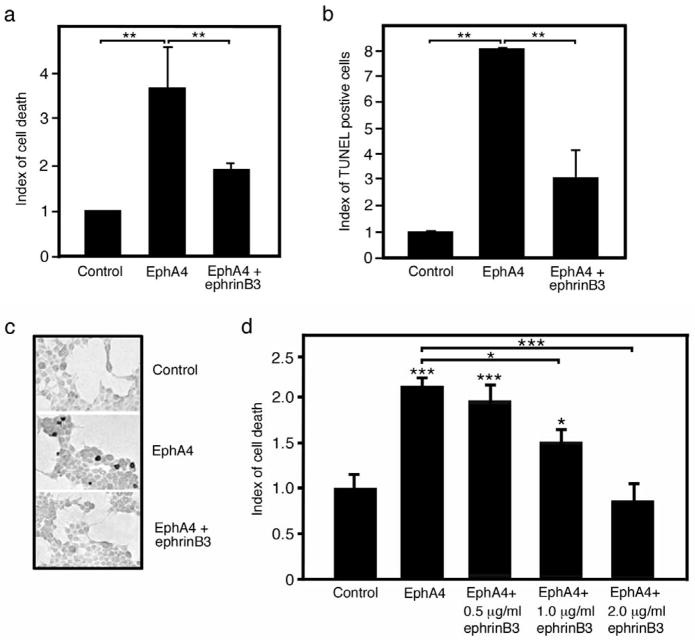
Figure 1.
EphA4 enhances apoptotic cell death that is blocked in a dose-dependent manner by ephrinB3. (a) HEK 293T cell cultures over-expressing EphA4 showed a higher number of dead cells as measured by Trypan blue exclusion. Addition of pre-clustered soluble ephrinB3 in the medium was able to reduce the amount of cells stained by Trypan blue. (b) EphA4 over-expression leads to an increase in TUNEL-positive cells 48 h after transfection. The number of TUNEL-positive cells is reduced upon addition of soluble ephrinB3 in the medium. (c) Representative pictures of TUNEL staining (quantification shown in (b)). The TUNEL-positive cells appear dark (a few are seen in the middle panel). (d) EphrinB3 blocked EphA4 induce cell death in a dose-dependent manner. The experiments were performed at least three times and the bars on the graphs represent the means of three separate wells (one representative experiment shown). * p<0.05; ** p<0.01; *** p<0.001.
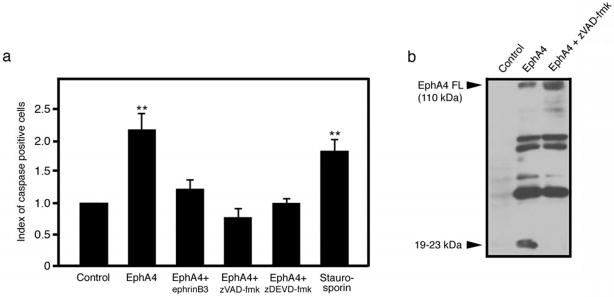
Figure 2.
EphA4 induces caspase activation. (a) Caspase activity was increased as a result of EphA4 over-expression. This increase in caspase activity was reduced by adding ephrinB3 to the cell medium or by incubating the cells with the caspase inhibitor z-VAD-fmk or caspase-3 inhibitor zDEVD-fmk. Staurosporin was used as a positive control to induce apoptosis. The experiment was performed at least three times and the bars on the graphs represent the means of three separate wells (one representative experiment shown). (b) EphA4 cleavage fragments were observed 48 hours after over-expression. These small fragments (19–23 kDa) are no longer present upon addition of the caspase inhibitor z-VAD-fmk, supporting caspase cleavage of EphA4. **p<0.01.
Pro-apoptotic functions of EphA4 require caspase cleavage
To further elucidate the molecular mechanisms of EphA4-induced cell death, we studied the involvement of caspases. Treatment with the broad range caspase inhibitor z-VAD-fmk and the caspase-3 inhibitor zDEVD-fmk blocked EphA4-induced cell death (Fig. 2a), supporting a caspase-dependent mechanism. Dependence receptors have been shown to require a preliminary caspase cleavage of their intracellular domain to trigger cell death [11]. As shown in Fig. 2b, Western blot analysis following full-length EphA4 expression in HEK293T cells revealed lower migrating bands at 19–23 kDa as a result of EphA4 cleavage that is inhibited by zVAD-fmk.
To further establish a direct cleavage of the EphA4 intracellular domain by caspases, the last 446 C-terminal amino acids were transcribed and translated in vitro. The product was incubated with purified active caspase-3 or caspase-8. Figure 3a shows that the intracellular domain of EphA4 was cleaved by caspase-3 but not by caspase-8, and this resulted in cleavage product(s) migrating at approximately 19–23 kDa. This demonstrated that EphA4 is a substrate for caspase-3-like caspases, and that it has at least one caspase cleavage site in its intracellular domain as shown for the vast majority of dependence receptors [7]. Since caspases cleavage sites have been shown to contain an aspartic acid residue in the P1 position [14], we generated D>N mutants in the EphA4 region encompassing the last 250 amino acids to match the size of the cleavage fragment. The mutation of residues D773/774N prevented cleavage by caspase-3 in vitro (Fig. 3b).
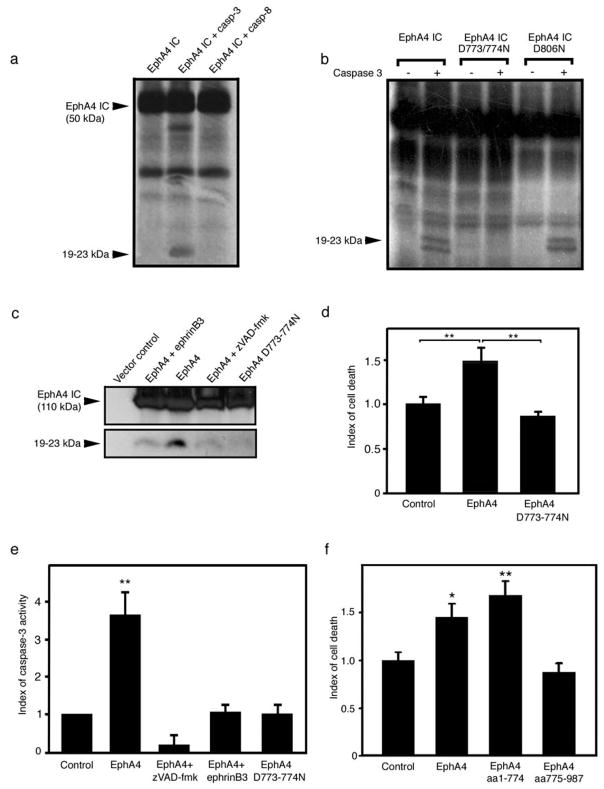
Figure 3.
EphA4 is a substrate for caspase-3 like caspase. (a) The intracellular fragment of EphA4 (aa 571–987) was expressed in vitro and incubated with caspases. Caspase-3, but not caspase-8, triggered the release of smaller fragments (19–23 kDa). The other bands present on the blots most likely reflect aspecific translation initiated at different methionine residues, as shown and described previously [13]. (b) Mutants D>N were generated to locate the caspase cleavage site. Mutation of residues D773/774 prevented the release of the small 19–23 kDa fragments, where lower molecular weight bands were resolved using a gradient gel. The mutant D806N is shown as a control to illustrate the specificity of cleavage. (c) EphrinB3 could block EphA4 cleavage similar to application of zVAD-fmk and the D773/774N mutant EphA4 receptor. (d) The mutant EphA4 D773/774N does not induce significantly greater cell death than the control. (e) The mutant EphA4 D773/774N does not induce greater caspase activation than the control. The levels of caspase activation are similar to the ones observed in the presence of ephrinB3. (f) The pro-apoptotic domain of EphA4 is located upstream from the cleavage site. The two fragments that would be generated upon caspase cleavage (aa1–774 and aa775–987) were over-expressed separately, cell death was observed in the amino terminal fragment but not the carboxy terminal fragment. *p<0.05; **p<0.01.
The overall dependence receptor notion hypothesizes that the caspase cleavage of the intracellular domain of dependence receptors is inhibited in the presence of their ligand [13, 15]. We then analyzed whether EphA4 cleavage could also be blocked by application of ephrinB3. As shown in Fig. 3c, application of ephrinB3 strongly inhibited the apparition of the 19–23kDa, which was similar to inhibition by zVAD-fmk and the D773/774 mutation.
In most cases, caspase cleavage of dependence receptors leads to the unveiling and release of a pro-apoptotic Addiction/Dependence Domain (ADD) [7]. We then assessed whether the caspase-cleavage of EphA4 is required for cell death induction. Forced expression of the D773/774N mutant form of EphA4 in HEK 293T cells was not able to trigger cell death (Fig. 3d) or caspase activation (Fig. 3e). We therefore transfected cells with constructs encoding for the two fragments that would be generated after caspase cleavage: an N-terminal ‘truncated’ protein encompassing amino acids 1–774 and a C-terminal fragment encompassing amino acids 775–987. Expression of the N-terminal fragment induced cell death similar to full length EphA4, whereas the C-terminal fragment showed no significant effect (Fig. 3f). This suggested that the ADD domain is located N-terminal to the D773/774 caspase cleavage site on the EphA4 protein, and is similar in that regard to the Neogenin, Patched or DCC dependence receptors [8, 13, 15].
EphA4-mediated apoptosis is independent of receptor phosphorylation
To further evaluate whether EphA4 activation is critical for inhibiting its pro-apoptotic effects, we examined the requirement for EphA4 phosphorylation. Upon stimulation with its ligand, EphA4 is known to undergo oligomerization that results in trans-phosphorylation of receptor dimers, however, it is a well-recognized concept that over-expression of receptor tyrosine kinases can lead to auto-phosphorylation. To address whether autophosphorylation can affect EphA4 pro-apoptotic activity, we evaluated to role of EphA4 phosphorylation using a kinase-dead EphA4 mutant (EphA4 K653M) and a mutant where two juxtamembrane tyrosines are mutated to phenylalanine (EphA4 Y596/602F). The juxtamembrane tyrosine residues are highly conserved among receptor tyrosine kinases, and have been shown to control auto-inhibition of receptor tyrosine kinases in general [16] and Eph receptors [17, 18]. To confirm the anti-phosphorylation potential of the two mutant receptors, we transfected HEK 293T cells with wild type EphA4 (V5-tagged), EphA4 Y596/602F (V5-tagged), or EphA4 K653M (untagged) receptors (Fig. 4a). Immunoprecipitation (IP) blots demonstrated that all three receptors were expressed in HEK 293T cells following transient-transfection, but only wild type EphA4 was phosphorylated. Cell death assays demonstrated that the two mutant EphA4 receptors retained their ability to induce cell death as compared to wild type EphA4 (Fig. 4b), and application of ephrinB3 could inhibit EphA4-induced cell death in both the wild type and kinase-dead mutant (Suppl. Fig. 3). Furthermore, analysis of the D773/774 mutant EphA4 receptor showed similar phosphorylation levels to wild type EphA4 (Fig. 4c), suggesting that EphA4 phosphorylation and activation are not implicated in the pro-apoptotic activity of EphA4. Moreover, the lack of cell death induction by the D773/774N mutant is not due to an impairment of activation, which may occur because of the location of the mutation in the activation loop, as EphA4 retained the ability to be phosphorylated by ephrinB3 (Fig. 4d).
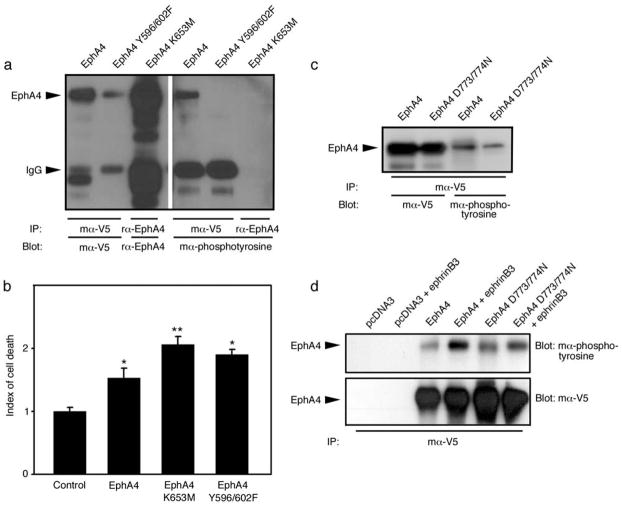
Figure 4.
The kinase activity of EphA4 is not necessary for cell death induction. Two mutants were compared to the wild type form: a kinase dead mutant (K653M) and a mutant with two mutated juxtamembrane tyrosine residues (Y596/602F) that prevent full intracellular domain activation. (a) Over-expression of EphA4 in HEK 293T cells leads to phosphorylation of the receptor, whereas the two mutants do not show tyrosine phosphorylation. (b) Despite their lack of tyrosine phosphorylation, the two EphA4 mutants are still able to induce cell death like the wild type form when over-expressed. (c) The D773/774N mutant EphA4 receptor could be phosphorylated when incubated with ephrinB3. (d) Phosphorylation of EphA4 D773/774N is increased after ephrinB3 addition. Lanes 1, 2: pcDNA 3; lanes 3, 4: EphA4-V5; lanes 5, 6: EphA4 D773/774N-V5. Lanes 2, 4 and 6: stimulation with ephrinB3. *p<0.05; **p<0.01.
EphA4 functions as a dependence receptor for neural progenitor cells that reside in the adult SVZ
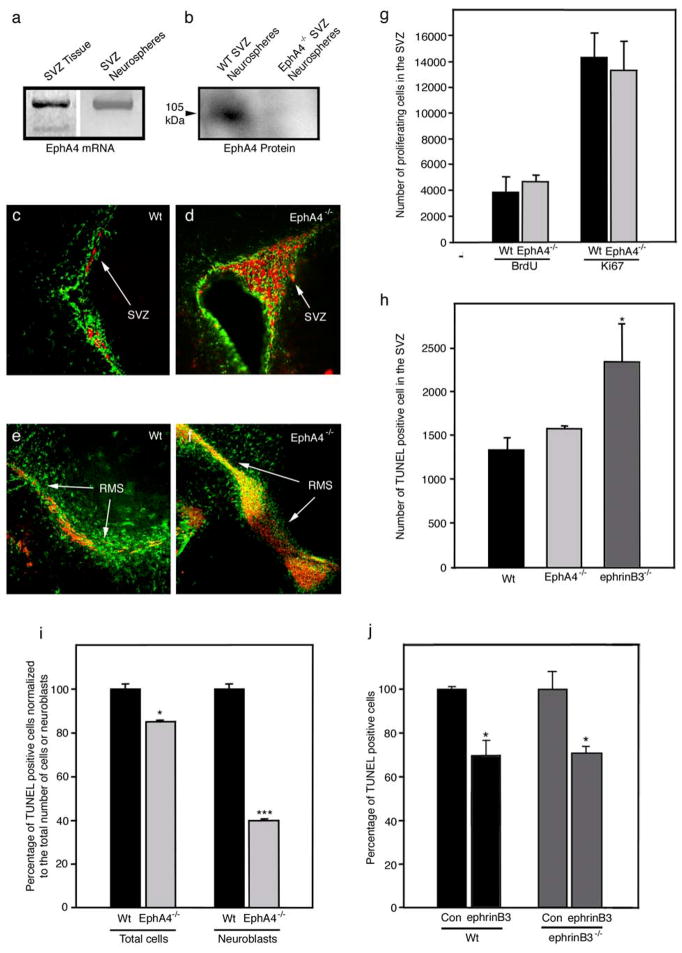
Previously we had observed an increase in TUNEL-positive cells in the germinal zone of ephrinB3−/− mice [4], suggesting that EphA4 may function to induce cell death in the absence of ephrinB3. To test this hypothesis, we evaluated the survival of cells residing in the SVZ in the absence of EphA4. Neural progenitor cells were isolated from SVZ tissues and grown as neurospheres. Tissue extracts and purified neurospheres were found to express EphA4 mRNA (Fig. 5a) and protein (Fig. 5b). Immunohistological examination of the SVZ area revealed a striking phenotype around the lateral ventricles, where excessive numbers of PSA-NCAM-positive neuroblasts accumulated in the SVZ and rostral migratory stream (RMS) of EphA4−/− mice as compared to wild-type (Wt) littermates (Fig. 5c–f). To examine whether the increased numbers of neuroblasts resulted from increased proliferation and/or decreased cell death, we quantified the numbers of BrdU- and Ki67-labeled cells (markers for proliferation) and TUNEL-labeled cells (marker of cell death) in the SVZ using non-biased stereological measurements. Quantification of Ki67 staining or BrdU incorporation demonstrated that the observed increase in neuroblasts numbers did not result from increased proliferation (Fig. 5g). Our initial examination of TUNEL-positive cells revealed a significant increase in the ephrinB3−/− mice, while EphA4−/− mice had similar levels to Wt controls (Fig. 5h). Although, when TUNEL-positive cells were standardized against the difference in total cell numbers between Wt and EphA4−/− mice, there was a significant reduction in the percentage of TUNEL-positive cells associated with the absence of EphA4 (Fig. 5i). This would suggest that the increase in neuroblasts in EphA4−/− mice may reflect, in part, a reduction in cell death in the adult SVZ. To provide additional gain-of-function support, we infused ephrinB3 back into the SVZ of both Wt and ephrinB3−/− mice in an attempt to reduce cell death. Figure 5j shows that animals receiving soluble ephrinB3 for 1 week had a reduction in the percentage of TUNEL-positive cells in both ephrinB3−/− and Wt mice. These findings support the dependence receptor role for EphA4 in cells that reside in the adult SVZ, and the ability of ephrinB3 to regulate neurogenesis.
Figure 5.
EphA4−/− animals have an enlarged SVZ and RMS. (a) EphA4 is expressed in the SVZ tissue and SVZ progenitor cells grown as neurospheres in vitro. (b) EphA4 protein is expressed in progenitor cells grown as neurospheres, but is absent in neurospheres derived from EphA4−/− mice. The EphA4−/− mice show an accumulation of PSA-NCAM-positive (red) neuroblasts (d) as compared to wild type littermates (c) The green staining corresponds to GFAP. The RMS of EphA4−/− animals is also enlarged and contains more neuroblasts (f) compared to wild type littermates (e). (g) Proliferation in the SVZ of EphA4−/−, analyzed by BrdU incorporation or Ki67 staining, was found to be unaffected in the EphA4−/− mice. (h) EphA4−/− mice have a similar number of dying cells in the SVZ (labeled by TUNEL), whereas ephrinB3−/− show a significant increase in cell death in the SVZ. However, compared to the total cell population or the neuroblasts population of the SVZ, less dying cells are present in the EphA4−/− SVZ. (j) Infusion of soluble ephrinB3-Fc into the lateral ventricle of ephrinB3−/− and wild type mice reduces the amount of TUNEL-positive cells, showing that it can act as an anti-apoptotic factor in vivo.
DISCUSSION
In the present article, we demonstrate that EphA4 shares characteristics similar to the ones observed for a new group of proteins called dependence receptors. EphA4 is able to trigger caspase-dependent cell death, which can be specifically blocked by its ligand ephrinB3. EphA4 is itself a substrate of a caspase-3-like caspase and its cleavage at amino acid position D773/774 is necessary for the induction of cell death. One potential role for EphA4 as a dependence receptor is to regulate neurogenesis in the adult SVZ, where the absence of its ligand ephrinB3 leads to increased cell death and the absence of EphA4 leads to excessive numbers of neuroblasts.
Recent studies have implicated Eph receptors in regulating cell death. EphA7 was shown to initiate cell death in neuronal progenitors when activated by ephrinA5 in the embryo [6]. This pro-apoptotic effect of ephrinA5 contradicts our dependence receptor mechanism cell death, where ephrinB3 is anti-apoptotic, suggesting that all Eph receptors do not function as dependence receptors. Recently, the DART motif has been identified in the trans-membrane region of dependence receptors [12]. This motif is present on EphA4 but not all Eph receptors (G. del Rio, personal communication). The function of the DART motif is unknown but could regulate homomultimerization of dependence receptors. Indeed it is thought that oligomerization caused by ligand binding may prevent monomer-induced cell death, as was shown for the p75 receptor [11, 19]. This hypothesis is reinforced by our finding that mutant forms of EphA4 that are unable to trans-phosphorylate are still able to initiate cell death, suggesting that the activation of the receptor is not critical for induction of cell death. TrkC kinase activity was also found to be dispensable in triggering apoptosis [9].
For most dependence receptors, the mechanism(s) of caspase binding, signal transduction, and caspase activation remain ill-defined. However, for many dependence receptors caspase cleavage leads to the release of a pro-apoptotic addiction/dependence domain (ADD). Our studies demonstrate that following caspase cleavage the C-terminus fragment is inactive, whereas the remaining N-terminus ‘truncated’ EphA4 receptor initiates the apoptotic cascade. This suggests that the ADD domain is upstream of the D773/774 cleavage site, which is similar to the dependence receptor Neogenin [8]. Although, the possibility exists that there is another cleavage site upstream of D773/774 that releases a second fragment and contains the ADD domain. However, it is clear that D773/774 cleavage is required for induction of EphA4-mediated cell death. This non-canonical cleavage site is located within the activation loop of the kinase domain, although the mutant protein exhibits tyrosine phosphorylation in both non-stimulated and ephrinB3 stimulated conditions. This demonstrates that the D773/774 mutant retains the ability to be phosphorylated, however, analysis of the kinase-dead mutants strongly support a kinase independent role of EphA4 in the apoptotic process through the elimination of the caspase cleavage site. The dependence receptors DCC, Ret, Met and TrkC all contain two cleavage sites [7, 9]. Another intriguing observation is that not all ligands are able to block the pro-apoptotic function of EphA4. Unlike ephrinB3, the A-class ligands ephrinA1 and ephrinA4, which bind EphA4 with good affinity in vitro, were not able to inhibit cell death. It is unclear why ephrinB3 and not ephrinA1 or A4 can inhibit EphA4-mediated cell death, however, minor differences in structure and binding may play a role to regulate caspase-3 cleavage. Furthermore, while in vitro binding assays have shown strong binding affinities between EphA4 and the three ephrins, less is know about physiological interactions. What is known is that there is strong biological relevance of ephrinB3-EphA4 interactions, where many abnormalities associated with the ephrinB3−/− mice phenocopy the EphA4−/− mice.
Dependence receptors are now recognized as potential tumor suppressors [7], and both DCC and Unc5H are considered tumor suppressors in colorectal malignancies [20]. Recently, over-expression of EphA2 has been shown to trigger apoptosis in cancer cell lines [21], supporting the dependence receptor role of Eph receptors. In addition, EphA4 is down-regulated in invasive forms of breast cancer [22] and in liver and kidney cancer [23]. EphA4 was also lost in metastatic melanoma [24], and this finding is in agreement with the hypothesis that malignant metastatic tumors may thrive in the absence of pro-apoptotic dependence receptors [25]. Reductions in EphB2, EphB3 or EphB4 expression correlate with tumor progression and malignancy in colorectal cancers [26–29], while EphB over-expression reduces cell growth in certain cancer cell lines [28–30]. Like EphA4, EphB3 also contains the DART motif and may be a putative new dependence receptor [12]. Finally, the pro-apoptotic role of Eph receptors is also supported by ephrin siRNA knockdown studies where cell growth is retarded [31].
The identification of EphA4 as a pro-apoptotic dependence receptor in the adult SVZ is an intriguing mechanism for the regulation of neural progenitor cell numbers. This suggests that EphA4 expressing NPCs require at least intermittent interactions with ephrins to survive. EphrinB3 is localized to regions surrounding the SVZ and RMS, but not within these neurogenic regions [4]. In the absence of EphA4, we observe a large increase of the neuroblast population in both the SVZ and the RMS. This phenotype has also been observed in mice lacking the pro-apoptotic genes Bax and Bak [32–34]. It is noteworthy that like the EphA4−/− mice, the Bax−/− and Bak−/− mice did not exhibit any change in cell proliferation. We can not exclude the possibility that defects in cell migration may also play a role in the build up of neuroblasts in the EphA4−/− mice. Impaired migration has been found in the Bax−/− mice [32], but it is difficult to separate defects in migration from apoptosis when defects in programmed cell death can lead to increased cell numbers that may indirectly alter migratory potential (i.e. bottleneck phenomenon). For these reasons, additional studies are required to address migratory defects in EphA4−/− mice.
Supplementary Material
Acknowledgments
We thank Jessica Salinas and Catherine Mermet-Bouvier for excellent technical assistance. This work was supported by the CNRS (PM), Ligue contre le Cancer (PM), NIH (PM), Miami Project to Cure Paralysis (DJL), NIH/NINDS NS049545 and NS30291 (DJL), and US Army W81XWH-05-1-0061 (DJL); Ralph C. Wilson Sr./Jr. Medical Research Foundation (DJL).
References
- 1.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 2.Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–7. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 3.Holmberg J, Armulik A, Senti KA, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan JG, Frisen J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–71. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricard J, Salinas J, Garcia L, Liebl DJ. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol Cell Neurosci. 2006;31:713–22. doi: 10.1016/j.mcn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Aoki M, Yamashita T, Tohyama M. EphA receptors direct the differentiation of mammalian neural precursor cells through a mitogen-activated protein kinase-dependent pathway. J Biol Chem. 2004;279:32643–50. doi: 10.1074/jbc.M313247200. [DOI] [PubMed] [Google Scholar]
- 6.Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–50. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- 7.Bredesen DE, Mehlen P, Rabizadeh S. Apoptosis and dependence receptors: a molecular basis for cellular addiction. Physiol Rev. 2004;84:411–30. doi: 10.1152/physrev.00027.2003. [DOI] [PubMed] [Google Scholar]
- 8.Matsunaga E, Tauszig-Delamasure S, Monnier PP, Mueller BK, Strittmatter SM, Mehlen P, Chedotal A. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004;6:749–55. doi: 10.1038/ncb1157. [DOI] [PubMed] [Google Scholar]
- 9.Tauszig-Delamasure S, Yu LY, Cabrera JR, Bouzas-Rodriguez J, Mermet-Bouvier C, Guix C, Bordeaux MC, Arumae U, Mehlen P. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci U S A. 2007;104:13361–6. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tulasne D, Deheuninck J, Lourenco FC, Lamballe F, Ji Z, Leroy C, Puchois E, Moumen A, Maina F, Mehlen P, Fafeur V. Proapoptotic function of the MET tyrosine kinase receptor through caspase cleavage. Mol Cell Biol. 2004;24:10328–39. doi: 10.1128/MCB.24.23.10328-10339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehlen P, Bredesen DE. The dependence receptor hypothesis. Apoptosis. 2004;9:37–49. doi: 10.1023/B:APPT.0000012120.66221.b2. [DOI] [PubMed] [Google Scholar]
- 12.del Rio G, Kane DJ, Ball KD, Bredesen DE. A novel motif identified in dependence receptors. PLoS ONE. 2007;2:e463. doi: 10.1371/journal.pone.0000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395:801–4. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 14.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–11. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 15.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–6. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard SR. Juxtamembrane autoinhibition in receptor tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:464–71. doi: 10.1038/nrm1399. [DOI] [PubMed] [Google Scholar]
- 17.Binns KL, Taylor PP, Sicheri F, Pawson T, Holland SJ. Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol Cell Biol. 2000;20:4791–805. doi: 10.1128/mcb.20.13.4791-4805.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wybenga-Groot LE, Baskin B, Ong SH, Tong J, Pawson T, Sicheri F. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell. 2001;106:745–57. doi: 10.1016/s0092-8674(01)00496-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang JJ, Rabizadeh S, Tasinato A, Sperandio S, Ye X, Green M, Assa-Munt N, Spencer D, Bredesen DE. Dimerization-dependent block of the proapoptotic effect of p75(NTR) J Neurosci Res. 2000;60:587–93. doi: 10.1002/(SICI)1097-4547(20000601)60:5<587::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, Bredesen DE, Scoazec JY, Mehlen P. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–4. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 21.Dohn M, Jiang J, Chen X. Receptor tyrosine kinase EphA2 is regulated by p53-family proteins and induces apoptosis. Oncogene. 2001;20:6503–15. doi: 10.1038/sj.onc.1204816. [DOI] [PubMed] [Google Scholar]
- 22.Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318:882–92. doi: 10.1016/j.bbrc.2004.04.102. [DOI] [PubMed] [Google Scholar]
- 23.Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–9. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 24.Easty DJ, Mitchell PJ, Patel K, Florenes VA, Spritz RA, Bennett DC. Loss of expression of receptor tyrosine kinase family genes PTK7 and SEK in metastatic melanoma. Int J Cancer. 1997;71:1061–5. doi: 10.1002/(sici)1097-0215(19970611)71:6<1061::aid-ijc24>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–58. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 26.Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, Gallinger S, Pals S, Clevers H. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–30. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 27.Guo DL, Zhang J, Yuen ST, Tsui WY, Chan AS, Ho C, Ji J, Leung SY, Chen X. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours. Carcinogenesis. 2006;27:454–64. doi: 10.1093/carcin/bgi259. [DOI] [PubMed] [Google Scholar]
- 28.Jubb AM, Zhong F, Bheddah S, Grabsch HI, Frantz GD, Mueller W, Kavi V, Quirke P, Polakis P, Koeppen H. EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res. 2005;11:5181–7. doi: 10.1158/1078-0432.CCR-05-0143. [DOI] [PubMed] [Google Scholar]
- 29.Davalos V, Dopeso H, Castano J, Wilson AJ, Vilardell F, Romero-Gimenez J, Espin E, Armengol M, Capella G, Mariadason JM, Aaltonen LA, Schwartz S, Jr, Arango D. EPHB4 and survival of colorectal cancer patients. Cancer Res. 2006;66:8943–8. doi: 10.1158/0008-5472.CAN-05-4640. [DOI] [PubMed] [Google Scholar]
- 30.Huusko P, Ponciano-Jackson D, Wolf M, Kiefer JA, Azorsa DO, Tuzmen S, Weaver D, Robbins C, Moses T, Allinen M, Hautaniemi S, Chen Y, Elkahloun A, Basik M, Bova GS, Bubendorf L, Lugli A, Sauter G, Schleutker J, Ozcelik H, Elowe S, Pawson T, Trent JM, Carpten JD, Kallioniemi OP, Mousses S. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer. Nat Genet. 2004;36:979–83. doi: 10.1038/ng1408. [DOI] [PubMed] [Google Scholar]
- 31.Iiizumi M, Hosokawa M, Takehara A, Chung S, Nakamura T, Katagiri T, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, Nakagawa H. EphA4 receptor, overexpressed in pancreatic ductal adenocarcinoma, promotes cancer cell growth. Cancer Sci. 2006;97:1211–6. doi: 10.1111/j.1349-7006.2006.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim WR, Kim Y, Eun B, Park OH, Kim H, Kim K, Park CH, Vinsant S, Oppenheim RW, Sun W. Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. J Neurosci. 2007;27:14392–403. doi: 10.1523/JNEUROSCI.3903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindsten T, Golden JA, Zong WX, Minarcik J, Harris MH, Thompson CB. The proapoptotic activities of Bax and Bak limit the size of the neural stem cell pool. J Neurosci. 2003;23:11112–9. doi: 10.1523/JNEUROSCI.23-35-11112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi J, Parada LF, Kernie SG. Bax limits adult neural stem cell persistence through caspase and IP3 receptor activation. Cell Death Differ. 2005;12:1601–12. doi: 10.1038/sj.cdd.4401676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.