Abstract
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a precancerous lesion with a well-described progression to carcinoma. This case report describes a 61-year-old woman with a history significant for multiple cancers and a confirmed germline mutation of MSH2, a mismatch repair gene responsible for Lynch syndrome, who was also found to have an IPMN of the pancreas. Phenotypic manifestations of Lynch syndrome in this patient included multiple adenomas and adenocarcinomas of the colon as well as several other Lynch syndrome-associated cancers. The patient’s adenocarcinoma of the colon and IPMN of the pancreas showed identical immunohistochemical staining profiles with loss of expression of MSH2 and MSH6 proteins as well as high levels of microsatellite instability. The immunohistochemical staining and microsatellite instability patterns of the adenocarcinoma of the colon and IPMN gives strong evidence to support the consideration of IPMN as part of the spectrum of lesions found in Lynch syndrome.
Keywords: Intraductal papillary mucinous neoplasm (IPMN), pancreatic cancer, Lynch syndrome, hereditary nonpolyposis colorectal cancer (HNPCC), MSH2
Introduction
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC), is an autosomal dominant condition caused by germline mutations of mismatch repair genes including MLH1, MSH2, MSH6 and PMS2.18 Microsatellite instability (MSI) testing and immunohistochemical analysis (IHC) on pathologic specimens are used to identify patients who have defective mismatch repair mechanisms. Patients with a high level of microsatellite instability and/or a loss of expression of the mismatch repair proteins in their tumor specimens are more likely to have a disease-causing mutation in the mismatch repair genes responsible for their tumor formation. In order to further establish the diagnosis of Lynch syndrome, further testing can be done to identify the specific germline mutation.
Phenotypically, Lynch syndrome is characterized by early onset colonic adenomas and adenocarcinomas. Previously described Lynch syndrome-associated extracolonic tumors include endometrial, ovarian, small intestinal, gastric and transitional cell carcinomas of the ureter and renal pelvis.11, 19, 20 Pancreatic carcinoma was first described in relation to Lynch syndrome in 1985 by Lynch et al. 12 Since this publication, evidence for the inclusion of pancreatic carcinoma in the Lynch syndrome cancer spectrum has been conflicting.1, 13, 21 Notably, pancreatic cancer is included as an extracolonic tumor associated with Lynch syndrome in the revised Bethesda guidelines.17
There are two primary precursors to pancreatic cancer: pancreatic intraepithelial neoplasms (panIN) and intraductal papillary mucinous neoplasms (IPMN). PanIns are noninvasive microscopic epithelial neoplasms, located in the smaller pancreatic ducts, characterized by cytologic and architectural atypia. Point mutations in the KRAS2 gene, as well as inactivation of the p16/CDKN2A, tp53 and SMAD4 genes are involved in the development of these lesions. In contrast IPMNs are grossly visible, noninvasive, mucin-producing epithelial neoplasms that involve the main pancreatic duct and its side branches. Various molecular alterations, including gene mutations in KRAS2, LKB1 and PIK3CA, have been reported in the development of IPMNs.8 In contrast to pancreatic adenocarcinoma, a relationship between IPMN and Lynch syndrome has not been described.
Case Report
The patient is a Caucasian female who first presented to our clinic at the age of 55. Her first diagnosis of cancer was a brain tumor of unknown pathologic type at approximately 12 years of age. Her gynecological malignancies have included a stage 1A endometroid ovarian cancer at 39, and a separate primary stage 1A endometrial cancer that was incidentally found in the pathology specimen of her right salpingooophorectomy and hysterectomy as treatment for her ovarian cancer. At 43 years of age she was diagnosed with an infiltrating ductal carcinoma of the breast. Dermatological cancer diagnoses have included metastatic melanoma of the thigh at 42 years of age, multiple basal cell carcinomas at 54 and 55 and a nasal tip squamous cell carcinoma at 58.
At 50 years of age the patient was diagnosed with an adenocarcinoma contained within an adenoma at the splenic flexure. At age 52, the patient was found to have another polyp suspicious for an intramucosal adenocarcinoma without submucosal invasion. As a result of these adenocarcinomas, the patient underwent a right subtotal colectomy. Following the surgery, the patient had normal surveillance of her rectal remnant without evidence of malignancy. At age 61, the patient was again diagnosed with an invasive adenocarcinoma as well as a villous adenoma in the rectum. She was recommended to undergo an abdominoperineal resection.
A pre-operative abdominal CT scan showed a 0.8 by 0.7 cm low attenuation lesion in the distal body of the pancreas. A MRI done for further evaluation of this lesion noted two cystic lesions within the pancreas at the distal tail measuring 1.2 cm and at the junction of the tail and body of the pancreas measuring 2.3 cm. On MRCP images, these cystic lesions were noted to communicate with the pancreatic ductal system and were suggestive of IPMN. In addition, there were several smaller cystic areas within the body of the pancreas which likely represented small IPMN.
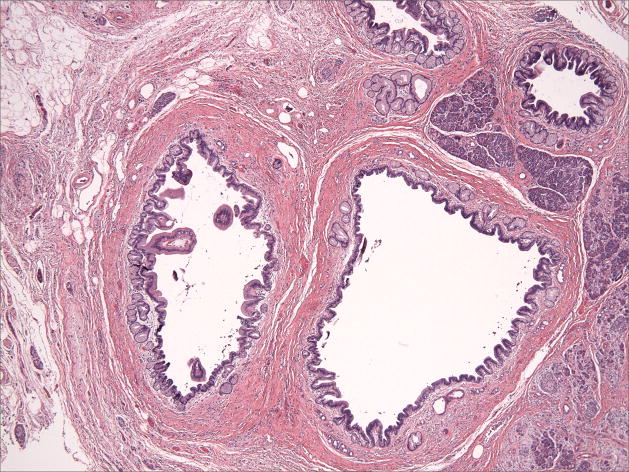
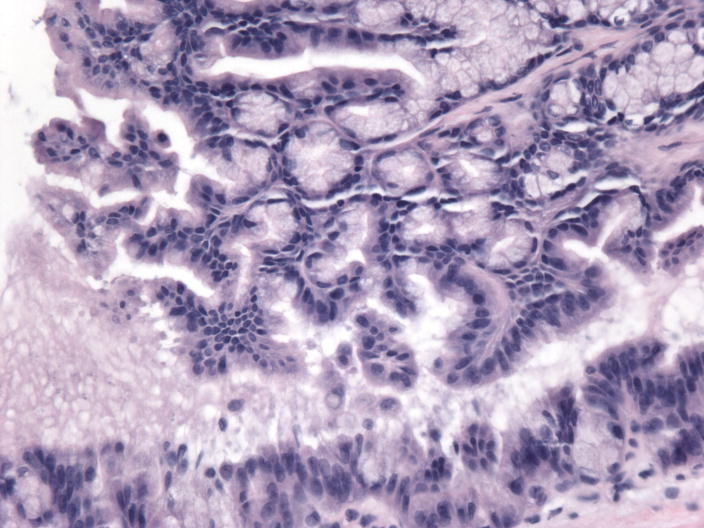
The patient underwent an abdominoperineal resection with completion of her colectomy and end ileostomy as well as a partial pancreatectomy and splenectomy. The post-operative pathologic specimen showed a rectal adenocarcinoma with negative margins and no nodal involvement. The pathologic specimen from the tail of the pancreas showed IPMN of gastric foveolar-type with moderate dysplasia and no invasive carcinoma. The pancreatic duct appeared mildly dilated (measuring up to 1.2 cm in diameter) and cystic, filled with clear serous contents. No other gross lesions including masses were identified in the pancreatic parenchyma. The specimen showed chronic pancreatitis as well as the presence of multifocal Pancreatic Intraepithelial Neoplasia (PanIN) 1-2. Lymphovascular, venous and perineural invasion were not identified.
Family history for this patient is unavailable as she was adopted and has no knowledge of her biological family’s medical history. She does have a 21-year-old son who has no history of cancer and is alive and well. He has declined genetic testing.
Materials and Methods
The patient was hesitant to undergo genetic testing for many years. At age 60, the patient decided to proceed and a peripheral blood sample was drawn for BRCA1, BRCA2, MLH1 and MSH2 germline mutations. DNA from white blood cells was extracted and purified, amplified by polymerase chain reaction, and directly sequenced.
Microsatellite instability testing was performed on the patient’s resected colon and pancreas specimens. DNA was separately prepared from microdissected normal and tumor cells and analyzed by a PCR based assay using fluorescent-labeled primers to 10 different microsatellite DNA loci (4 mononucleotide repeats, BAT 25, BAT 26, BAT 40, BAT34c4; 4 dinucleotide repeats, D17S250, D5S346, D18S55, D10S197, ACTC; and a penta-tetra-mononucleotide marker, MYCL1). The normal and tumor allele patterns were compared for each marker.
Immunohistochemical staining for mismatch repair proteins, MLH1, MSH2, MSH6 and PMS2, was performed on sections of the patient’s resected colon and pancreas specimens.
Results
The patient was negative for mutations in her BRCA1, BRCA2 and MLH1 genes. However, she was found to have a germline mutation in the MSH2 gene, which was a mutation that resulted in deletion of exons 8 to 10 leading to premature truncation of the MSH2 protein and was felt to be deleterious. She was also found to have a second mutation in the MSH2 gene termed W764C that was of uncertain significance.
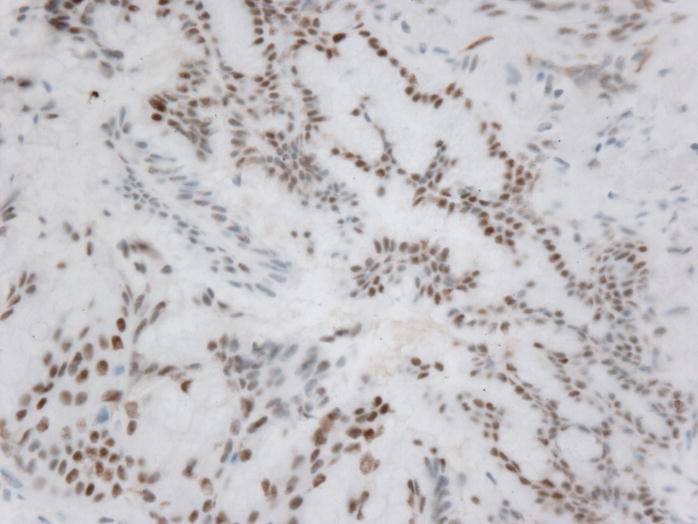
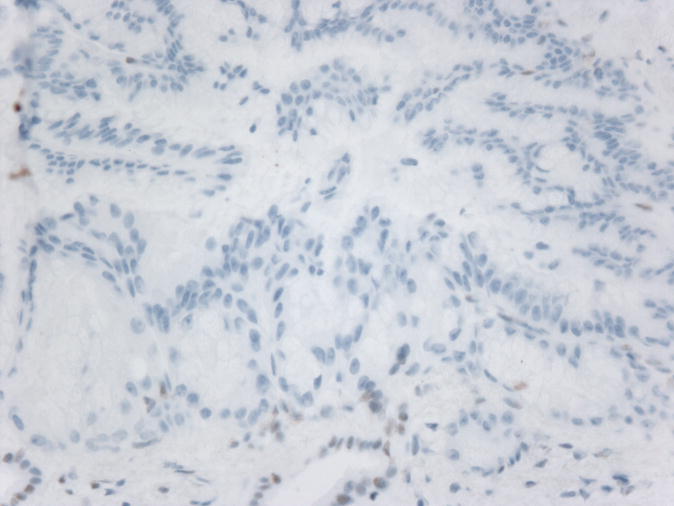
Microsatellite instability was detected in 6 of 6 microsatellite markers in the colonic adenocarcinoma and in 4 of 9 microsatellite markers in the IPMN indicating that both specimens had high levels of microsatellite instability. Immunohistochemical studies on the colonic adenocarcinoma indicated a loss of MSH2 and MSH6 expression with intact MLH1 and PMS2 staining. Interestingly, immunohistochemical studies on the IPMN indicated an identical staining pattern. (Figure 1) All specimens had appropriate internal controls.
Figure 1.
Microphotographs of the IPMN A) Low-Power H&E staining B) High-power H&E staining C) Intact MLH1 immunohistochemical staining, D) Absence of MSH2 immunohistochemical staining
Discussion
We report the first case of an IPMN of the pancreas occurring in a patient with Lynch syndrome, in whom a mismatch repair gene germline mutation has been identified. Despite her unknown family history due to adoption, the patient’s clinical history, which includes a wide range of cancers diagnosed at young ages, suggested a hereditary cancer syndrome. In this patient, positive testing for an MSH2 germline mutation confirmed the clinical suspicion of Lynch syndrome. In addition to her germline mutation, the patient’s resected colon specimen showed loss of expression of the MSH2 protein and a high level of microsatellite instability. This loss of expression as well as a high level of microsatellite instability in the tumor tissue provides further evidence of the mutation in her MSH2 gene and the diagnosis of Lynch syndrome.
A pathologic association between IPMN and Lynch syndrome has been a source of controversy in the past. Mucinous noncystic (colloid) carcinoma of the pancreas, a variant of ductal adenocarcinoma that has been shown to arise from IPMN, was demonstrated in one of eight cases to show a high level of microsatellite instability.9, 10 Additionally, multiple case reports have demonstrated microsatellite instability as well as MLH1 and MSH2 gene mutations in medullary carcinoma of the pancreas, a newly described variant of adenocarcinoma with an unknown relationship to IPMN.2, 5, 22 Another study looking at pancreatic adenocarcinoma showed that 13% of sporadic pancreatic cancer cases were MSI high and that these samples were associated with poor differentiation. This study also looked at a subset of 3 patients with confirmed MLH1 germline mutations and found that the pancreatic cancers in these patients had a high level of microsatellite instability and were poorly differentiated. This study concluded that pancreatic adenocarcinomas with MSI-H represent a distinct oncogenic pathway, but this study did not comment on a relationship between IPMNs and pancreatic carcinoma.23
A study looking at MMR protein staining of IPMNs directly showed presence of staining of MLH1 and MSH2 in 100% of samples. The conclusion from this study was that MMR does not play a significant role in the pathogenesis of IPMNs. However, this study analyzed random pathology samples and not specifically samples from patients with Lynch syndrome. 7 The pancreatic resection specimen of the patient described here showed a loss of expression of the MSH2 protein, as well as a high level of MSI, which strongly suggest the presence of an underlying MSH2 gene mutation as the cause of her pancreatic cells undergoing malignant change. Because mutations in the MSH2 gene are known to cause Lynch syndrome, evidence of an MSH2 gene mutation in this patient’s pancreatic specimen suggests that IPMN be considered part of the spectrum of cancers found in Lynch syndrome families.
A population-based study estimated the incidence of extrapancreatic neoplasms in patients with IPMN to be 10%.14 Retrospective studies have shown that IPMN is associated with extrapancreatic neoplasms, specifically colorectal adenomas and adenocarcinomas as well as gastric carcinomas.4, 16 This finding suggests that patients with IPMN may have an increased genetic susceptibility for tumor formation. Some have proposed that IPMN be considered a manifestation of attenuated familial adenomatous polyposis, whereas others have suggested it be considered part of the Peutz-Jeghers syndrome cancer spectrum.3, 15 This case report suggests that IPMN may also be part of the extracolonic tumors found in Lynch syndrome. Evidence of a mutation known to cause Lynch syndrome-associated tumors found in both this patient’s germline and pancreatic lesion supports this claim. Furthermore, this is supported by the fact that colorectal adenomas and adenocarcinomas as well as gastric carcinomas, the two most common extrapancreatic neoplasms in individuals with IPMN, are both found with increased frequency in Lynch syndrome families.
This case report is complicated by the patient’s extensive cancer history because some of her cancers, in addition to her IPMN, cannot be explained by her Lynch syndrome mutation. Specifically, infiltrating ductal carcinoma of the breast, as was seen in this patient, is not part of the classic tumor spectrum in Lynch syndrome. Additionally, not enough information could be collected on the patient’s childhood brain tumor. However, brain tumors have been described as part of Lynch syndrome in the subset of Turcot’s syndrome, which involves a primary tumor of the central nervous system and multiple concurrent colorectal adenomas.6
We report evidence supporting the inclusion of IPMN in the growing list of extracolonic Lynch syndrome-associated tumors. It is important to identify patients with familial cancer syndromes in order to recommend appropriate screening intervals and modalities. IPMN is a precancerous condition that can be diagnosed with noninvasive radiology or ERCP and treated with pancreatic resection to prevent progression into carcinoma. Further studies are necessary to determine the prevalence of IPMN in Lynch syndrome families.
Acknowledgments
We would like to thank Rachel Rucker-Schmidt, MD and Jason Hornick, MD, PhD for their assistance in obtaining the pathology images.
Supported in part by NIH grant K24-113433
Abbreviations
- IPMN
Intraductal papillary mucinous neoplasm
Footnotes
No conflicts of interest exist
References
- 1.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Banville N, Geraghty R, Fox E, et al. Medullary carcinoma of the pancreas in a man with hereditary nonpolyposis colorectal cancer due to a mutation of the MSH2 mismatch repair gene. Hum Pathol. 2006;37:1498–1502. doi: 10.1016/j.humpath.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Chetty R, Salahshor S, Bapat B, et al. Intraductal papillary mucinous neoplasm of the pancreas in a patient with attenuated familial adenomatous polyposis. J Clin Pathol. 2005;58:97–101. doi: 10.1136/jcp.2004.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi MG, Kim SW, Han SS, et al. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51–56. doi: 10.1001/archsurg.141.1.51. discussion 56. [DOI] [PubMed] [Google Scholar]
- 5.Goggins M, Offerhaus GJ, Hilgers W, et al. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. Poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+ Am J Pathol. 1998;152:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton SR, Liu B, Parsons RE, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332:839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 7.Handra-Luca A, Couvelard A, Degott C, et al. Correlation between patterns of DNA mismatch repair hmlh1 and hmsh2 protein expression and progression of dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch. 2004;444:235–238. doi: 10.1007/s00428-003-0966-0. [DOI] [PubMed] [Google Scholar]
- 8.Hruban RH, Maitra A, Kern SE, et al. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831–849. doi: 10.1016/j.gtc.2007.08.012. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luttges J, Beyser K, Pust S, et al. Pancreatic mucinous noncystic (colloid) carcinomas and intraductal papillary mucinous carcinomas are usually microsatellite stable. Mod Pathol. 2003;16:537–542. doi: 10.1097/01.MP.0000072748.65178.2F. [DOI] [PubMed] [Google Scholar]
- 10.Luttges J, Zamboni G, Longnecker D, et al. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942–948. doi: 10.1097/00000478-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Lynch HT, de la Chapelle A. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet. 1999;36:801–818. [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch HT, Voorhees GJ, Lanspa SJ, et al. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: a family study. Br J Cancer. 1985;52:271–273. doi: 10.1038/bjc.1985.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mecklin JP, Jarvinen HJ. Tumor spectrum in cancer family syndrome (hereditary nonpolyposis colorectal cancer) Cancer. 1991;68:1109–1112. doi: 10.1002/1097-0142(19910901)68:5<1109::aid-cncr2820680535>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Riall TS, Stager VM, Nealon WH, et al. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204:803–813. doi: 10.1016/j.jamcollsurg.2007.01.015. discussion 813–804. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017–2022. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999;94:470–473. doi: 10.1111/j.1572-0241.1999.879_h.x. [DOI] [PubMed] [Google Scholar]
- 17.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 19.Vasen HF, Wijnen JT, Menko FH, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996;110:1020–1027. doi: 10.1053/gast.1996.v110.pm8612988. [DOI] [PubMed] [Google Scholar]
- 20.Watson P, Lynch HT. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993;71:677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008 doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilentz RE, Goggins M, Redston M, et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. Am J Pathol. 2000;156:1641–1651. doi: 10.1016/S0002-9440(10)65035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto H, Itoh F, Nakamura H, et al. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res. 2001;61:3139–3144. [PubMed] [Google Scholar]