Abstract
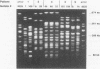
Escherichia coli is the most common gram-negative organism associated with bacteremia. While recurrent E. coli urinary tract infections are well-described, recurrent E. coli bacteremia appears to be uncommon, with no episodes noted in multiple series of patients with gram-negative bacteremias. We report on 5 patients with recurrent bloodstream infections identified from a series of 163 patients with E. coli bacteremia. For each patient, the isolates from each episode were analyzed by pulsed-field gel electrophoresis (PFGE) and ribotyping and for the presence of E. coli virulence factors. For each of four patients, the index and recurrent episodes of bacteremia represented the same strain as defined by PFGE, and the strains were found to carry one or more virulence factors. The remaining patient, with two episodes of bloodstream infection separated by a 4-year interval, was infected with two isolates that did not carry any virulence factors and that were clonally related by ribotype analysis but differed by PFGE. All five patients had either a local host defense defect (three patients) or impaired systemic defenses (one patient) or both (one patient). Thus, recurrent E. coli bacteremia is likely to represent a multifactorial process that occurs in patients with impaired host defenses who are infected with virulent isolates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Arthur M., Dunn R., Kim C., Selander R. K., Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990 Feb;161(2):230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- Archambaud M., Courcoux P., Labigne-Roussel A. Detection by molecular hybridization of pap, afa, and sfa adherence systems in Escherichia coli strains associated with urinary and enteral infections. Ann Inst Pasteur Microbiol. 1988 Sep-Oct;139(5):575–588. doi: 10.1016/0769-2609(88)90156-1. [DOI] [PubMed] [Google Scholar]
- Arthur M., Arbeit R. D., Kim C., Beltran P., Crowe H., Steinbach S., Campanelli C., Wilson R. A., Selander R. K., Goldstein R. Restriction fragment length polymorphisms among uropathogenic Escherichia coli isolates: pap-related sequences compared with rrn operons. Infect Immun. 1990 Feb;58(2):471–479. doi: 10.1128/iai.58.2.471-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Johnson C. E., Rubin R. H., Arbeit R. D., Campanelli C., Kim C., Steinbach S., Agarwal M., Wilkinson R., Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Cloning of the aerobactin-mediated iron assimilation system of plasmid ColV. J Bacteriol. 1983 Feb;153(2):1111–1113. doi: 10.1128/jb.153.2.1111-1113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics. 1981 Jul;98(3):467–490. doi: 10.1093/genetics/98.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte function in vitro. Infect Immun. 1982 Sep;37(3):966–974. doi: 10.1128/iai.37.3.966-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont H. L., Spink W. W. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958-1966. Medicine (Baltimore) 1969 Jul;48(4):307–332. doi: 10.1097/00005792-196907000-00003. [DOI] [PubMed] [Google Scholar]
- Grüneberg R. N. Relationship of infecting urinary organism to the faecal flora in patients with symptomatic urinary infection. Lancet. 1969 Oct 11;2(7624):766–768. doi: 10.1016/s0140-6736(69)90478-4. [DOI] [PubMed] [Google Scholar]
- Herthelius M., Möllby R., Nord C. E., Winberg J. Amoxicillin promotes vaginal colonization with adhering Escherichia coli present in faeces. Pediatr Nephrol. 1989 Oct;3(4):443–447. doi: 10.1007/BF00850224. [DOI] [PubMed] [Google Scholar]
- Johnson C. E., Maslow J. N., Fattlar D. C., Adams K. S., Arbeit R. D. The role of bacterial adhesins in the outcome of childhood urinary tract infections. Am J Dis Child. 1993 Oct;147(10):1090–1093. doi: 10.1001/archpedi.1993.02160340076018. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., Carling P. C., McCabe W. R. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med. 1980 Mar;68(3):332–343. doi: 10.1016/0002-9343(80)90101-1. [DOI] [PubMed] [Google Scholar]
- Lomberg H., Hanson L. A., Jacobsson B., Jodal U., Leffler H., Edén C. S. Correlation of P blood group, vesicoureteral reflux, and bacterial attachment in patients with recurrent pyelonephritis. N Engl J Med. 1983 May 19;308(20):1189–1192. doi: 10.1056/NEJM198305193082003. [DOI] [PubMed] [Google Scholar]
- Maslow J. N., Brecher S. M., Adams K. S., Durbin A., Loring S., Arbeit R. D. Relationship between indole production and differentiation of Klebsiella species: indole-positive and -negative isolates of Klebsiella determined to be clonal. J Clin Microbiol. 1993 Aug;31(8):2000–2003. doi: 10.1128/jcm.31.8.2000-2003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow J. N., Mulligan M. E., Adams K. S., Justis J. C., Arbeit R. D. Bacterial adhesins and host factors: role in the development and outcome of Escherichia coli bacteremia. Clin Infect Dis. 1993 Jul;17(1):89–97. doi: 10.1093/clinids/17.1.89. [DOI] [PubMed] [Google Scholar]
- Maslow J. N., Mulligan M. E., Arbeit R. D. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin Infect Dis. 1993 Aug;17(2):153–164. doi: 10.1093/clinids/17.2.153. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullus K., Hörlin K., Svenson S. B., Källenius G. Epidemic outbreaks of acute pyelonephritis caused by nosocomial spread of P fimbriated Escherichia coli in children. J Infect Dis. 1984 Nov;150(5):728–736. doi: 10.1093/infdis/150.5.728. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Young L. S., Martin W. J., Meyer R. D., Weinstein R. J., Anderson E. T. Gram-negative rod bacteremia: microbiologic, immunologic, and therapeutic considerations. Ann Intern Med. 1977 Apr;86(4):456–471. doi: 10.7326/0003-4819-86-4-456. [DOI] [PubMed] [Google Scholar]