Abstract
Abstract The effect of the immunomodulatory chemotherapeutic agent cyclophosphamide (CTX) on tumor growth was investigated in primary and metastatic intracerebral and subcutaneous rat xenograft models. Nude rats were treated with CTX (100 mg/kg, intraperitoneally) 24 hours before human ovarian carcinoma (SKOV3), small cell lung carcinoma (LX-1 SCLC), and glioma (UW28, U87MG, and U251) tumor cells were inoculated subcutaneously, intraperitoneally, or in the right cerebral hemisphere or were infused into the right internal carotid artery. Tumor development was monitored and recorded. Potential mechanisms were further investigated. Only animals that received both CTX and Matrigel showed consistent growth of subcutaneous tumors. Cyclophosphamide pretreatment increased the percentage (83.3% vs 0%) of animals showing intraperitoneal tumors. In intracerebral implantation tumor models, CTX pretreatment increased the tumor volume and the percentage of animals showing tumors. Cyclophosphamide increased lung carcinoma bone and facial metastases after intra-arterial injection, and 20% of animals showed brain metastases. Cyclophosphamide transiently decreased nude rat white blood cell counts and glutathione concentration, whereas serum vascular endothelial growth factor was significantly elevated. Cyclophosphamide also increased CD31 reactivity, a marker of vascular endothelium, and macrophage (CD68-positive) infiltration into glioma cell-inoculated rat brains. Cyclophosphamide may enhance primary and metastatic tumor growth through multiple mechanisms, including immune modulation, decreased response to oxidative stress, increased tumor vascularization, and increased macrophage infiltration. These findings may be clinically relevant because chemotherapy may predispose human cancer subjects to tumor growth in the brain or other tissues.
Introduction
Metastatic spread, not primary tumor burden, is the leading cause of cancer death. Animal models of human metastatic tumors are an important tool for evaluating potential therapies; however, for many human tumor cells, xenografted human tumor growth in immunodeficient nude rats can be unsuccessful, inconsistent, or slow. This effect is particularly problematic in brain tumor models, in which tumor growth cannot easily or inexpensively be monitored externally.
Cancer treatments have well-known toxic adverse effects; however, opposite effects that enhance malignancy or metastasis are not well understood. Chemotherapy can increase tumor angiogenesis by mobilizing endothelial precursor cells in the bone marrow to migrate to the tumor [1,2]. Cyclophosphamide (CTX, Cytoxan) is a common chemotherapeutic agent that has immunomodulatory effects in addition to its direct cytotoxic activity. In the clinic, secondary malignancies have developed in some patients treated with CTX used alone or in association with other antineoplastic drugs and/or modalities [3–6]. In this report, secondary malignancies caused by CTX treatment were not observed. However, CTX pretreatment supported metastasis formation, which often is not observed in xenograft models [7,8].
Cyclophosphamide pretreatment has been used as a mechanism to improve the anticancer efficacy of viral oncolytic gene therapy [9–11]. The authors attribute the increased efficacy to a reduction in the innate immune response elicited by the virus that would normally reduce the quantity and spread of virus particles in the brain. It is known that a single dose of CTX can decrease total splenocyte number and CD4+25+ regulatory T cells [12–14]. Recently, Yamauchi et al. [15] reported that CTX pretreatment induced human fibrosarcoma cancer metastasis in a mouse model. In another mouse model, formation of lung metastases was also enhanced up to 1000-fold by CTX pretreatment [16]. In both studies, CTX (200 mg/kg, intraperitoneally (i.p.)) was given to the mouse 24 hours before tumor cell injection. Because the half-life of CTX is approximately 17 minutes, the drug should not affect the viability of tumor cells injected 24 hours later [17]. Nude (rnu/rnu) rats lack a thymus but maintain innate immune activities, including natural killer cells. Similar to the mouse models, we hypothesized that using CTX pretreatment (24 hours before tumor cell inoculation) to reduce the innate immune response might improve the growth and/or metastasis of human tumor xenograft models in nude rats.
Although CTX has known immunomodulatory effects, changes in tumor growth may also be attributable to alternate mechanisms. Glutathione (GSH) is a major endogenous antioxidant, with important roles in detoxifying free radicals and reactive oxygen species [18]. Decreased GSH and/or increased oxidative stress may impact expression of vascular endothelial growth factor (VEGF), the major angiogenic factor during epithelial carcinogenesis in a large number of human cancers and metastases [19,20]. The growth of malignant tissues and tumor metastases are dependent on adequate vascularization. The objective of this study was to investigate if single-dose pretreatment with the immunomodulatory chemotherapeutic agent CTX improved tumor growth in subcutaneous, intracerebral, and metastatic nude rat xenograft models. Our second goal was to investigate potential mechanisms involved in this effect.
Materials and Methods
The care and use of the animals was approved by the institutional animal care and use committee and was under the supervision of the Department of Comparative Medicine at Oregon Health and Science University. Female (200–250 g) nude (rnu/rnu) and heterozygous (rnu/wt) rats were selected from the breeding colony maintained in the animal facility at Oregon Health and Science University. All animals were housed in individually hanging wire-bottom cages supplied with an automated watering system in a room with a 12-hour light-dark cycle maintained at 22 ± 2°C. Food and water were supplied to all rats ad libitum. Cyclophosphamide powder was purchased from Bristol-Myers Squibb Company (Princeton, NJ). The powder was resuspended with sterile saline before use.
Rat Xenografted Tumor Models
Human cancer cell lines used in this study were human ovarian carcinoma (SKOV3), small cell lung carcinoma (LX-1 SCLC), and glioma (UW28, U87MG, and U251). All cells were cultured with proper medium supplemented with serum and antibiotics. SKOV3 and U87MG cells were obtained from the American Type Culture Collection (Manassas, VA), UW28 and U251 human glioma cells were obtained from Dr. Ali-Osman (Duke University), and LX-1 was originally obtained from Mason Research Institute (Worcester, MA). Cells were harvested immediately before implantation and were used only if viability exceeded 90%. Rats were pretreated with CTX (100 mg/kg, i.p.) or saline 24 hours before tumor cell inoculation.
Subcutaneous tumor model
A total of 2.5 x 107 cells were mixed (ratio, 1:1) with Matrigel Basement Membrane Matrix (BD Biosciences, Bedford, MA), and the mixture was injected subcutaneously into the left or right flank. Tumor growth was measured every 3 to 4 days using a dial caliper. The subcutaneous tumor volume was calculated as follows: (tumor length)2 x tumor width/2. Animals were killed at 35 days after tumor implantation.
Intraperitoneal tumor model
A total of 2.5 x 107 SKOV3 human ovarian cells were injected into the peritoneal cavity. Animals were monitored and killed at predetermined time points (8–12 weeks) or when clinical symptoms indicated.
Intracerebral tumor model
Rats were anesthetized with ketamine (60 mg/kg, i.p.) and diazepam (7.5 mg/kg, i.p.). Cells (106 in 12–15 µl) were injected into the caudate nucleus in the right caudate putamen (vertical, bregma — 6.5 mm; lateral, bregma — 3.1 mm) as previously described [21,22]. Intracerebral tumor growth was monitored by magnetic resonance imaging (MRI) and histology.
Hematogenous metastasis model
Rats were anesthetized with isoflurane (5% induction, 2% maintenance Aerrane; Anaquest, Inc., Madison, WI), and LX-1 SCLC cells (1–5 x 106 in 1 ml saline) were infused retrograde into the right internal carotid artery as previously described [21]. Systemic and intracerebral tumor growth was monitored by MRI and histology.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed on intracerebral and hematogenous models at time points determined by prior studies with the individual tumor types. Rats were anesthetized using i.p. medetomidine (0.6 mg/kg Domitor; Orion Pharma, Espoo, Finland) and ketamine (15 mg/kg) and were imaged on a 3-T MRI scanner (SiemensMagnetom Trio, Erlangen, Germany) using a custom rat head transmitter-receiver coil as previously described [22]. The 3-T imaging sequences were as follows: T1-weighted spin echo with relaxation time (TR) = 750 milliseconds and echo time (TE) = 12 milliseconds; T2-weighted turbo spin echo (TR = 5430 milliseconds, TE = 78 milliseconds); and fluid-attenuated inversion recovery (TR = 9280 milliseconds, TE = 89 milliseconds, inversion time = 2100 milliseconds). The voxel size was 0.26 x 0.26 x 2 mm for coronal scans. T1-weighted scans were acquired before and after gadolinium (Omniscan; Amersham Health AS, Oslo, Norway) at a dose of 0.1 to 0.3 mmol/kg injected into a femoral vein. After MRI, medetomidine was reversed using atipamezole (Antisedan; Orion Pharma, Espoo, Finland).
Histology and Immunohistochemistry
Animals were killed at predetermined time points or when clinical symptoms indicated. Brains were excised and fixed in 10% buffered formalin for vibratome sectioning, 100 µm in the coronal plane. For intracerebral tumor volumetrics, every sixth brain section was stained with hematoxylin and then imaged at high resolution (30 µm pixel diameter) on flatbed scanner (Expression 1640XL; Epson, Long Beach, CA) using Adobe Photoshop software. Tumor volume was assessed using NIH Image software. Immunohistochemistry was performed by incubating the brain slices with proper dilutions (1:50 to 1:2000) of primary antibodies. Mouse antihuman nuclei monoclonal antibody (clone 3E1.3) was purchased from Chemicon/Millipore (Temecula, CA). Anti-CD31 (sc-46694), CD68 (sc-7084), and VEGF (sc-1836) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Then tissue slices were further incubated with the appropriate biotinylated secondary antibody and visualized with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) using diaminobenzidine. A control for immunostaining omitted the primary antibody to demonstrate diaminobenzidine reactivity. Images were obtained with an Axiocam digital camera mounted on a Zeiss Axioplan microscope (Carl Zeiss Co., Oberkochen, Germany).
Rat Hematology, GSH, and VEGF Measurements
For blood count analysis, rat whole blood (0.5 ml) was collected in EDTA microtubes before treatment and at 1, 3, 7, and 14 days after administration of CTX (100 mg/kg, i.p.). Blood counts were analyzed in duplicate on a Hemavet 850 counter (CDC Technologies Inc., Oxford, CT). Rat serum was collected by centrifugation (2000 rpm at 4°C) and stored at -20°C for future use.
Rat serum GSH content was measured using the Quantichrom GSH assay (BioAssay Systems, Hayward, CA). Serum samples (250 µl of 1:5 dilution, in duplicate) were evaluated according to the manufacturer's protocol. The GSH concentration was quantitated by comparing the 405-nm optical density of the sample with a GSH standard.
Rat serum VEGF level was measured using the Quantikine sandwich ELISA kit (R&D Systems, Minneapolis, MA). Standard, control, and serum samples (50 µl each, in duplicate) were added to a 96-well plate precoated with a specific antibody against rat VEGF, and the assay was performed as described by the manufacturer. The serum VEGF concentration was quantitated by comparing the optical density of the sample at 450 nm with a standard generated according to the manufacturer's instructions.
Statistical Analysis
The results were expressed as mean ± SEM, and the significance of the difference between the mean values of the treated animals and those of controls was determined by the Student's t test. The level of significance was corrected by multiplying the P value by the number of comparisons performed (n) according to Tukey's correction. The paired Student's t test was used to compare the values of the parameters before and after treatment. For data analysis, one-way analysis of variance test was performed by comparing the different arms of treatment of the single variables. Significance was determined at the 5% level, two-sided.
Results
Cyclophosphamide Enhanced Subcutaneous and Intraperitoneal Tumor Growth
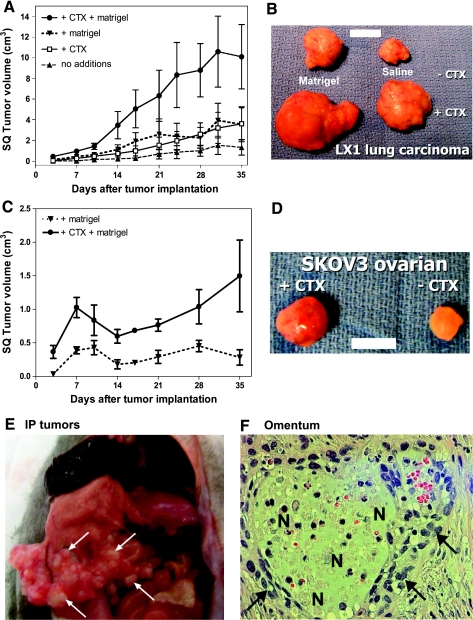
To investigate the effect of CTX on subcutaneous tumor development, we pretreated female nude rats with CTX (100 mg/kg, i.p.) 24 hours before the injection of tumor cells, with or without Matrigel, into the left or right flank. Matrigel alone produced a subcutaneous mass immediately after injection but reabsorbed and disappeared within 3 days (the first day for tumor measurement). With the LX-1 human SCLC cells, small subcutaneous tumors that were often palpable but not measurable grew in the absence of CTX or Matrigel, with final volume of 1.5 ± 0.8 cm3 at 35 days (n = 5; Figure 1A). Addition of either Matrigel or CTX moderately enhanced LX-1 subcutaneous tumor growth (3.6 ± 1.7 and 3.6 ± 1.5 cm3 at 35 days, respectively). The combination of CTX pretreatment and Matrigel synergistically increased LX-1 subcutaneous tumor growth (10.1 ± 3.1 cm3; Figure 1, A and B and Table 1). Subcutaneous tumors in the +CTX group showed increased surface vascularity (Figure 1B).
Figure 1.
Effect of cyclophosphamide pretreatment on the growth of subcutaneous and intraperitoneal tumors. Female nude rats were pretreated with CTX (100 mg/kg, i.p.) or saline 24 hours before cell implantation. (A–D) Subcutaneous tumor growth after inoculation of 250 µl (2.5 x 107) of tumor cells mixed with 250 µl of Matrigel or saline was measured by a dial caliper twice per week. (A) LX-1 SCLC subcutaneous tumor development (mean ± SEM tumor volume, n = 5). (B) Representative LX-1 SCLC subcutaneous tumors excised from animals five weeks after inoculation, from the +/- Matrigel and +/- CTX groups. Bar, 1 cm. (C) SKOV3 ovarian carcinoma subcutaneous tumor development (mean ± SEM tumor volume, n = 5). (D) Representative SKOV3 ovarian carcinoma subcutaneous tumors excised from animals five weeks after inoculation from +/- CTX groups. Bar, 1 cm. (E.F) Intraperitoneal tumor growth after inoculation of 250 µl (2.5 x 107) of SKOV3 ovarian tumor cells. (E) Representative SKOV3 ovarian intraperitoneal tumors nine weeks after inoculation in a +CTX rat. Arrows indicate tumor. (F) Histologic micrograph of omentum from a CTX treated animal. Arrows indicate irregular anaplastic cells; N indicates multifocal areas of necrosis and inflammation. Original magnification, x200.
Table 1.
Summary of Cyclophosphamide Pretreatment on Different Rat Tumor Models.
| Tumor Model | Cell Lines (Timeline) | Treatment | Animal with Tumor (%) | Tumor Volume |
| Subcutaneous | SCLC LX-1 (5 wk) | No additions | 4/5 (80%) | 1.5 ± 0.8 cm3 |
| Matrigel | 4/5 (80%) | 3.6 ± 1.7 cm3 | ||
| Cytoxan | 5/5 (100%) | 3.6 ± 1.5 cm3 | ||
| Cytoxan + Matrigel | 5/5 (100%) | 10.1 ± 3.1 cm3 | ||
| Ovarian SKOV3 (5 wk) | No cytoxan | 3/5 (60%) | 0.3 ± 0.1 cm3 | |
| Cytoxan | 5/5 (100%) | 1.5 ± 0.5 cm3 | ||
| Intraperitoneal | Ovarian SKOV3 (9 wk) | No cytoxan | 0/2 (0%) | n.d. |
| Cytoxan | 5/6 (83.3%) | n.d. | ||
| Intracerebral | Lymphoma MC116 (3–4 wk) | No cytoxan | 16/19 (84.2%) | 48.4 ± 66.2 mm3 |
| Glioma U87 (8 wk) | Cytoxan | 12/13 (92.3%) | 33.0 ± 16.9 mm3 | |
| No cytoxan | 5/8 (62.5%) | 14.6 ± 13.1 mm3 | ||
| Glioma U251 (8–12 wk) | Cytoxan | 8/8 (100%) | 79.8 ± 33.5 mm3 | |
| No cytoxan | 8/12 (66.7%) | 3.2 ± 1.6 mm3 | ||
| Glioma UW28 (3 wk) | Cytoxan | 8/8 (100%) | 67.5 ± 33.9 mm3 | |
| No cytoxan | 6/6 (100%) | 71.8 ± 23.2 mm3 | ||
| SCLC LX-1 (3 wk) | No cytoxan | 8/8 (100%) | 29.1 ± 4.1 mm3 | |
| Hematogenous | SCLC LX-1 (6 wk) | No cytoxan | Brain 0/5 (0%) | n.d. |
| Other* 2/5 (20%) | n.d. | |||
| Cytoxan | Brain 2/10 (20%) | n.d. | ||
| Other* 10/10 (100%) | n.d. |
n.d. indicates not determined; wk, week(s).
Animals developed facial or/and bone malignancy other than CNS tumor after intracarotid tumor cell injection.
Without the addition of Matrigel, SKOV3 ovarian carcinoma cells did not form subcutaneous tumors in nude rats (not shown). Cyclophosphamide pretreatment in combination with Matrigel significantly enhanced SKOV3 tumor growth compared with Matrigel alone (1.5 ± 0.5 vs 0.3 ± 0.1 cm3; Figure 1C and Table 1). Without CTX pretreatment, SKOV3 tumor appeared largely avascular; tumor vascularization was prominent in the CTX pretreatment group (Figure 1D). The decrease in tumor volume 7 to 14 days after tumor implantation might indicate loss of effectiveness of CTX in this model.
Without CTX pretreatment, SKOV3 ovarian carcinoma cells did not form intraperitoneal tumors in nude female rats. After CTX pretreatment, five of six rats (83.3%) developed multiple intraperitoneal ovarian tumor nodules (Figure 1E and Table 1). Tumors were found at the mesentery region along the basal stomach and duodenum area. In omentum, multifocal areas of necrosis, inflammation, and irregular anaplastic cells were found, typical of tumor invasion (Figure 1F). These results suggest that CTX pretreatment enhanced both subcutaneous and intraperitoneal tumor growth.
Cyclophosphamide Enhanced Intracerebral Tumor Growth and Hematogenous Metastases
Several human tumor cell lines consistently form intracerebral tumors when implanted in the caudate nucleus in nude rat brain in the absence of CTX (Table 1). LX-1 human SCLC intracerebral tumors grow quickly and consistently [21], with a volume of 29.1 ± 4.1 mm at 12 days after implantation (Table 1). In contrast, MC116 human B-lymphoma cells show inconsistent intracerebral growth [22]. Pretreatment with CTX (100 mg/kg, i.p.) increased the percentage of animals showing MC116 brain tumor and improved the consistency of growth (Table 1).
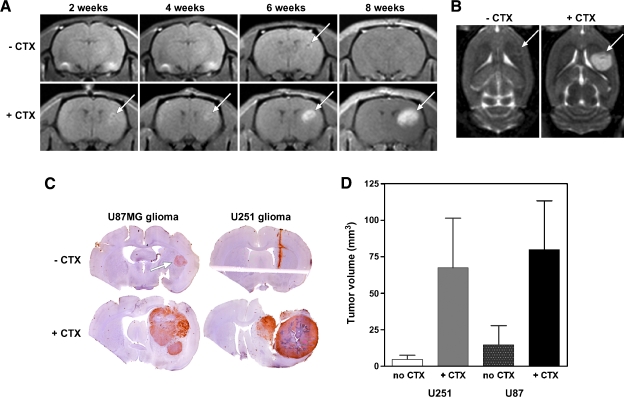
We tested one glioma model, human UW28 cells, that rapidly formed large tumors in nude rat brain without CTX pretreatment, with a volume of 71.8 ± 23.2 mm3 (n = 6) at 3 weeks after implantation (Table 1). Two other glioma models, U87MG and U251, showed slow and inconsistent growth. Pretreatment with CTX reduced the time delay to tumor detection by MRI and increased the percentage of animals showing tumors in both models. In the U87MG intracerebral glioma model, in the absence of CTX pretreatment, no tumor was detected until 6 to 8 weeks after implantation by MRI using either T1-weighted sequences with gadolinium enhancement (Figure 2A, top images) or T2-weighted sequences (Figure 2B, left panel). In CTX-treated rats, U87MG intracerebral tumors could be detected by MRI as early as 2 weeks after implantation and progressively developed through 8 weeks (Figure 2A, bottom images and Figure 2B, right).
Figure 2.
Effect of cyclophosphamide on the growth of implanted intracerebral glioma models. Female nude rats were pretreated with CTX (100 mg/kg, i.p.) or saline 24 hours before stereotactic implantation of 106 human U87MG or U251 glioma cells into the caudate nucleus in the right cerebral hemisphere. (A) T1-weighted coronal scans with gadolinium contrast, from a rat without CTX (top) or with CTX pretreatment (bottom). Magnetic resonance imaging was performed every 2 weeks after inoculation of U87MG cells. (B) T2-weighted axial scans from the same animals at 8 weeks after tumor implantation. (C) Representative U87MG (left) and U251 (right) tumors in the.CTX (top) and +CTX (bottom) treatment groups. Coronal 100-µm vibratome sections were stained with antihuman nuclei antibody with a hematoxylin background. (D) Bar graph (mean ± SEM) of U87MG (left) and U251 (right) brain tumor volumes in rats pretreated with or without CTX (n = 5–6 per group). Brains were harvested for tumor volumetrics at 8 weeks (U87MG) or at 8 to 12 weeks (U251) after cell implantation.
Representative histologic findings in the glioma models is shown in Figure 2C. Upon sacrifice, most animals receiving U87MG cells without CTX pretreatment showed either no tumor (two of six animals) or small brain tumors (three of six animals), and only one rat had a large tumor (Figure 2D and Table 1). A broad range of tumor size was found in the CTX-treated animals, with a mean tumor volume of 79.8 ± 33.5 mm3 at 6 to 8 weeks after implantation, and all animals showed tumor growth. Similar effects of CTX were observed in the U251 glioma model (Figure 2, C and D, and Table 1). Without CTX pretreatment, only four of six rats showed brain tumor at 6 to 8 weeks after implantation, with a mean tumor volume of 4.7 ± 2.9 mm3. With CTX pretreatment, U251 glioma grew in 100% of animals with a mean volume of 67.5 ± 33.9 mm3 (n = 5). Owing to the small sample size of the groups (n = 5–6) and high variability, the CTX effect was not significant in either the U87MG or the U251 groups; however, statistical significance (P = .0085) was found between no CTX and CTX-treated animals if both U87MG and U251 models were combined.
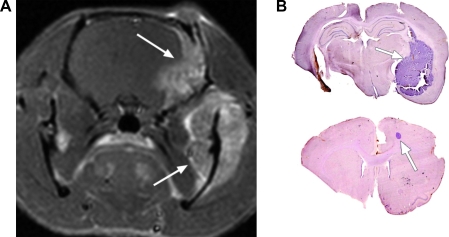
In humans, brain metastases of systemic tumors occur through a complicated sequence of intravasation, tissue localization, extravasation, and growth that is not well understood. To mimic this process, we injected LX-1 human SCLC cells into the right internal carotid artery in nude rats. Without CTX pretreatment, two of five rats (40%) had bone and facial metastases within 4 to 8 weeks of tumor cell inoculation; none showed brain metastases (Table 1). With CTX pretreatment, 10 of 10 rats developed bone and facial metastases after intra-arterial LX-1 cell injection. The MRI in Figure 3A shows a tumor in the mandibular region and another in the dura extending into the skull of one rat, although no tumors were found in brain parenchyma. Two (20%) of the CTX-treated animals showed brain metastases (Figure 3B and Table 1). Our data suggested that CTX pretreatment increased the occurrence of metastatic tumor growth from tumor cells circulating in the vasculature. The mechanisms involved in the difference of CTX effect among bone, facial, and brain metastases are unknown and need to be further investigated.
Figure 3.
Hematogenous metastases in nude rats. Female nude rats were pretreated with CTX (100 mg/kg, i.p.) or saline 24 hours before infusion of 106 LX-1 human SCLC cells into the right internal carotid artery. (A) Gadolinium-enhanced T1-weighted MRI showing a mandibular tumor (lower arrow) and metastasis in the dura extending into the skull (upper arrow). (B) Histologic micrographs of LX-1 SCLC brain metastases (arrows) in two CTX-pretreated rats. Arrows indicate tumor.
Mechanisms of CTX Enhancement of Tumor Growth
A single dose of CTX (100 mg/kg, i.p.) decreased nude rat total white blood cell counts (45.9 ± 2.9% of baseline on day 1, 30.5 ± 6.3% on day 3), lymphocytes (46.4 ± 7.3% and 54.9 ± 8.9%), and neutrophils (45.7 ± 3.4% and 22.5 ± 4.1%) (Figure 4A). Blood counts rebounded at day 10 and were back to baseline at day 21. The lymphocytopenia noted at baseline is common in nude rats. With CTX treatment, lymphocyte counts were further reduced and almost undetectable in some rats. No effect of CTX on erythrocytes and platelets was found.
Figure 4.
Effect of cyclophosphamide on nude rat blood counts, serum VEGF, and GSH levels. Female nude rats (n = 4–5) were treated with CTX (100 mg/kg, i.p.). At the indicated time points, 0.5 to 1 ml of whole blood was collected, analyzed in duplicate on a Hemavet 850 counter for blood counts (A), and serum was assayed for VEGF (B) and GSH (C) levels using commercial kits as described in the Materials and Methods section. For blood count analysis, data are presented as the percent of baseline counts (Day 0) for each animal for total white blood cell, neutrophils, and lymphocytes. Significant differences from Day 0 are indicated by *(P < .05) and **(P < .01).
Treatment with CTX elevated the serum VEGF level from a basal level of 3.2 ± 1.3 pg/ml to 13.1 ± 2.3, 6.1 ± 0.9, and 37.3 ± 14.3 pg/ml at days 1, 3, and 7, respectively (Figure 4B). Then serum VEGF level returned to baseline at day 14 (3.1 ± 1.4 pg/ml). Owing to the small sample size (n = 4–5) and individual variations, only the day 7 time point was found to be significantly elevated from baseline (P = .0066). At this same 7-day time point, serum GSH concentrations were significantly reduced (39.0 ± 1.4 µM; P = .0112) compared with other time points (56.3–58.3 µM; Figure 4C). These results suggest that a single dose of CTX might create an immune and angiogenic microenvironment suitable for tumorigenesis.
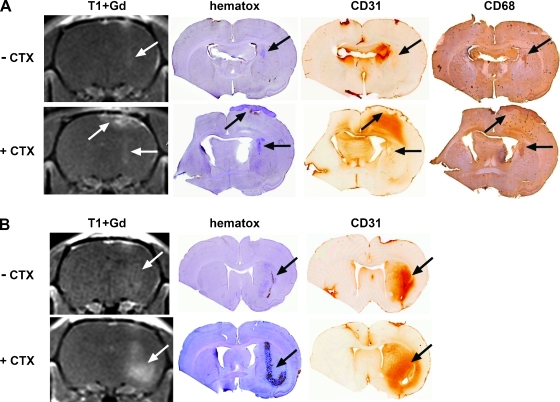
We hypothesized that transient suppression of host immune responses by CTX treatment could limit the infiltration of inflammatory cells into brain tissues. We further hypothesized that CTX might transiently enhance intracerebral tumor growth in immune-competent animals. To test these hypotheses, we evaluated for the presence of tumor and inflammation at day 7 after intracerebral inoculation of U87MG human glioma cells in both nude rats and genetically matched immune-competent heterozygous (rnu/wt) rats. We found that CTX pretreatment actually enhanced the brain inflammation response as determined by a hyperintense T2-weighted MRI signal in the inoculation area with gadolinium enhancement on T1 weighted MRI scans (Figure 5, A and B). The volume of the brain showing a signal change was larger in heterozygous rats (Figure 5B) than in nude rats (Figure 5A). Staining for CD31 (platelet endothelial cell adhesion molecule, a marker of neovascularization) was more intense and widespread in CTX-treated nude and heterozygous rats compared with their untreated counterparts. Staining for VEGF was higher in the CTX-treated rat brain but was not clearly cell-associated. Immunohistochemistry for macrophages (CD68) was elevated in the rat brains with CTX treatment (Figure 5A). Consistent with our previous results, the intracerebral U87MG tumor volume was larger in the CTX-treated nude rats than in the control rat even at the very early stage (7 days; Figure 5B). Heterozygous rats showed no tumor growth in the absence of CTX. Interestingly, CTX-treated heterozygous rats did develop some tumor mass along the inoculation track (Figure 5B). The presence of tumor cells was confirmed by staining for human nuclear antigen.
Figure 5.
Effect of cyclophosphamide on immune cell infiltration in rat brain. Male nude rats (A) and immune-competent heterozygous rats (B) were treated with saline (top panels) or CTX (100 mg/kg, i.p.; bottom panels) 24 hours before inoculation of U87MG human glioma cells in the caudate nucleus in the right hemisphere. At 7 days after cell implantation, MRI was performed and brains were immediately assessed for histology and immunocytochemistry. Images shown are T1-weighted MRI with gadolinium contrast (T1+Gd), hematoxylin histology (hematox), CD31 (neovascularization), and CD68 (macrophage) immunohistochemistry. Arrows indicate tumor.
Discussion
For many human tumor cells, xenografted human tumor growth in immunodeficient nude rats can be unsuccessful, inconsistent, or slow. We hypothesized that impairment of the innate immune response with systemic chemotherapy may increase the incidence and growth of metastases in brain and other tissues.
We initially hypothesized that the tumor-enhancing effects of CTX were due to the depletion of circulating white blood cells. The time course of immune cell depletion in our study was similar to that of previous studies in mice [23] and rats [24]. The cell type specificity of the immunomodulation by CTX is unclear. Cyclophosphamide decreased mouse total splenocyte number by 50% within 3 to 4 days after treatment, particularly CD19+ splenocytes and also decreased CD4+25+ regulatory T cells [12,13]. Alternatively, CTX may decrease the numbers of CD8+ T cells while actually increasing CD11b+ cells and CD44hi CD8+ memory lymphocytes [14]. Metronomic low-dose CTX selectively depleted regulatory T cells in cancer patients but also restored Tand natural killer effector functions [14,25]. Thus, it is clear that CTX can impact immune cells, although the exact immunomodulatory effects are not completely elucidated.
Although immune cell depletion supports the hypothesis that CTX acts by reducing innate immunity, the cell infiltration studies in rat brain do not. We found that CTX pretreatment leads to a widespread increase in MRI enhancement in the inoculated cerebral hemisphere after tumor cell implantation. Immunostaining suggests that this involves macrophage (CD68) infiltration into the brain. Macrophages seem to be directly involved in tumor progression and metastasis [26]. They may promote angiogenesis by producing angiogenic growth factors, including VEGF, and proteinases such as matrix metalloproteinase 9 [27]. Therefore, macrophages may both increase vascularization and enhance tumor cell movement toward and intravasation into more vessels. This places macrophages at the center of an invasion microenvironment.
Vascular endothelial growth factor plays an important role in tumor growth and metastasis, as an essential regulator of normal and abnormal blood vessel growth. Our study showed that CTX treatment elevated the VEGF concentration in normal rat serum by 11.6-fold at 1 week. Increased vascularization was seen on the surface of subcutaneous tumors (especially in SKOV3 ovarian tumor) in CTX-pretreated animals. Immunostaining for CD31 and VEGF was elevated in the rat brain at 7 days after the inoculation of human glioma in the CTX treatment group. CD31 is a multifunctional molecule involved in inflammation and vascular biology and is a marker for neovascularization [28,29]. These observations led to the hypothesis that CTX induces tumor growth secondary to modification of the vasculature with VEGF. In support of this hypothesis, Park et al. [30] demonstrated that CTX induced VEGF mRNA and protein expression in rat thymus. In contrast, others have shown that CTX inhibited basic fibroblast growth factor-stimulated neovascularization in the mouse, suggesting that it acts as an antiangiogenic agent [31].
Cyclophosphamide has also been shown to mobilize myeloid-derived suppressor and endothelial progenitor cells positive for CD34, CD133, and VEGF receptor 2, which may be associated with increased levels of VEGF [32]. The circulating endothelial progenitor cells are recruited to tumor capillaries and obviously play an important role in vascularization of malignant tumors for neo-angiogenesis [1,33]. Vascular endothelial growth factor receptor 2 was found to mediate macrophage infiltration into orthotopic pancreatic tumors in mice [34]. Similarly, we found increased macrophage infiltration into rat brain in Figure 5A with CTX treatment. Others have shown a positive correlation between circulating endothelial progenitor cell levels and tumor volume found in mouse models [35–37]. Other studies suggest that CTX may enhance metastasis due to vascular endothelial cell injury [38,39] or inhibition of a host-based cancer-killing process [15]. The mobilization of progenitor cells by CTX may attribute additional or alternative mechanisms involved in enhancement of tumor growth and metastasis.
Glutathione is an endogenous antioxidant involved in multiple cellular detoxification activities. Decreased GSH synthesis may be a response to CTX-induced oxidative stress [40]. Cyclophosphamide has been reported to increase [41,42] and decrease [43] GSH levels in various animal models, but the effect is variable depending on the tissue and time point tested. In patients with chronic lymphocytic leukemia, CTX caused a decrease in serum and lymphocyte GSH concentrations [44,45]. Impaired free radical scavenging activity in response to CTX may favor tumor growth [18].
Secondary malignancies caused by CTX have not been observed in our animal xenograft models but are seen infrequently in humans [3–6]. We suggest that in our studies, CTX did not cause new tumor neoplastic transformation but rather induced conditions that allowed the local and/or distant outgrowth of existing tumor cells in both CNS and non-CNS sites. In support of this, we found that CTX pretreatment increased the occurrence of brain metastasis from 0% to 20% and facial/bone metastasis from 20% to 100% in our LX-1 SCLC hematogenous model. Thus, in addition to promoting local tumor growth, CTX pretreatment supports metastasis formation, which is often not observed in xenograft models [7,8]. Together, our results indicate that CTX may enhance primary and metastatic tumor growth through multiple mechanisms. Lymphodepletion, decreased GSH, elevated VEGF, increased CD31-positive brain immunoreactivity, and macrophage infiltration after CTX treatment may provide the environment for increased brain and/or other tissue tumor growth.
A significant question is whether CTX can promote tumor growth and/or CNS metastasis in humans. The active metabolite of CTX has limited permeability across the blood-brain barrier and is unlikely to affect micrometastases within the sanctuary of the CNS [46]. The unexpected tumor growth- and metastasis-enhancing effect of CTX should be taken into consideration when deciding which cytotoxic chemotherapy treatment will be selected and applied to cancer patients.
Acknowledgments
The authors thank Sheila Taylor for her technical assistance.
Abbreviations
- CTX
cyclophosphamide
- GSH
glutathione
- MRI
magnetic resonance imaging
- SCLC
small cell lung carcinoma
- TE
echo time
- TR
relaxation time
- VEGF
vascular endothelial growth factor
Footnotes
Funding: This work was supported by a Veterans Administration Merit Review grant and National Institutes of Health grants NS33618, NS53468, and NS44687 from the National Institute of Neurological Disorders and Stroke to E.A.N.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 2.Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, Kerbel RS. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 3.Malik SW, Myers JL, DeRemee RA, Specks U. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am J Respir Crit Care Med. 1996;154:1851–1856. doi: 10.1164/ajrccm.154.6.8970380. [DOI] [PubMed] [Google Scholar]
- 4.Bernard-Marty C, Mano M, Paesmans M, Accettura C, Munoz-Bermeo R, Richard T, Kleiber K, Cardoso F, Lobelle JP, Larsimont D, et al. Second malignancies following adjuvant chemotherapy: 6-year results from a Belgian randomized study comparing cyclophosphamide, methotrexate and 5-fluorouracil (CMF) with an anthracycline-based regimen in adjuvant treatment of nodepositive breast cancer patients. Ann Oncol. 2003;14:693–698. doi: 10.1093/annonc/mdg204. [DOI] [PubMed] [Google Scholar]
- 5.Stein JP, Skinner EC, Boyd SD, Skinner DG. Squamous cell carcinoma of the bladder associated with cyclophosphamide therapy for Wegener's granulomatosis: a report of 2 cases. J Urol. 1993;149:588–589. doi: 10.1016/s0022-5347(17)36156-6. [DOI] [PubMed] [Google Scholar]
- 6.Stillwell TJ, Benson RC, Jr, DeRemee RA, McDonald TJ, Weiland LH. Cyclophosphamide-induced bladder toxicity in Wegener's granulomatosis. Arthritis Rheum. 1988;31:465–470. doi: 10.1002/art.1780310402. [DOI] [PubMed] [Google Scholar]
- 7.Dore JF, Bailly M, Bertrand S. Metastases of human tumors in experimental animals. Anticancer Res. 1987;7:997–1003. [PubMed] [Google Scholar]
- 8.Kamb A. What's wrong with our cancer models? Nat Rev Drug Discov. 2005;4:161–165. doi: 10.1038/nrd1635. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, Harsh GRT, Louis DN, Bartus RT, Hochberg FH, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 10.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65:11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 11.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, Kaur B, Louis DN, Weissleder R, Caligiuri MA, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 13.Motoyoshi Y, Kaminoda K, Saitoh O, Hamasaki K, Nakao K, Ishii N, Nagayama Y, Eguchi K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep. 2006;16:141–146. [PubMed] [Google Scholar]
- 14.Honeychurch J, Glennie MJ, Illidge TM. Cyclophosphamide inhibition of anti-CD40 monoclonal antibody-based therapy of B cell lymphoma is dependent on CD11b+ cells. Cancer Res. 2005;65:7493–7501. doi: 10.1158/0008-5472.CAN-04-3808. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi K, Yang M, Hayashi K, Jiang P, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 2008;68:516–520. doi: 10.1158/0008-5472.CAN-07-3063. [DOI] [PubMed] [Google Scholar]
- 16.van Putten LM, Kram LK, van Dierendonck HH, Smink T, Fuzy M. Enhancement by drugs of metastatic lung nodule formation after intravenous tumour cell injection. Int J Cancer. 1975;15:588–595. doi: 10.1002/ijc.2910150408. [DOI] [PubMed] [Google Scholar]
- 17.Kline I, Gang M, Tyrer DD, Mantel N, Venditti JM, Goldin A. Duration of drug levels in mice as indicated by residual antileukemic efficacy. Chemotherapy. 1968;13:28–41. doi: 10.1159/000220528. [DOI] [PubMed] [Google Scholar]
- 18.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y. Tumor angiogenesis and therapy. Biomed Pharmacother. 2005;59(Suppl 2):S340–S343. doi: 10.1016/s0753-3322(05)80070-8. [DOI] [PubMed] [Google Scholar]
- 20.Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13:1845–1857. doi: 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- 21.Neuwelt EA, Pagel MA, Kraemer DF, Peterson DR, Muldoon LL. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther. 2004;309:594–599. doi: 10.1124/jpet.103.063347. [DOI] [PubMed] [Google Scholar]
- 22.Soussain C, Muldoon LL, Varallyay C, Jahnke K, DePaula L, Neuwelt EA. Characterization and magnetic resonance imaging of a rat model of human B-cell central nervous system lymphoma. Clin Cancer Res. 2007;13:2504–2511. doi: 10.1158/1078-0432.CCR-06-2379. [DOI] [PubMed] [Google Scholar]
- 23.Anton E. Ultrastructural changes of stromal cells of bone marrow and liver after cyclophosphamide treatment in mice. Tissue Cell. 1997;29:1–9. doi: 10.1016/s0040-8166(97)80066-3. [DOI] [PubMed] [Google Scholar]
- 24.Nygaard UC, Lovik M. Blood and spleen lymphocytes as targets for immunotoxic effects in the rat-a comparison. Toxicology. 2002;174:153–161. doi: 10.1016/s0300-483x(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 25.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 27.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 29.Kalinowska A, Losy J. PECAM-1, a key player in neuroinflammation. Eur J Neurol. 2006;13:1284–1290. doi: 10.1111/j.1468-1331.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 30.Park HJ, Kim MN, Kim JG, Bae YH, Bae MK, Wee HJ, Kim TW, Kim BS, Kim JB, Bae SK, et al. Up-regulation of VEGF expression by NGF that enhances reparative angiogenesis during thymic regeneration in adult rat. Biochim Biophys Acta. 2007;1773:1462–1472. doi: 10.1016/j.bbamcr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 32.Mauro E, Rigolin GM, Fraulini C, Sofritti O, Ciccone M, De Angeli C, Castoldi G, Cuneo A. Mobilization of endothelial progenitor cells in patients with hematological malignancies after treatment with filgrastim and chemotherapy for autologous transplantation. Eur J Haematol. 2007;78:374–380. doi: 10.1111/j.1600-0609.2007.00831.x. [DOI] [PubMed] [Google Scholar]
- 33.Ribatti D. The involvement of endothelial progenitor cells in tumor angiogenesis. J Cell Mol Med. 2004;8:294–300. doi: 10.1111/j.1582-4934.2004.tb00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 35.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 37.Goon PK, Lip GY, Boos CJ, Stonelake PS, Blann AD. Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia. 2006;8:79–88. doi: 10.1593/neo.05592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwamoto Y, Fujita Y, Sugioka Y. YIGSR, a synthetic laminin peptide, inhibits the enhancement by cyclophosphamide of experimental lung metastasis of human fibrosarcoma cells. Clin Exp Metastasis. 1992;10:183–189. doi: 10.1007/BF00132750. [DOI] [PubMed] [Google Scholar]
- 39.Carmel RJ, Brown JM. The effect of cyclophosphamide and other drugs on the incidence of pulmonary metastases in mice. Cancer Res. 1977;37:145–151. [PubMed] [Google Scholar]
- 40.Manda K, Bhatia AL. Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol. 2003;19:367–372. doi: 10.1023/b:cbto.0000013342.17370.16. [DOI] [PubMed] [Google Scholar]
- 41.Abraham P, Sugumar E. Increased glutathione levels and activity of PON1 (phenyl acetate esterase) in the liver of rats after a single dose of cyclophosphamide: a defense mechanism? Exp Toxicol Pathol. 2008;59:301–306. doi: 10.1016/j.etp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Lopez SG, Luderer U. Effects of cyclophosphamide and buthionine sulfoximine on ovarian glutathione and apoptosis. Free Radic BiolMed. 2004;36:1366–1377. doi: 10.1016/j.freeradbiomed.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 43.Hosseinimehr SJ, Karami M. Chemoprotective effects of captopril against cyclophosphamide-induced genotoxicity in mouse bone marrow cells. Arch Toxicol. 2005;79:482–486. doi: 10.1007/s00204-005-0655-7. [DOI] [PubMed] [Google Scholar]
- 44.Bakan N, Taysi S, Yilmaz O, Bakan E, Kuskay S, Uzun N, Gundogdu M. Glutathione peroxidase, glutathione reductase, Cu-Zn superoxide dismutase activities, glutathione, nitric oxide, and malondialdehyde concentrations in serumof patients with chronic lymphocytic leukemia. Clin Chim Acta. 2003;338:143–149. doi: 10.1016/j.cccn.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Silber R, Farber CM, Papadopoulos E, Nevrla D, Liebes L, Bruck M, Brown R, Canellakis ZN. Glutathione depletion in chronic lymphocytic leukemia B lymphocytes. Blood. 1992;80:2038–2043. [PubMed] [Google Scholar]
- 46.Genka S, Deutsch J, Stahle PL, Shetty UH, John V, Robinson C, Rapoport SI, Greig NH. Brain and plasma pharmacokinetics and anticancer activities of cyclophosphamide and phosphoramide mustard in the rat. Cancer Chemother Pharmacol. 1990;27:1–7. doi: 10.1007/BF00689268. [DOI] [PubMed] [Google Scholar]