Abstract
Standard cell culture conditions do not reflect the physiological environment in terms of oxygen tension (20% vs 3%). The effects of lowering oxygen tension on cell proliferation in culture can be beneficial as well as detrimental depending on the cell line studied, but the molecular mechanism underlying such effects is not fully understood. We observed that the proliferative capacity of the rat cell lines NRK and INS-1 was inhibited when cultured under 3% oxygen as compared to 20% oxygen. Suppression of proliferation in NRK cells was accompanied by induction of DNA double strand breaks whereas in INS-1 cells it was accompanied by up-regulation of p53 and p27. Although Sirt1 was up-regulated in both cell lines by 3% oxygen the effects on antioxidant enzymes (MnSOD, CuZnSOD and catalase) were cell line specific. Marked up-regulation of heme oxygenase-1 (HO-1) was detected in both NRK and INS-1 cells when cultured in 3% oxygen. HO-1 expression can be readily induced by exposure to hydrogen peroxide in culture. These results suggest that reduced oxygen tension suppresses the proliferative capacity of these two cell lines through a stress response that is similar to an oxidative stress response but the molecular events that lead to the reduced cell proliferation are cell line specific.
Keywords: Oxygen tension, Cell proliferation, Oxidative stress, DNA damage, Antioxidant enzymes, Heme oxygenase-1, Sirt1, NRK, INS-1
Introduction
Standard in vitro culture is generally performed at atmospheric oxygen tension (∼ 20% O2) which is higher than that of the in vivo environment [1]. There is increasing evidence showing that such higher than physiological levels of oxygen tension can affect the growth, metabolism and functions of many cell lines in vitro [2]. For example, oxygen modulates growth of human skin fibroblasts with elevated oxygen tensions being inhibitory [3]. Furthermore, proliferative life span of human fibroblasts is inversely related to ambient oxygen tension [4,5]. Elevation of oxygen tension from 5% to atmospheric oxygen level can significantly alter morphology, growth and function of skin microvascular endothelial cells [6]. Moreover, mouse embryonic fibroblasts (MEFs) undergo senescence when cultured under standard culture conditions whereas such senescence can be abolished by culturing them in 3% O2 which is closer to physiological oxygen levels [7]. Increased oxidative stress may play a role in the effects observed in in vitro cultures under atmospheric oxygen tension. Indeed, MEFs accumulate high levels of oxidative DNA damage when cultured in 20% oxygen as compared to 3% oxygen [7].
Recently, however, adverse effects of reduced oxygen tension on the growth of some cell lines have been observed. Human T cell clones showed shorter lifespans and reduced proliferative capacities under reduced oxygen tension when compared to clones grown under standard culture conditions [8]. Similarly, culture under a low oxygen tension (5% O2) suppressed the proliferation of mouse embryonic stem cells [9]. However, it is poorly understood as to how reduced oxygen tension adversely affects cell proliferation in culture.
The aim of this study was to investigate the effects of low oxygen culture conditions on cell proliferation of two rat cell lines and examine molecular events that may underlie such effects. Here we show that the proliferative capacity of two rat cell lines, NRK (a kidney cell line) and INS-1 (an insulin-secreting cell line), was suppressed under 3% O2 conditions. This was accompanied by a stress response which is similar to that induced by oxidative stress. We also show differential effects of ambient oxygen tensions on DNA damage, cell-cycle regulatory proteins and some key antioxidant enzymes in these two cell lines.
Materials and methods
Cell culture
NRK cells were obtained from the America Type Culture Collection (CRL-6509, ATCC). The NRK cells were grown in Dulbecco's modified Eagle medium (DMEM; D6546, Sigma) supplemented with 10% fetal bovine serum (F7524, Sigma), 2 mM l-glutamine (G7513, Sigma) and 1% penicillin–streptomycin (P0781, Sigma). The INS-1 cells were grown in RPMI medium (R5886, Sigma) containing 10% fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 50 μM 2-mercaptoethanol, and 1% penicillin–streptomycin. The NRK and INS-1 cells were seeded at 1 × 104 cells per 60 mm dish and maintained in a humidified CO2 incubator (37 °C, 5% CO2) under oxygen tension of 3% and 20% respectively. 3% O2 conditions were achieved by using nitrogen gas to suppress the ambient oxygen in a multi-gas cell culture incubator (Sanyo Biomedical). The cells were cultured under these two oxygen conditions for 5 weeks and the cell number was determined from direct cell counts obtained using a hemocytometer. A sufficient number of dishes were prepared at the beginning of the experiments so that weekly cell counting did not involve repeated passage and splitting for the duration of 5 weeks. Thus the only variable for comparison of cell growth was the difference in oxygen tensions.
H2O2 treatment
IMR-90 cells at population doubling (PD) 24.5 were obtained from the America Type Culture Collection (ATCC). The cells were grown in ATCC modified Eagle's minimal essential medium supplemented with 10% fetal bovine serum (FBS, ATCC), 100 U/ml penicillin and 0.1 mg/ml streptomycin (penicillin–streptomycin solution from Invitrogen). Cells for H2O2 treatment were prepared from the exponential phase around PD 30. H2O2 treatment was carried out 24 h after seeding by incubating 2 × 106 cells in 100-mm dishes in 13 ml of the culture medium containing 600 μM H2O2 for 2 h. The cells were then re-fed with medium containing no H2O2 and incubated until harvesting.
NRK cells (5 × 105 cells/60 mm dish) were treated with H2O2 for 2 h and then cultured in H2O2-free medium for additional 5 h before harvesting. INS-1 cells tended to detach from the dish surface upon H2O2 treatment. Therefore INS-1 cells were treated with lower concentrations of H2O2 (60 and 120 μM) and incubated without changing the H2O2-containing medium until harvesting. All the above H2O2 treatment experiments were carried out under culture conditions of 20% oxygen tension.
Western blotting
Whole cell lysates were prepared by scraping and then homogenizing cells in Laemmli buffer [0.12 M Tris, pH 6.8, 4% (w/v) SDS, 20% (v/v) glycerol with protease inhibitors (cocktail from Sigma)]. Protein concentration was determined by the bicinchoninic acid (BCA) method (Sigma) with BSA as a standard. Protein (15 μg) was separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. Blots were probed with the following antibodies: anti-γH2AX (JBW301, Upstate), anti-p53 (MAB1746, R&D Systems), anti-p27 (ab7961, Abcam), anti-heme oxygenase-1 (ab13243, Abcam), anti-Sirt1 (07-131, Upstate), anti-MnSOD (06-984, Upstate), anti-CuZnSOD (AF3787, R&D Systems), anti-catalase (ab16731, Abcam) and anti-PDGF receptor α (sc-338, Santa Cruz). The bound primary antibodies were detected by horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences), followed by enhanced chemiluminescence (Amersham Biosciences). The densities of the bands were quantified using an Alpha Imager (Alpha Innotech Corporation, San Leandro, CA).
Immunofluorescence analysis
Cells on coverslips were fixed in 4% paraformaldehyde in PBS for 10 min followed by one wash with TBS [50 mM Tris–HCl (pH7.4), 150 mM NaCl], permeabilization in 0.2% Triton X-100 in PBS for 5 min, three washes with TBS and quenching in fresh 0.1% sodium borohydride in TBS. Coverslips were blocked with blocking buffer (10% horse serum, 1% BSA, 0.02% NaN3, 1 × PBS) for 1 h, washed with TBS and incubated with anti-γH2AX (07-164, Upstate) in 1% BSA in TBS overnight at 4 °C. After washing, the cells were incubated with 1:400 dilution of Alexa Fluor®555 (A21428, Molecular Probes) in the presence of DAPI for 45 min at room temperature in the dark, washed with TBS, air dried in the dark, mounted on glass slides using the ProLongTM Antifade Kit (Molecular Probes) and inspected with a Zeiss LSM510 Meta confocal laser scanning microscope.
Results
Effects of oxygen tension on cell proliferation
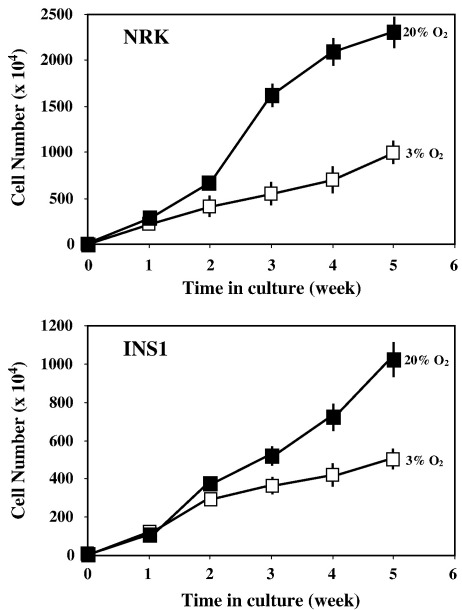
Comparison of cell proliferation under 3% and 20% oxygen tensions revealed that the proliferative capacity of NRK and INS-1 was suppressed by 3% oxygen tension (Fig. 1). The suppression of cell proliferation was not apparent in the first week of culture but became increasingly significant in the subsequent 4 weeks (Fig. 1).
Fig. 1.
Proliferation of NRK and INS-1 cells cultured under 3% and 20% oxygen tensions. Cells were seeded 1 × 104 cells/60 mm dish and the cell number was then counted at the indicated times. Means of three experiments are shown. Closed square, 20% O2; open square, 3% O2 (± SEM indicated by error bars).
Effects of oxygen tension on DNA double strand breaks
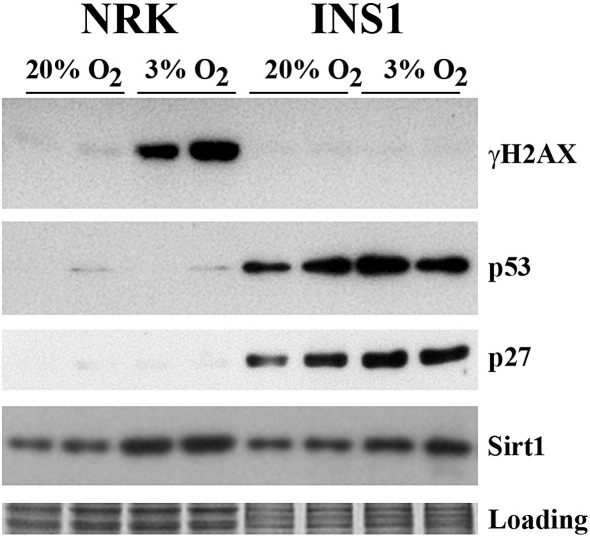
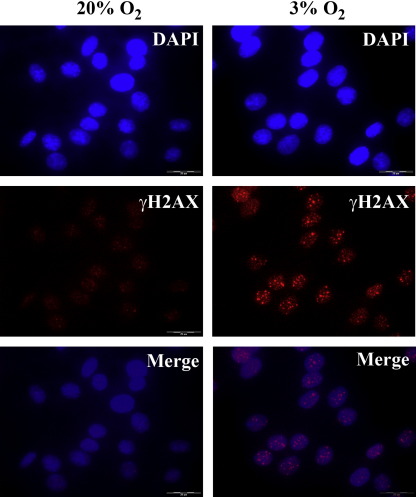
Western blot analysis revealed that γH2AX was markedly induced (19-fold increase) in NRK cells when cultured under 3% oxygen tension, but this induction was absent in INS-1 cells (Fig. 2). γH2AX is a phosphorylated histone protein H2AX, which is a biomarker of DNA double strand breaks [10]. Consistent with the observation by Western blotting, DNA damage foci were readily detected in the nuclei of NRK cells when cultured under 3% oxygen tension but absent in INS-1 cells (Fig. 3, data not shown for INS-1 cells). The appearance of some fainter foci in the NRK cells at 20% oxygen is likely due to the potential DNA replication-associated lesions that may occur in S-phase during the cell cycle [11].
Fig. 2.
Effects of oxygen tension on γH2AX, cell-cycle regulatory proteins and Sirt1 levels. After culturing under 3% and 20% oxygen tensions for 5 weeks NRK and INS-1 cells were harvested and two replicates for each condition were analysed by Western blotting using primary antibodies as indicated. Equal loading was confirmed by Coomassie blue staining.
Fig. 3.
Detection of DNA double strand breaks in NRK cells. After culturing under 3% and 20% oxygen tensions for 5 weeks NRK cells were fixed and stained with antibodies against γH2AX. Nuclei were revealed by DAPI staining.
Effects of oxygen tension on Sirt1 expression
Sirt1 is a deacetylase that regulates various physiological responses including cell fate, stress and ageing [12]. The above finding that 3% oxygen tension induced DNA double strand breaks, together with recent observations that Sirt1 is involved in DNA damage response and DNA repair [13,14] prompted us to determine the expression of Sirt1 in these two cell lines. As shown in Fig. 2, the expression of Sirt1 was elevated by 3% oxygen tension in both NRK (1.8-fold increase) and INS-1 cells (1.4-fold increase).
Effects of 3% oxygen tension on the protein levels of p53 and p27
As γH2AX and DNA damage foci were not detectable in INS-1 cells despite their proliferation being suppressed in 3% oxygen (Figs. 1 and 2), we then investigated levels of cell-cycle regulatory proteins p53 and p27. As shown in Fig. 2 levels of p53 and p27 were elevated in INS-1 cells by 3% oxygen tension (1.6- and 1.7-fold increase respectively). However, these two proteins were barely detectable in NRK cells (Fig. 2). We failed to detect p21 in these two cell lines under the two different oxygen tensions (data not shown).
Effects of oxygen tension on expression of heme oxygenase-1 and induction of the enzyme by oxidative stress
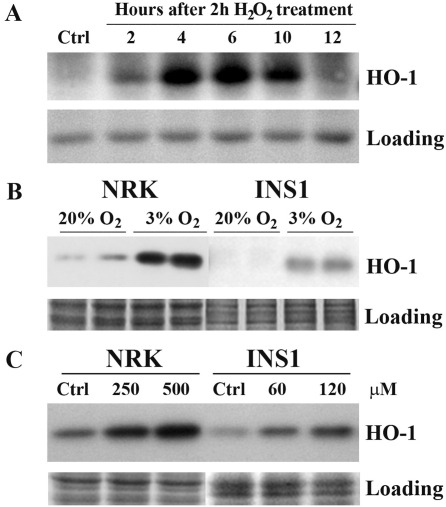
We previously demonstrated that oxidative stress by hydrogen peroxide induced DNA double strand breaks which resulted in premature senescence in human fibroblasts [15,16]. As shown in Fig. 4A oxidative stress rapidly induced heme oxygenase-1 expression in human fibroblast IMR-90 cells. Interestingly heme oxygenase-1 was markedly induced by 3% oxygen tension in both NRK (6.4-fold increase) and INS-1 cells (13.2-fold increase) (Fig. 4B). Similar to the observation in IMR-90 cells heme oxygenase-1 in NRK and INS-1 cells were also readily induced by H2O2 treatment (Fig. 4C).
Fig. 4.
Induction of heme oxygenase-1 (HO-1) by H2O2 treatment and by 3% oxygen tension. (A) Human fibroblast IMR-90 cells were treated with 600 μM of H2O2 for 2 h and then cultured in H2O2-free medium and harvested at the indicated time points for Western blotting. Culture conditions for this experiment were 20% oxygen tension. (B) NRK and INS-1 cells were cultured under 3% and 20% oxygen tensions for 5 weeks and then harvested for Western blotting. Two replicates for each condition were analysed. (C) NRK cells were treated with the indicated concentrations of H2O2 for 2 h and then cultured in H2O2-free medium for a further 5 h until harvested for Western blotting. INS-1 cells were treated with the indicated concentrations of H2O2 for 7 h before harvested for Western blotting. Culture conditions for this experiment were 20% oxygen tension. Equal loading was confirmed using an anti-actin antibody and Coomassie blue staining respectively.
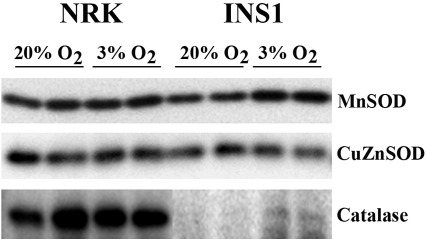
Effects of 3% oxygen tension on antioxidant enzymes
The above observation that heme oxygenase-1 was induced in 3% oxygen prompted us to assess the effects of the two different oxygen tensions on expression of antioxidant enzymes. Western blot analysis revealed that levels of MnSOD, CuZnSOD and catalase in NRK cells were not affected by the two different oxygen tensions (Fig. 5). However, the expression of catalase and MnSOD in INS-1 cells were noticeably up-regulated by 3% oxygen tension: whilst undetectable under 20% oxygen conditions, catalase became clearly detectable under 3% oxygen and MnSOD showed a 1.4-fold increase (Fig. 5).
Fig. 5.
Effects of oxygen tension on expression levels of antioxidant enzymes. After culturing under 3% and 20% oxygen tensions for 5 week NRK and INS-1 cells were harvested and two replicates for each condition were analysed by Western blotting using primary antibodies as indicated. Equal loading was confirmed by Coomassie blue staining.
Discussion
In this study we investigated the effects of two ambient oxygen tensions on cell proliferation of a rat kidney epithelial cell line, NRK and a rat insulinoma cell line, INS-1. Contrary to our expectations 3% oxygen tension suppressed the proliferative capacity of these two cell lines as compared to the standard 20% oxygen culture conditions. This is surprising considering the fact that 3% oxygen tension more accurately reflects physiological oxygen levels whereas atmospheric oxygen tension of 20% is regarded as hyperoxic and can cause oxidative stress to cells [17]. Nevertheless, similar suppressive effects of low oxygen tensions on cell proliferation have been observed in human T cell clones [8] and mouse embryonic stem cells [9]. Interestingly molecular mechanisms that underlie the suppression of the proliferative capacity in NRK and INS-1 cell lines are different. The suppression of proliferation of NRK cells is accompanied by induction of DNA double strand breaks whereas in INS-1 cells the suppression was related to the elevation of inhibitory cell-cycle regulatory proteins p53 and p27.
Although the molecular pathways linking low oxygen tension and induction of DNA double strand breaks in NRK cells and elevation of p53 and p27 in INS-1 cells remain to be established, the induction of heme oxygenase-1 (HO-1) in both cell lines suggested that a stress response pathway is activated. HO-1 is an enzyme that catalyzes the degradation of heme by opening its tetrapyrrole ring structure to yield biliverdin, free iron and carbon monoxide [18]. Biliverdin is rapidly reduced by biliverdin reductase to bilirubin, which has antioxidant effects [18]. HO-1 can be induced by various stressors including oxidative stress and the induction of HO-1 is suggested to have some beneficial effects on protection of cells against oxidative stress as an adaptive cellular defense mechanism [19-23]. The induction of HO-1 by hydrogen peroxide together with the observation that HO-1 was induced in both rat cell lines under 3% oxygen suggest that 3% oxygen tension may cause a stress response that is similar to an oxidative stress response in these two rat cell lines. In agreement with this suggestion we observed elevated expression of Sirt1 in these two cell lines cultured in 3% oxygen. As Sirt1 is involved in promoting DNA repair and cell survival [13,24], it is likely that Sirt1 is up-regulated to mitigate the adverse effects imposed by 3% oxygen tension. However, it is worth noting that HO-1 has an antiproliferative effect which is likely to be mediated by the production of carbon monoxide [23]. The antiproliferative effect of HO-1 was reported in vascular smooth muscle cells and was suggested to be through the activation of cGMP and p38 MAP kinase [25] and by autocrine regulation of specific components of the cell-cycle machinery to limit vascular smooth muscle cell proliferation [26].
Changing ambient oxygen tension has frequently been used as a method of applying a controlled level of oxidative stress to cells [27,28]. However, the results obtained in this study demonstrated that 3% oxygen tension exerted deleterious effects on cell proliferative capacity suggesting that low oxygen tension, despite being closer to physiological levels, is not always beneficial to cell growth in culture. It has been reported that low oxygen tension can cause a down-regulatory effect on receptor binding and expression in certain cell lines [29]. We previously observed that PDGF receptor α decreased markedly in human fibroblasts during cellular senescence and suggested that this decrease might account for, at least in part, the loss of proliferative capacity in the senescent cells [30]. When we measured the expression of PDGF receptor α in these two rat cell lines in this study we found that its expression was not affected by the two different oxygen tensions used (data not shown). However, it remains to be investigated whether binding of PDGF to its receptor, or indeed binding of other growth factors to their receptors, or the expression of other growth factor receptors in these two cell lines are affected by different ambient oxygen tensions.
Superoxide dismutase (MnSOD and CuZnSOD) and catalase are enzymes that catalyze the breakdown of superoxide into hydrogen peroxide and further into water and oxygen and thus are the major antioxidant defense system for protection of oxidative damage in the cells. The lack of effects of the two different oxygen tensions on three major antioxidant enzymes (MnSOD, CuZnSOD and catalase) in NRK cells may suggest that alteration in metabolism of reactive oxygen species (ROS) is not responsible for the reduced cell proliferative capacity in this cell line. However, 3% oxygen tension caused up-regulation of MnSOD and catalase in the INS-1 cell line. As INS-1 is a cell line derived from rat insulinoma, this is in line with an earlier report that antioxidant enzyme activities respond differently to ambient oxygen tension in tumor and normal cells with MnSOD being more readily inducible in transformed cells [31].
The observations made in this study suggest that the cellular response to oxygen tension is not as straightforward as often assumed. 3% oxygen may indeed be closer to physiological oxygen levels for most cells in vivo, except some cell types such as lung fibroblasts and corneal epithelia which normally reside in tissues with physiological oxygen pressures much closer to atmospheric 20%. Such a physiologically appropriate oxygen tension does not always result in a growth superior to that attainable under any other conditions in which the cells could be grown.
In summary, this study demonstrated that low oxygen tension suppressed the cell proliferative capacity in NRK and INS-1 cell lines. This is accompanied by induction of DNA double strand breaks in NRK cells and up-regulation of p53 and p27 in INS-1 cells. Thus, although low oxygen tension induced a common stress response pathway (up-regulation of heme oxygenase-1) that is similar to an oxidative stress response in both cell lines, the molecular events that lead to the reduced cell proliferation in these two cell lines are cell line specific.
Acknowledgments
This work was supported by the BBSRC, the European Union and the British Heart Foundation. We thank Dr. Fiona Gribble for kindly providing INS-1 cells.
References
- 1.Sullivan M., Galea P., Latif S. What is the appropriate oxygen tension for in vitro culture? Mol. Hum. Reprod. 2006;12:653. doi: 10.1093/molehr/gal081. [DOI] [PubMed] [Google Scholar]
- 2.Csete M. Oxygen in the cultivation of stem cells. Ann. N. Y. Acad. Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 3.Balin A.K., Pratt L. Oxygen modulates the growth of skin fibroblasts. In Vitro Cell Dev. Biol. Anim. 2002;38:305–310. doi: 10.1290/1071-2690(2002)038<0305:OMTGOS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q., Fischer A., Reagan J.D., Yan L.J., Ames B.N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balin A.K., Fisher A.J., Anzelone M., Leong I., Allen R.G. Effects of establishing cell cultures and cell culture conditions on the proliferative life span of human fibroblasts isolated from different tissues and donors of different ages. Exp. Cell Res. 2002;274:275–287. doi: 10.1006/excr.2002.5485. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L., Dosanjh A., Chen H., Karasek M. Divergent effects of extracellular oxygen on the growth, morphology, and function of human skin microvascular endothelial cells. J. Cell Physiol. 2000;182:134–140. doi: 10.1002/(SICI)1097-4652(200001)182:1<134::AID-JCP15>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duggan O., Hyland P., Annett K., Freeburn R., Barnett C., Pawelec G., Barnett Y. Effects of a reduced oxygen tension culture system on human T cell clones as a function of in vitro age. Exp. Gerontol. 2004;39:525–530. doi: 10.1016/j.exger.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Kurosawa H., Kimura M., Noda T., Amano Y. Effect of oxygen on in vitro differentiation of mouse embryonic stem cells. J. Biosci. Bioeng. 2006;101:26–30. doi: 10.1263/jbb.101.26. [DOI] [PubMed] [Google Scholar]
- 10.Stucki M., Jackson S.P. gammaH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair (Amst) 2006;5:534–543. doi: 10.1016/j.dnarep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Bartek J., Lukas C., Lukas J. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 12.Michan S., Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong J., Juhn K., Lee H., Kim S.H., Min B.H., Lee K.M., Cho M.H., Park G.H., Lee K.H. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp. Mol. Med. 2007;39:8–13. doi: 10.1038/emm.2007.2. [DOI] [PubMed] [Google Scholar]
- 14.Gorospe M., de Cabo R. AsSIRTing the DNA damage response. Trends Cell Biol. 2008;18:77–83. doi: 10.1016/j.tcb.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J.H., Ozanne S.E., Hales C.N. Heterogeneity in premature senescence by oxidative stress correlates with differential DNA damage during the cell cycle. DNA Repair (Amst) 2005;4:1140–1148. doi: 10.1016/j.dnarep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Chen J.H., Stoeber K., Kingsbury S., Ozanne S.E., Williams G.H., Hales C.N. Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J. Biol. Chem. 2004;279:49439–49446. doi: 10.1074/jbc.M409153200. [DOI] [PubMed] [Google Scholar]
- 17.Davies K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 18.Galbraith R. Heme oxygenase: who needs it? Proc. Soc. Exp. Biol. Med. 1999;222:299–305. doi: 10.1177/153537029922200313. [DOI] [PubMed] [Google Scholar]
- 19.Deshane J., Wright M., Agarwal A. Heme oxygenase-1 expression in disease states. Acta Biochim. Pol. 2005;52:273–284. [PubMed] [Google Scholar]
- 20.Oztezcan S., Kirgiz B., Unlucerci Y., Dogru-Abbasoglu S., Bilge H., Uysal M., Aykac-Toker G. Heme oxygenase induction protects liver against oxidative stress in X-irradiated aged rats. Biogerontology. 2004;5:99–105. doi: 10.1023/B:BGEN.0000025073.46100.01. [DOI] [PubMed] [Google Scholar]
- 21.Pascal T., Debacq-Chainiaux F., Boilan E., Ninane N., Raes M., Toussaint O. Heme oxygenase-1 and interleukin-11 are overexpressed in stress-induced premature senescence of human WI-38 fibroblasts induced by tert-butylhydroperoxide and ethanol. Biogerontology. 2007;8:409–422. doi: 10.1007/s10522-007-9084-8. [DOI] [PubMed] [Google Scholar]
- 22.Ryter S.W., Choi A.M. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid. Redox. Signal. 2002;4:625–632. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- 23.Courtney A.E., Maxwell A.P. Heme oxygenase 1: does it have a role in renal cytoprotection? Am. J. Kidney Dis. 2008;51:678–690. doi: 10.1053/j.ajkd.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 24.Chen W.Y., Wang D.H., Yen R.C., Luo J., Gu W., Baylin S.B. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Otterbein L.E., Zuckerbraun B.S., Haga M., Liu F., Song R., Usheva A., Stachulak C., Bodyak N., Smith R.N., Csizmadia E., Tyagi S., Akamatsu Y., Flavell R.J., Billiar T.R., Tzeng E., Bach F.H., Choi A.M., Soares M.P. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat. Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 26.Peyton K.J., Reyna S.V., Chapman G.B., Ensenat D., Liu X.M., Wang H., Schafer A.I., Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002;99:4443–4448. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]
- 27.von Zglinicki T., Saretzki G., Docke W., Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 28.Ho H.Y., Cheng M.L., Cheng P.F., Chiu D.T. Low oxygen tension alleviates oxidative damage and delays cellular senescence in G6PD-deficient cells. Free Radic. Res. 2007;41:571–579. doi: 10.1080/10715760601184819. [DOI] [PubMed] [Google Scholar]
- 29.Falanga V., Takagi H., Ceballos P.I., Pardes J.B. Low oxygen tension decreases receptor binding of peptide growth factors in dermal fibroblast cultures. Exp. Cell Res. 1994;213:80–84. doi: 10.1006/excr.1994.1175. [DOI] [PubMed] [Google Scholar]
- 30.Chen J.H., Ozanne S.E., Hales C.N. Analysis of expression of growth factor receptors in replicatively and oxidatively senescent human fibroblasts. FEBS Lett. 2005;579:6388–6394. doi: 10.1016/j.febslet.2005.09.102. [DOI] [PubMed] [Google Scholar]
- 31.Allen R.G., Balin A.K. Effects of oxygen on the antioxidant responses of normal and transformed cells. Exp. Cell Res. 2003;289:307–316. doi: 10.1016/s0014-4827(03)00279-9. [DOI] [PubMed] [Google Scholar]