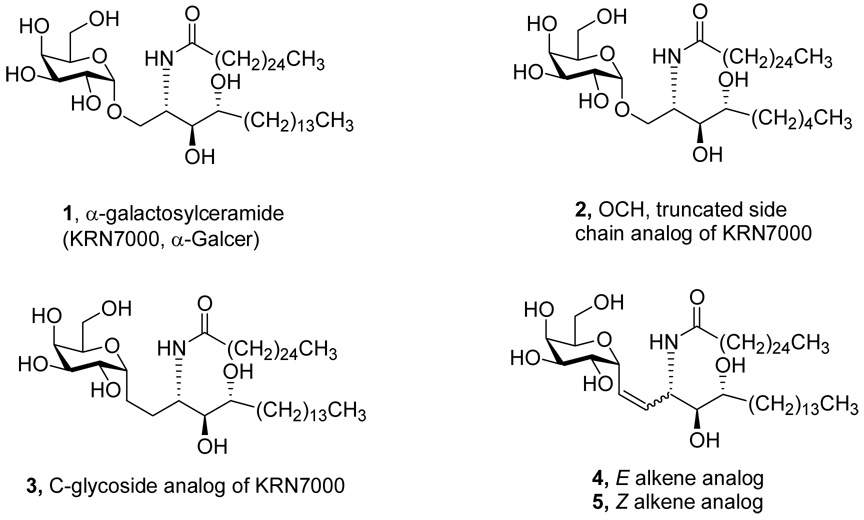
In the early 90’s, researchers at Kirin Pharma reported the results of their studies of glycolipid extracts of Agelas Mauritianus, an Okinawan sponge. An optimized synthetic material, α-galactosylceramide 1 (Figure 1, also known as KRN7000 and α-Galcer), a slightly simplified version of the active materials found in the sponge extracts, displayed potent anti-tumor activity in a whole animal murine assay. i
Figure 1.
α-galactosylceramide 1 (KRN7000) and its analogues
An intensive series of biological experiments in the ensuing decade unveiled the activity of the α-Galcer as stemming from its immunostimulant activity. ii Thus, α-Galcer is not cytotoxic, and high-throughput cell-based assays would not have revealed its activity. The molecular basis for α-Galcer stimulation of the immune system is its initial binding to a protein receptor, named CD1d, found on the surface of antigen presenting cells. The complex between the receptor and the α-Galcer has been crystallized and analyzed by X-ray diffraction and shows that two lipid chains of the ceramide are buried in two hydrophobic channels of the protein and that the 2,3 hydroxyls of galactose and the 3 hydroxyl of the phytosphingosine side-chain participate in hydrogen bonds with the protein. iii This binding motif leaves the 4 and 6 hydroxyls of the α-Galcer available to be recognized by receptors on natural killer T cells. In a very recent disclosure, the crystal structure of the triple complex of CD1d/ α-galcer/NKT receptor shows the binding of the gal-4-OH to the NKT, but the 6-OH seems to be free. iv The effect of the second protein molecule’s recognition of the initial complex is to produce a powerful surge of cytokines interferon-γ, (IFNγ), interleukin-4 (IL-4) and interleukin-12 (IL-12) which then signal for the eventual cytotoxic immune response. Extensive analog studies in the O-glycoside series revealed the importance of the galactose configuration and the free hydroxyls. The only allowed substitution of the galactose OH’s is N-acyl at the 6-position of galactose. Variation of lipid chain length and degrees of unsaturation in both the phytosphingosine and N-acyl side-chains are permissible. v In most cases, small changes in cytokine levels are the result; but in one striking example, shortening the lipid of the phytosphingosine from 14 to 5 carbons (Figure 1, compound 2, named as OCH) stimulates almost exclusive production of IL-4 with a great diminution of IFNγ and IL-12. In immunology terms, this change is said to favor a TH2 immune response whereas the reverse, a decrease in IL-4 and an increase in the other cytokines is called a TH1 response. In the mouse, the TH2 effect offers protection against autoimmune diseases while the TH1 pathway protects against “foreign” pathogens.
The investigation of α-Galcer in our laboratory began with the concept that the C-glycoside 3 might provide a more deep-seated change in the structure-activity profile. Aside from the obvious stability to enzymatic hydrolysis provided by replacement of anomeric O by CH2, the important changes are (i) the loss of one hydrogen-bond acceptor and (ii) the destabilization of the galactose chair through replacement of axial O-anomeric stabilization with axial CH2 steric repulsion. In the event, the C-glycoside 3 was prepared and we were rewarded with striking enhancement of in vivo activity in blocking malaria and melanoma in mice. Assay for cytokines revealed that IFNγ and IL-12 levels were about the same as for the O-glycoside while the IL-4 titre was dramatically reduced. We also studied the E and Z alkene analogs 4 and 5 and found that the E material 4 was active whereas the Z analog 5 was essentially inert in our assays (Figure 1).vi
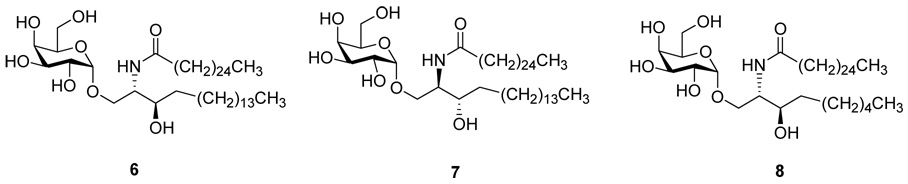
Since stereochemistry in both the galactose and in the linker region had been found to be a critical element for activity, we deemed it worthwhile to examine the effects of stereoisomers in the phytosphingosine chain. There had been an early report of a study in the O-series where the 4 possible stereoisomers 6 bearing the 4-deoxyphytosphingosine chain were studied.vii Interestingly, the only isomer other than the natural 2S, 3R to show tumor growth inhibitory effects was 7 with the phytosphingosine in the unnatural, enantiomeric 2R, 3S form. Also, there has been a recent evaluation of the natural 4-deoxy series with the exact analogs of both α-Galcer and OCH. The activities 6 and 8 in the 4-deoxy series were similar to those of the parent materials (Figure 2).viii
Figure 2.
4-deoxy analogs 6, 7 and 8 of α-Galcer and OCH
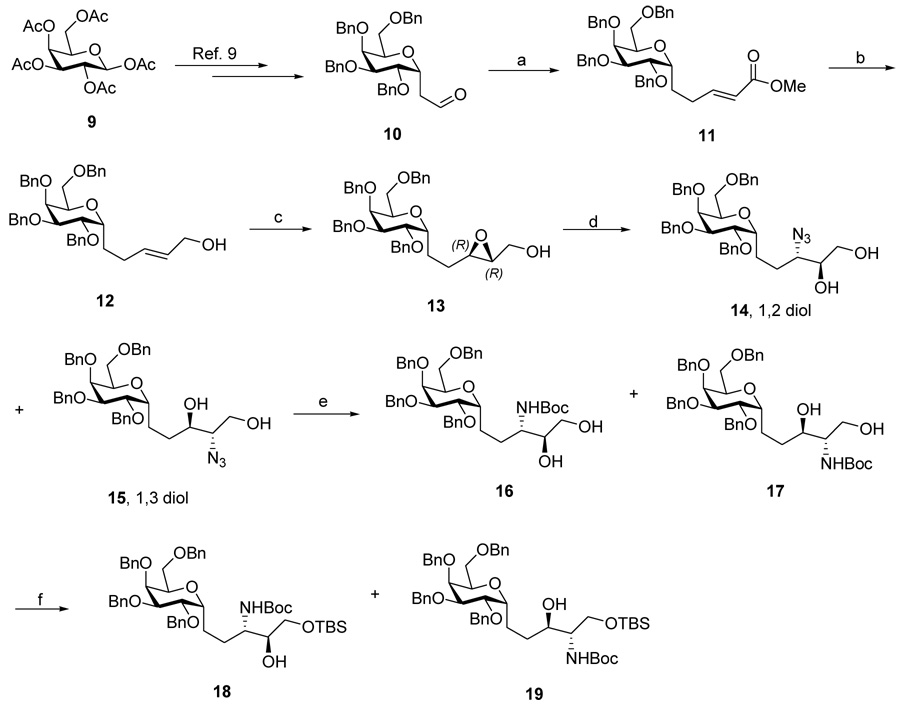
Since our earlier preparations of C-glycoside 3 had been convergent and involved coupling of intact phytosphingosines with galactose, they would not be suitable for our proposed stereoisomerism study. Thus, we embarked on a scheme where the phytosphingosine was elaborated from a pre-formed C-glycoside which we now describe.ix
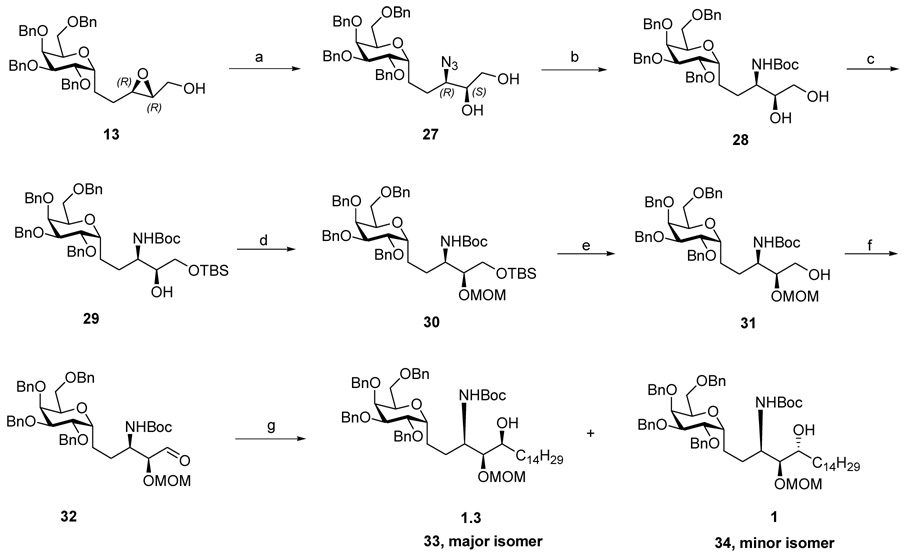
The known aldehyde 10 was obtained in 5 steps from commercially available β-D-galactose pentaacetate 9 according to a literature procedure (Scheme 1).10 Homologation of 10 via Wadsworth-Horner-Emmons reaction afforded (E)-α, β unsaturated ester 11. DIBAL-H reduction gave the (E)-allylic alcohol 12 in 92% yield. Sharpless asymmetric epoxidation (SAE)11 using substoichiometric amount of catalysts (50 mol% TTIP, 60 mol% D-(-)-DIPT) ensured conversion of 12 to (2R, 3R) epoxy alcohol 13 in high enantiomeric excess (ee>95%). Opening the (2R, 3R) epoxy alcohol 13 using NaN3/NH4Cl in a simple SN2 conversion provided an inseparable mixture of desired 3-azido-1,2 vicinal diol 14 and by-product 1,3 diol 15 in 90% yield.12 The 8/1 ratio of 14/15 was determined at a later stage. Thus, the mixture of azides was reduced with P(Me)3 to provide amine intermediate,13 which was then protected with (t-Boc)2O to afford an inseparable mixture of regioisomers 16 and 17. To our delight, selective protection of the primary hydroxyl group as its TBS ether gave a separable mixture of 18 and 19 in a ratio of 8/1 in 90% yield. To assign their structures, 18 and 19 were deprotected with TBAF and the products were treated with NaIO4. Only a compound derived from 1,2 diol could be cleaved by NaIO4 to give the corresponding aldehyde while the 1,3 diol could not be cleaved by NaIO4. In this way, 18 was confirmed to be the 1,2-vicinal diol.
Scheme 1.
Regents and conditions: (a). (MeO)2POCHCO2Me, MeCN, r.t, overnight, 89%. (b). DIBAL-H, CH2Cl2, −78°C, 2h, 90%. (c).TTIP,D-(-)-DIPT,TBHP, 4 A MS, CH2Cl2, −20 °C, 18h, 70%. (d). NaN3/NH4Cl, MeOH/H2O(8:1), 80°C, overnight, 93%. (e). i. P(Me)3, THF, overnight; ii.1N NaOH, 2h; iii. (t-Boc)2O, satd.NaHCO3, THF/1, 4-dioxane(1:1), r.t, overnight, 78% for three steps.(f). TBSCl, imidazole, CH2Cl2, r.t., 88%.
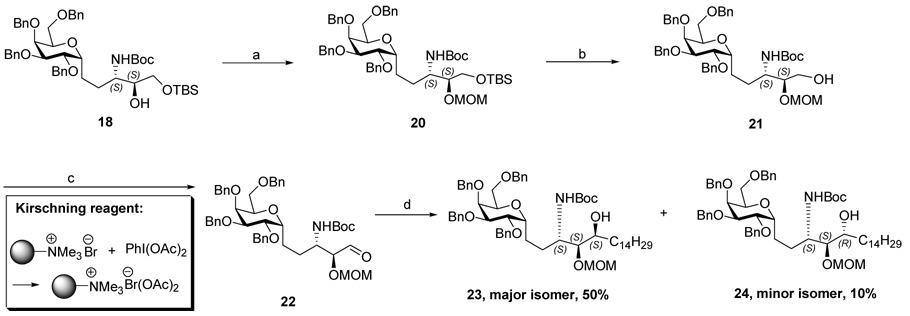
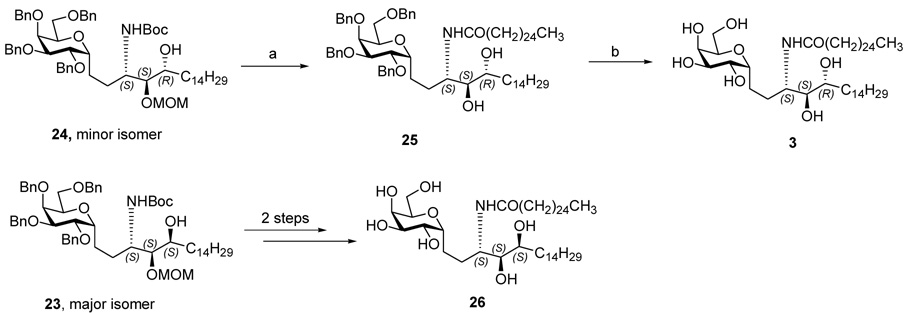
Protection of the secondary hydroxyl of compound 18 with MOM and removal of the TBS group gave primary alcohol 21 in high yield. Oxidation of alcohol to aldehyde 22 proceeded without epimerization at the neighboring α stereocenter using either Dess-Martin periodinane or a novel Kirschning solid phase oxidant, 14 both procedures taking place in high yield without causing epimerization at the α position. Inverse addition of the crude aldehyde 22 to freshly prepared tetradecyl magnesium bromide (C14H29MgBr) provided a separable mixture of 23 and 24 in a ratio of 5:1 and in 60% combined yield for two steps. Based on the “Cram chelation control rule”, 15 the stereochemistry of 23 and 24 was tentatively assigned as (3S, 4S, 5S) and (3S, 4S, 5R) respectively with the minor isomer 24 proposed as the desired, or natural, (3S, 4S, 5R) configuration at the three contiguous stereocenters. The structure assignment was confirmed later by correlation with the corresponding final products (Scheme 2)
Scheme 2.
Reagents and conditions: (a). MOMCl, DIPEA, CH2Cl2, 0°C -r.t, 12h, 95%. (b). TBAF, THF, r.t, 2 h, 100%. (c). Kischning reagent, TEMPO(cat.), CH2Cl2, rt, 2h, or Dess-Martin oxidation, CH2Cl2, rt, 1h, quantitative. (d). C14H29MgBr, THF, 0°C-rt., 2h, 58% for two steps.
Acidic hydrolysis of 24 removed MOM and Boc groups in one step and the resulting amine was converted to amide 25 in 80% yield. Hydrogenation of amide 25 using Pearlman’s catalyst (Pd(OH)2/C) in THF/EtOH solution afforded the fully deprotected C-glycoside analogue of KRN7000 3. Its NMR spectra matched with those of the previously synthesized C-glycoside analogue of KRN7000 (see supporting information). Similarly, the major isomor 23 was converted to the corresponding C-glycoside analogue 26, which is a 5-OH epimer of the target C-glycoside analogue of the KRN7000 (Scheme 3)
Scheme 3.
Reagents and conditions: (a). i. HCl, THF/MeOH,1,4-dioxane(1:1:1), r.t. overnight; ii. CH3(CH2)24COOPhNO2, DMAP, THF, r.t, 24 h, 72% for two steps. (b).H2, Pd(OH)2/C, THF/EtOH(1:1), 24h, 42%. For compound 26, 35% for three steps.
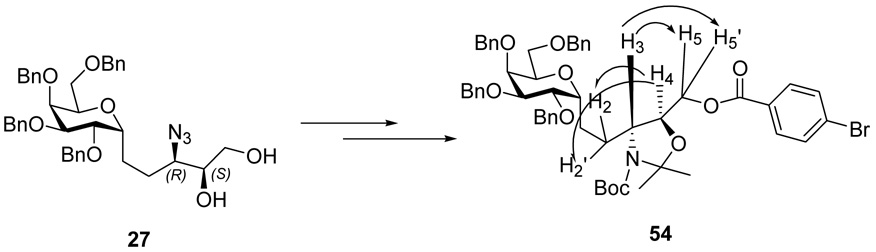
As described above, the key step of opening of epoxide 13 by sodium azide was not a very clean reaction, requiring extended reaction time in refluxing MeOH. Furthermore, about 10% of the regioisomeric azide (at C-4) which was obtained required separation via careful chromatography at a later stage. Therefore, we adopted the Ti(O-i-Pr)2(N3)2 conditions described by Sharpless.16 In our case, treatment of the (2R, 3R) epoxy alcohol 13 with Ti(O-i-Pr)2(N3)2 gave a single product which we assigned as the desired anti-3-azido-2-OH 14 on the basis of the hypothesis that ring opening of the oxirane was a single SN2 reaction as in the sodium azide experiment. Spectral comparison with authentic 14 was not definitive because the batch obtained in the sodium azide experiment was a mixture. In actual fact, the Ti(O-i-Pr)2(N3)2 single product was ultimately revealed to be 3-azido-1, 2 vicinal diol 27. As our synthesis proceeded via duplication of the sequence described above, detailed comparison of the final products derived from the Ti(O-i-Pr)2(N3)2 opening of the epoxy alcohol 13 did not match with those compounds derived from NaN3 opening. The correct structure assignment in this section (Scheme 4) reveals a series that is diastereomeric to those obtained from the sodium azide opening. The (2S, 3R) epoxy alcohol 27 was converted to aldehyde 32 in an identical manipulation sequence as described in the previous section. Grignard reaction between the aldehyde 32 and C14H29MgBr gave a separable mixture, and the ratio of the major isomer 33 and minor isomer 34 was 1.3:1. The stereochemical assignment was based on the “Cram-chelation control” model as was shown to be operative in the epimeric series (Scheme 4).
Scheme 4.
Reagents and conditions: (a) Ti(i-OPr)2(N3)2, Benzene, 70 °C,10 min, 64%. (b) i. P(Me)3, THF, r.t, 18h; ii. 1N NaOH, 2h; iii. (t-Boc)2O, THF/1,4 -dioxane(1:1), satd. NaHCO3, r.t,12h; 91% for 3 steps. (c) TBSCl, imidazole, CH2Cl2, r.t, 2 h, 99%. (d) MOMCl, DIPEA, CH2Cl2, 0°C-r.t, 24h, 99%. (e) TBAF, THF, 0°C-r.t, 2h, 92%. (f) Dess-Martin oxidation, CH2Cl2, r.t, 2h. (g) C14H29MgBr, THF, 0°C-r.t, 2 h, 50% combined yield for 2 steps.
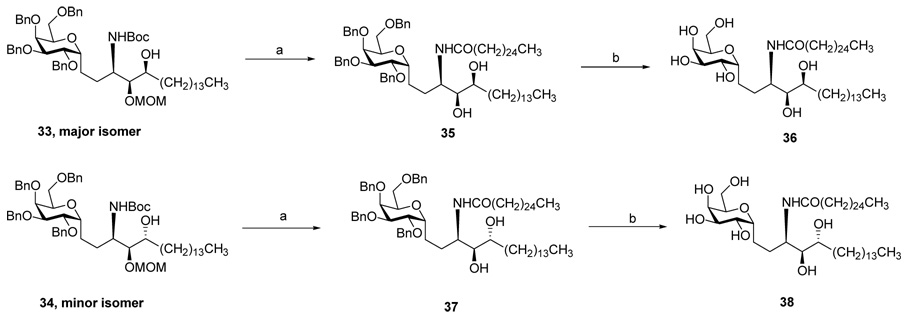
Both major isomer 33 and minor isomer 34 were converted to the corresponding C-glycoside analogues 36 and 38 respectively (Scheme 5). The revelation that neither of the NMR spectra of these two final products (see supporting information) matched with those of the α-C-Galcers from the sodium azide sequence forced us to assign their stereochemistries and to account for a divergence from the expected stereochemical outcome.
Scheme 5.
Reagents and conditions: (a) i. 6N HCl, THF/MeOH (1:1) overnight; ii. CH3 (CH2)24COOPhNO2, DMAP, THF, 24h. (b) Pd(OH)2 /C, THF/ EtOH (1:1), H2, 24h. 54% for 36, three steps from 33; 55% for 38, three steps from 34.
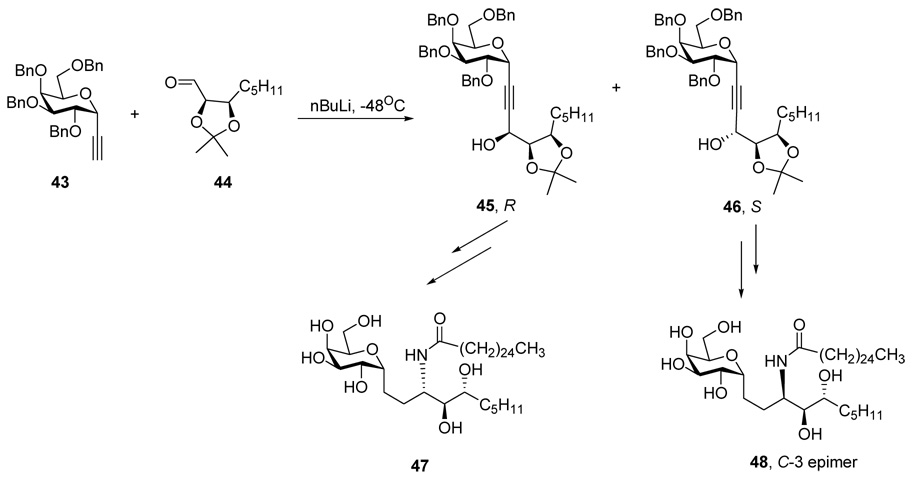
In fact, the C-glycoside analog 38, the minor isomer derived from the Ti-catalyzed sequence, was identical to the C–3 epimer 42 which had been obtained in earlier work from our group. This material arose from the linking of a phytosphingosine derivative 40, epimeric at C-3, to a galactose 39 via cross metathesis. The epimeric configuration of the amino group was unambiguous because it derived from natural phytosphingosine in which the primary alcohol had been oxidized to an aldehyde which had then been treated to form an epimeric mixture at the N-bearing carbon (Scheme 6)
Scheme 6.
Alternative synthesis of C-3 epimer 42 (Guangwu Chen, Hunter College)
The nmr spectrum of epimer 38 also closely matched the spectrum of the C-glycoside 48, a material of the same configuration prepared by Annoura, differing only in carbon chain length (Scheme 7). 18
Scheme 7.
Annoura et al.'s synthesis of C-glycoside analog of OCH and its C-3 epimer
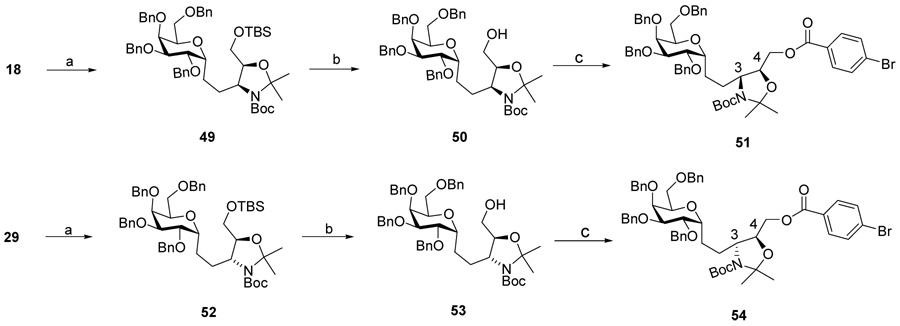
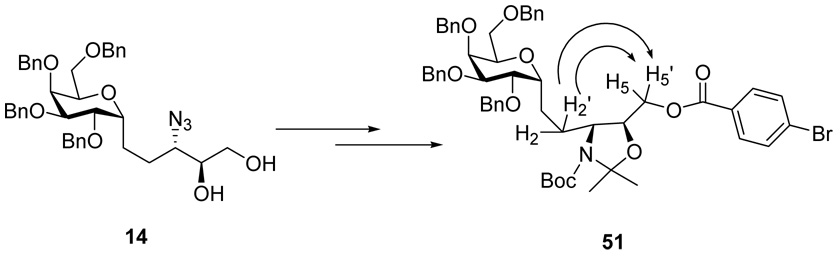
Therefore, it must have been the case that the Ti-aided azide-opening of epoxide 13 had occurred with retention of the 3-N stereo center. To obtain diagnostic NMR confirmation of stereochemistry, the trans- amino alcohol 18 and cis-amino alcohol 29, derived from opening of epoxide 13 with NaN3 and Ti-aided azide respectively, were transformed to the corresponding cis 5-membered oxazolidine benzoyl ester 51 and trans benzoyl ester 54(Scheme 8).
Scheme 8.
Reagents and conditions: (a). 2,2-dimethoxy propane, p-TsOH, CH2Cl2, 0°C-r.t; 75% for 49, 91% for 52. (b).TBAF, THF, 0°C-r.t; 95% for 50, 90% for 53. (c).4-BrPhCOCl, pyridine, CH2Cl2, r.t; 82% for 51, 90% for 54.
In its NOESY spectrum, strong correlation between H2, H2’ and H5, H5’of the p-bromo benzoyl ester 51 clearly demonstrated the cis relationship of these groups, therefore the corollary cis relationship of H3 and H4, thus establishes the anti relationship of the corresponding H3 and H4 in the 1,2-diol 14 before the heterocycle is formed (Figure 3).
Figure 3.
NOESY effect of 51, derived from NaN3 opening of epoxide 13
While for the p-bromo benzoyl ester 54, which was derived from Ti(O-i-Pr)2(N3)2 opening of epoxide 13, two sets of correlation between H2, H2’ and H4 protons, H3 and H5, H5’ protons were observed. No correlationship between H3 and H4 was detected, which was anticipated for a trans five-membered cyclic ring system (Figure 4). In this way, the stereochemical relationship between the 3-azide and 2-hydroxy of 1,2-diol 27 was proved to be syn, thus, the 3-C stereo center was proved unambiguously to be retained during Ti(O-i-Pr)2(N3)2 opening of epoxide 13.
Figure 4.
NOESY effect of 54, derived from Ti(O-i-Pr)2(N3)2 opening of epoxide 13
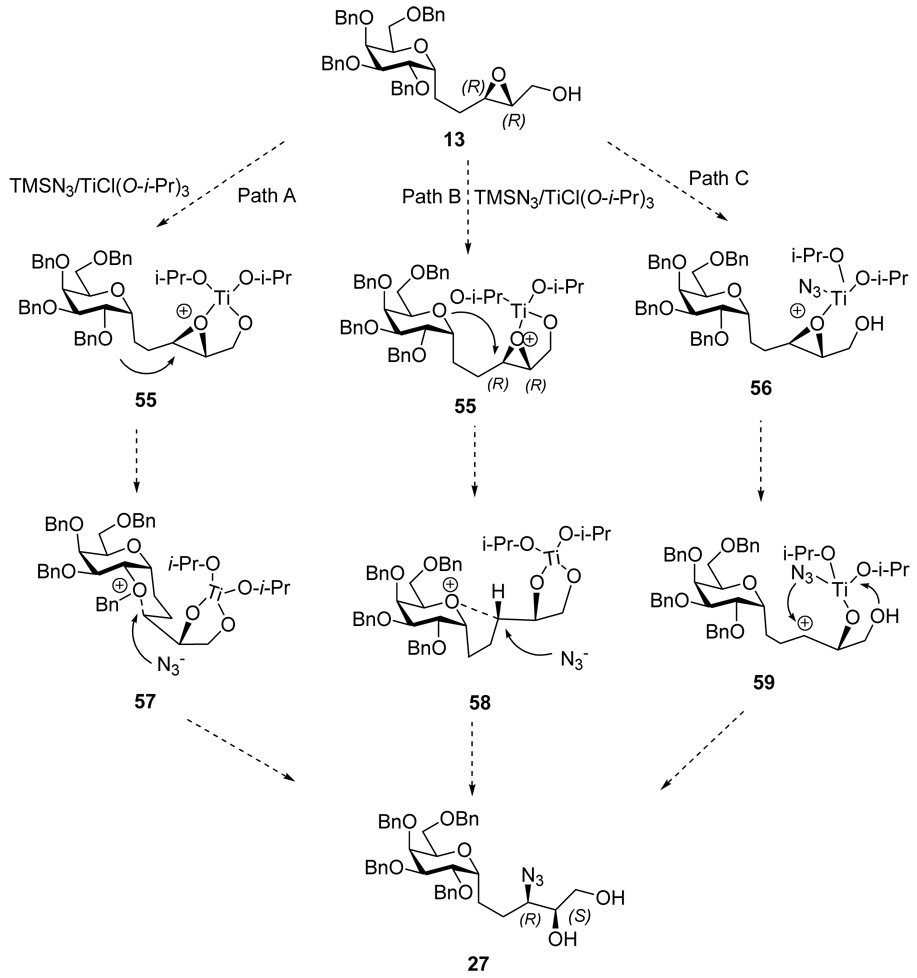
This epoxide opening with retention can be rationalized by invoking Ti-catalyzed intramolecular participation by the pyranosidic O or 2-BnO to form an intermediate oxonium ion (Scheme 9, path A or B, respectively), inverting the epoxide center, reminiscent of earlier work of Fraser-Reid.19 This would be followed by an intermolecular azide opening with a second inversion, i.e., net retention, to afford azido alcohol 27. It is also possible to rationalize the stereochemistry result by invoking a Ti-catalyzed epoxide opening/intramolecular delivery of azide from the Ti complex as suggested by Tan (path C) (Scheme 9).20 There is no simple experiment that will distinguish between these two retention pathways.
Scheme 9.
Mechanism for the retention of configuration of 3-N stereo center
Immunostimulant Activity
Our collaborating immunology group conducted in vitro assays for cytokine production using a system 3 separate NKT cell hybridomas paired with a CD1d antigen-presenting HeLa cell. With NKT line 829, the stereoisomers described (compound 26, 36 and 38) produced 40-fold less IFNγ than the positive control of α-Galcer. With NKT 912, our stereoisomers were 7-fold less productive and with NKT 926, they were 50-fold less productive. Further, there was no significant difference among these unnatural stereoisomers. 21
In summary, the C-glycoside analog 3 of α-galactosylceramide 1 (KRN7000) was synthesized in 19 linear steps in 3.3% overall yield through the Sharpless Asymmetric Epoxidation method as the controller of stereochemistry. Sodium azide opening of epoxide 13 gave anti vicinal azido diol 14 with inverted stereo center at C-3 but Ti(O-i-Pr)2(N3)2 opening of epoxide 13 provided syn vicinal azido diol 27 with retention of the C-3 configuration. This unusual phenomenon could be rationalized by related pathways which find literature precedent; and therefore this result should serve as a cautionary note for researchers planning to use Ti catalysis for epoxide opening.
Experimental
Instruments and Materials
NMR spectra were recorded with a QE 300MHz (1H) and 75 MHz (13C) with a TECMAG data system or Bruker 500 MHz (1H) and 125 MHz (13C) in deuterated solvents. The assignment of proton and carbon NMR peaks was supported by routine COSY and HSQC spectra and for some cases by NOESY spectra. Melting points were determined on a Fisher-Johns apparatus and were uncorrected Electrospray ionization (ESI) mass spectra experiments were performed at the Hunter College Mass Spectrometry Facility on an Agilent Technologies 1100 LC/MSD. Typical ESI method: solvent: 1/1 acetonitrile/water + 0.1% HOAc + 50µl NH4Ac, flow: 0.5 ml/min, positive ion mode, fragmentor voltage: 30–200V, drying gas at 175°C. All air –moisture sensitive reactions were performed under a positive pressure of dry N2 gas. All solvents and reagents were purified prior to use according to standard laboratory procedures. Low temperatures were recorded as bath temperatures. Thin layer chromatography analysis was carried out on precoated aluminium sheets of silica gel 60 F 254. UV light and vanillin, phosphomolybdic acid spray or DNP spray were used to visualize the components on the TLC plates. Flash column chromatography was carried out with silica gel 60 (230–400 mesh) purchased from ChemAbsorb, using ACS reagent grade petroleum ether, hexane, ethyl acetate, methylene chloride, chloroform, and methanol as eluents.
5-(3,4,5-Tris-benzyloxy-6-benzyloxymethyl-tetrahydro-pyran-2-yl)-pent-2-enoic acid methyl ester (11)
To a solution of crude 3–(3,4,5-Tris-benzyloxy-6-benzyloxymethyl-tetrahydro-pyran-2-yl)-propionaldehyde (10) (1.3 g, 2.3 mmol) in anhydrous MeCN (30 mL) was added methyl(triphenylphosphoronylidene)acetate (1.5 g, 4.5 mmol) in one portion at room temperature under N2. The solution was stirred overnight at room temperature. The mixture was diluted with CH2Cl2 (50 mL), washed with H2O (20 mL), extracted with CH2Cl2 (3 × 50 mL), dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. Purification by flash chromatography eluting with 30 % ethyl acetate/petroleum ether provided is,α β unsaturated ester 11 (1.27 g, 89% yield) as yellow oil. M/S: m/z 654 (M++NH4+). HRMS calcd for C40H44O7 636.3087, found 636.3085. 1H NMR (500MHz, CDCl3): δ 7.45-7.20 (m, 20H, Ph), 7.0-6.9 (d, t, J=15.6, 6.9Hz, 1H, CH=), 5.80 (d, J = 15.5 Hz, 1H, =CH), 4.76-4.78 (m, 8H,CH2Ph), 4.05-3.90 (m, 4H), 3.85-3.75 (m, 5H), 3.70-3.65 (m, 1H), 2.40-2.30 (m, 1H), 2.25-2.10 (m, 1H), 1.90-1.80 (m, 1H), and 1.65-1.55 (m, 1H). 13C NMR (125MHz, CDCl3): δ 167.2, 149.1, 138.7, 138.6, 138.5, 138.4, 128.6, 128.6, 128.5, 128.6, 128.5, 128.5, 128.4, 128.3, 128.1, 128.0, 128.0, 127.9, 127.9, 127.8, 127.7, 121.4, 77.4, 77.1, 76.8, 74.5, 73.4, 73.3, 73.2, 72.4, 67.8, 51.5 and 28.7.
5-(3,4,5-Tris-benzyloxy-6-benzyloxymethyl-tetrahydro-pyran-2-yl)-pent-2-en-1-ol (12)
α, β unsaturated ester 11 (1.20 g, 1.89 mmol) was dissolved in anhydrous CH2Cl2 (10 mL) and was cooled to −78 °C under N2. DIBAL-H (5.7 mL, 5.7 mmol, 1 M in hexane) was added dropwise over 10 min. After completion of addition, the reaction mixture was slowly warmed up to room temperature and was stirred for 2 h at room temperature. The solution was cooled to 0°C and MeOH (10 mL) was added dropwise followed by addition of satd. potassium sodium tartate solution (30 mL). The solution was stirred vigorously until two clear phases appeared. The mixture was extracted with ethyl acetate (3 × 50 mL). The combined organic layer was dried with Na2SO4, filtered and concentrated in vacuo. Purification by flash chromatography eluting with 30-50% ethyl acetate/petroleum ether afforded the allylic alcohol 12 (1.03 g, 90% yield) as pale yellow oil. M/S: m/z 626 (M++NH4+). HRMS calcd for C39H44O6 608.3138, found 608.3134. 1H NMR (500MHz, CDCl3): δ 7.35-7.20 (m, 20H, Ph), 5.71-5.65 (m, 2H, -CH=CH-), 4.75-4.52 (m, 8H, CH2Ph), 4.05 (m, 2H, -CH2OH), 3.99-3.90 (m, 3H), 3.85-3.75 (m, 2H), 3.73-3.67 (m, 1H), 3.65-3.3.0 (dd, J=10.3, 4.5 Hz, 1H), 2.20 (m, 1H), 2.05-1.99 (m, 1H), 1.74-1.70 (m, 1H), and 1.60-1.50 (br, 1H). 13C-NMR (125MHz, CDCl3): δ 138.8, 138.7, 138.5, 138.5, 132.7, 129.6, 128.6, 128.6, 128.6, 128.5, 128.4, 128.2, 128.0, 127.9, 127.9, 127.8, 127.8, 127.8, 127.7, 127.6, 77.0, 76.9, 74.7, 73.5, 73.4, 73.4, 73.3, 73.1, 72.3, 68.0, 63.8 and 28.7.
(3’R,4’R)-1-(3’,4’-oxiran-5’-hydroxy-pentanyl)-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (13)
A 100 mL round bottom flask containing powdered 4Å molecular sieves (4 g) was heated with a heating gun in vacuo for 10 min and was cooled down to room temperature under N2. The flask was then filled with dry CH2Cl2 (10 mL) and was cooled to −20°C. Titanium (IV) isopropoxide (0.4 mL, 1.35 mmol) and diisopropyl D-(-)-tartrate (0.28 mL, 1.62 mmol) were then added. The solution was stirred for 30 min at −20°C. tert-butyl hydroperoxide (0.54 mL, 2.7 mmol, 5 M in toluene) was added dropwise over a period of 10 min. The solution was stirred for another 30 min at −20°C. Allylic alcohol 12 (0.82 g, 1.35 mmol, predried with 4Å MS for 30 min) in dry CH2Cl2 (5 mL) was added dropwise. The solution was stirred at −20°C for 18 h (stored in freezer overnight) and was cooled to 0°C. H2O (10 mL) was added and the mixture was stirred for 30 min at 0°C, then 30% NaOH solution saturated by NaCl (10 mL) was added and the solution was stirred for 30 min at 0°C. The mixture was filtered through a pad of celite and the celite was washed with CH2Cl2 (50 mL). The organic phase was separated and the aqueous layer was extracted with CH2Cl2 (3 × 30 mL). The organic phase was combined and washed with brine (20 mL), dried with Na2SO4, filtered and concentrated in vacuo. The crude mixture was purified by flash chromatography eluting with 50% ethyl acetate/petroleum ether to provide epoxide 13 (0.50 g, 70% yield) as pale yellow oil. M/S: m/z 642 (M++NH4+). HRMS calcd for C39H44O7 624.3087, found 624.3082. 1H NMR (300MHz, C6D6): δ 7.0 (m, 20H, Ph), 4.45-4.10 (m, 8H, CH2Ph), 3.95-3.85 (m, 2H), 3.83-3.80 (m, 1H), 3.80-3.75 (m, 1H, -CH2OH), 3.70-3.60 (m, 1H, -CH2OH), 3.50-3.45 (m, 1H), 3.35-3.3.14 (m, 3H), 2.70 (m, 1H), 2.55-2.45 (m, 1H), 1.80-1.46 (m, 3H), and 1.40-1.11 (m, 2H). 13C NMR (125MHz, CDCl3): δ 138.7, 138.5, 138.4, 138.2, 128.5, 128.4, 128.4, 128.3, 128.2, 128.1, 128.0, 127.9, 127.8, 127.8, 127.8, 127.7, 76.9, 74.6, 73.7, 73.5, 73.4, 73.3, 73.2, 72.4, 68.0, 61.8, 58.5, 55.6, 28.2 and 23.4.
3-azido-1, 2 diol 14 and 2-azido-1, 3 diol 15
The (2R, 3R) epoxide 13 (23.8 g, 38 mmol) was dissolved in a mixed solution of MeOH/H2O (400 mL/50 mL, 8:1). NaN3 (12.4 g, 190 mmol) and NH4Cl (4.5 g, 84 mmol) were added in one portion. The solution was refluxed at 80°C under N2 for 16 h. The solution was cooled down to room temperature and the solvent was removed under reduced pressure. The mixture was diluted with ethyl acetate (200 mL), washed with H2O (200 mL). The aqueous layer was separated from organic layer and was extracted with ethyl acetate (3 × 150 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude mixture was purified by flash chromatography eluting with 30–50% ethyl acetate/petroleum ether to afford a mixture of 1, 2 diol 14 and 1, 3 diol 15 (23.6 g, 93% yield) as yellow oil. M/S: m/z 685 (M++NH4+). HRMS calcd for C39H45N3O7, 667.3258, found 667.3256. 1H NMR (500MHz, CDCl3): δ 7.34-7.24 (m, 20H, Ph), 4.74-4.49 (m, 8H, CH2Ph), 4.03-3.86 (m, 4H), 3.82-3.71 (m, 2H), 3.66-3.61 (m, 1H), 3.58-3.53 (m, 3H), 3.52-3.48 (m, 1H), 2.71 (br s, 1H, -OH), 1.96 (br s, 1H, -OH), 1.85-1.78 (m, 1H), 1.74-1.68 (m, 2H) and 1.51-1.42 (m, 1H). 13C NMR (125MHz, CDCl3): δ 138.5, 138.4, 138.2, 138.1, 128.5, 128.5, 128.4, 128.4, 128.1, 128.1, 128.0, 128.0, 127.9, 127.8, 127.7, 127.7, 127.6, 127.6, 74.4, 73.8, 73.3, 73.3, 73.2, 72.4, 67.7, 64.4, 63.2 and 27.1.
1, 2-diol 16 and 1, 3-diol 17
The mixture of 1,2 diol 14 and 1,3 diol 15 (24.6 g, 37 mmol) was dissolved in anhydrous THF (400 mL). A solution of P(Me)3 (220 mL, 221 mmol, 1 M in THF) was added dropwise at 0°C under N2 over 30 min. The solution was then stirred at room temperature overnight. A solution of NaOH (200 mL, 0.4 mol, 2 M) was added and the solution was stirred for 2 h. The mixture was extracted with ethyl acetate (3 × 200 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude amine was dissolved in a solution of THF/1, 4-dioxane (100 mL/100 mL, 1:1). A satd. solution of NaHCO3 (50 mL) was added followed by addition of (t-Boc)2O (11.1 mL, 48.1 mmol). The solution was stirred for 24 h at room temperature. The mixture was concentrated to a total volume of 50 mL under reduced pressure. The solution was extracted with ethyl acetate (3 × 200 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude mixture was purified by flash chromatography eluting with 30–50% ethyl acetate/petroleum ether to afford a mixture of 1, 2 diol 16 and 1, 3 diol 17 (21.3 g, 78% combined yield) as yellow oil. M/S: m/z 759 (M++NH4+). HRMS calcd for C44H55NO9, 741.3877, found 741.3874. 1H NMR (500MHz, CDCl3): δ 7.32-7.25 (m, 20H, Ph), 4.921 (d, J=8.4 Hz, 1H, NH), 4.71-4.44 (m, 8H, CH2Ph), 4.05 (bs, 1H), 3.97-3.93 (m, 3H), 3.74-3.73 (br s, 1H), 3.69 (br s, 1H), 3.59 (dd, J=10.4 Hz, 3.52 Hz, 1H), 3.54-3.42 (m, 3H), 3.21-3.19 (br s, 1H), 3.12 (br s, 1H), 2.66 (broad peak, OH), 2.41 (broad peak, OH), 1.87-1.86 (m, 1H), 1.71-1.69 (m, 1H), 1.58-1.55 (m, 1H), 1.43 (s, 9H, (CH3)3), and 1.36-1.28 (m, 1H). 13C NMR (125MHz, CDCl3): δ157.5, 138.7, 138.5, 138.4, 138.4, 128.6, 128.6, 128.5, 128.5, 128.3, 128.2, 128.1, 128.1, 128.0, 127.9, 127.8, 127.7, 127.7, 127.7, 80.1, 76.5, 74.6, 74.5, 73.4, 73.3, 73.2, 73.2, 72.4, 67.5, 63.1, 53.9 (NHCO), 53.1 (NHCO), 29.1, 28.5, 27.0, 24.6 and 20.9.
(3’S,4’S)-1-{3’-N-tertbutylcarbamate-4’-hydroxy-5’-(tert-Butyl-dimethyl-silanyloxy)-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (18)
The mixture of compounds 16 and 17 (11.8 g, 15.9 mmol) was dissolved in anhydrous CH2Cl2 (200 mL) at room temperature under N2. Imidazole (3.3 g, 48.0 mmol) and t-butyldimethylsilyl chloride (3.2 g, 20.7 mmol) were added in one portion. The solution was stirred for 1h at room temperature. The mixture was washed with satd. NH4Cl solution (200 mL), extracted with CH2Cl2 (3 × 150 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude mixture was purified by flash chromatography eluting with 10–20% ethyl acetate/petroleum ether to get the first fraction 19 (1.3 g, 10% yield) and the second fraction 18 (10.6 g, 78% yield), which is the desired product as yellow oil. M/S: m/z 873(M++NH4+). HRMS calcd for: C50H69NO9Si, 855.4742, found 855.4738. 1H NMR (500 MHz, CDCl3): δ 7.33-7.20 (m, 20H, Ph)), 4.99-4.97 (d, J=8.9 Hz, 1H, NH), 4.67-4.44 (m, 8H, CH2), 4.02-3.95 (bs, 1H), 3.92-3.91 (m, 1H), 3.87-3.86 (m, 1H), 3.81-3.77 (m, 1H), 3.72 (bs, 1H), 3.68-3.66 (m, 1H), 3.61-3.53 (m, 4H), 3.49-3.39 (m, 1H), 1.74-1.64 (m, 4H), 1.38 (s, 9H, (CH3)3), 0.89-0.70 (s, 9H, CH3), 0.02 (s, 6H, CH3). 13C NMR (125 MHz, CDCl3): δ 156.4, 138.8, 138.7, 138.6, 138.5, 128.6, 128.5, 128.5, 128.5, 128.4, 128.2, 128.1, 128.1, 128.0, 127.9, 127.8, 127.8, 127.7, 127.6, 79.3, 77.1, 74.7, 73.4, 73.4, 73.3, 72.3, 67.9, 64.7, 53.9, 28.6, 27.9, 26.1, 18.4, −5.2 and −5.3.
(3’S,4’S)-1-{3’-N-tert butyl carbamate-4’-methoxymethoxy-5’-(tert-Butyl-dimethyl-silanyloxy)-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (20)
The TBS protected ether 18 (5.8 g, 6.8 mmol) was dissolved in anhydrous CH2Cl2 (200 mL). Freshly distilled diisopropylethylamine (11.8 mL, 67.9 mmol) was added at 0°C under N2. Methoxymethyl chloride (2.1 mL, 27.2 mmol) was then added dropwise over 10 min. The solution was stirred at room temperature for 18 h. The reaction was quenched by addition of satd. NH4Cl solution (100 mL), extracted with CH2Cl2 (3 × 100 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude mixture was purified by flash chromatography eluting with 10–20% ethyl acetate/petroleum ether to get the fully protected compound 20 (5.79 g, 95%) as yellow oil. M/S: 917 m/z (M++NH4+). HRMS calcd for C52H73NO10Si, 899.5004, found 899.5007. 1H NMR (500MHz, CDCl3): δ 7.31-7.26 (m, 20H, Ph), 5.52-5.23 (d, J=8.5 Hz, 1H, NH), 4.69-4.47 (m, 10H, CH2Ph and OCH2O), 3.97 (br s, 2H), 3.89 (br s, 1H), 3.77-3.76 (m, 3H), 3.73-3.70 (m, 2H), 3.67-3.64 (m, 2H), 3.57-3.56 (m, 1H), 3.35 (s, 3H, OCH3), 1.68 (bs, 3H), 1.53 (s, 1H), 1.42 (s, 9H, (CH3)3), 0.89 (s, 9H, CH3), 0.05 (s, 3H, CH3), 0.04 (s, 3H, CH3). 13C NMR (125MHz, CDCl3): δ 156.0, 138.7, 138.6, 138.5, 138.4, 128.3, 128.2, 128.1, 128.0, 128.0, 128.0, 127.9, 127.7, 127.6, 127.5, 127.5, 127.2, 96.5, 79.4, 78.8, 77.4, 77.1, 74.8, 73.6, 73.4, 73.3, 73.2, 72.2, 68.0, 64.2, 55.8, 52.2, 28.7, 27.9, 18.4 and −5.9.
(3’S,4’S)-1-{3’-N-tert butyl carbamate-4’-methoxymethoxy-5’-hydroxy-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (21)
The fully protected compound 20 (4.6 g, 5.2 mmol) was dissolved in anhydrous THF (100 mL) and the solution was cooled to 0°C under N2. A solution of tetrabutylammonia floride (10.3 mL, 10.3 mmol, 1 M THF solution) was added. The mixture was stirred for 2.5 h at room temperature. The solution was washed with satd. NH4Cl solution (100 mL), extracted with ethyl acetate (3 × 100 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude mixture was purified by flash chromatography eluting with 20–60% ethyl acetate/petroleum ether to get the primary alcohol 21 (4.0 g, 100% yield) as white solid. M/S: 803 m/z (M++NH4+). HRMS calcd for C46H59NO10, 785.4139, found 785.4135. 1H NMR (500 MHz, CDCl3): δ 7.33-7.26 (m, 20H, Ph), 4.88 (d, J=8.9 Hz, 1H, NH), 4.71-4.47 (m, 10H, CH2Ph and OCH2O), 4.02 (br s, 1H), 3.96 (br s, 1H), 3.95-3.3.85 (m 2H), 3.72 (br s, 2H), 3.69-3.62 (m, 1H), 3.61-3.54 (m, 2H), 3.54-3.48 (m, 1H), 3.36 (s, 3H, OCH3), 3.35 (broad peak, 1H), 3.28 (m, 1H), 1.78 (br s, 1H), 1.71-1.69 (br s, 1H), 1.61-1.55 (br s, 1H), 1.43 (s, 9H, (CH3)3), 1.29-1.26 (br s, 1H). 13C NMR (125 MHz, CDCl3): δ 156.6, 138.8, 138.7, 138.5, 128.6, 128.5, 128.5, 128.4, 128.4, 128.3, 128.2, 128.1, 127.9, 127.8, 127.8, 127.8, 127.7, 96.9, 82.7, 79.7, 77.4, 77.1, 76.7, 74.7, 73.3, 73.3, 72.2, 67.8, 62.3, 55.9, 51.6, 28.6 and 26.9.
(3’S,4’S,5’S)-1-{3’-N-tert-butylcarbamate-4’-methoxymethoxy-5’-hydroxynonadecanyl}-(2,3,4,6-tetra-O-benzyl)-α-C-D-galactopyranoside 23
The primary alcohol 21 (0.6 g, 0.77 mmol) was dissolved in anhydrous CH2Cl2 (10 mL) under N2. Dess-Martin periodinane (0.42 g, 1 mmol) was added in one portion at room temperature. The solution was stirred for 45 min at room temperature and was diluted with CH2Cl2 (20 mL) followed by addition of satd. NaHCO3 solution (10 mL) and satd. Na2S2O3 solution (10 mL). The mixture was stirred until two clear phases appeared. The solution was extracted with CH2Cl2 (3 × 60 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude product was coevaporated with anhydrous toluene (3 × 10 mL) and was then put under high vacuum to afford the crude aldehyde 22 (0.55 g), which was used in the next step without further purification.
Kirschning Oxidation14: A mixture of the primary alcohol 21 (0.8 g, 0.8 mmol) and a polymer bound resin prepared according to literature procedure14 (1.4 g, 4.9 mmol) in dry CH2Cl2 (10 mL) with catalytic amount of 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO) under nitrogen was stirred at room temperature for 2h. After disappearance of starting material, the resin was washed with CH2Cl2 (3 × 30 mL) and the combined organic washings and filtrate was concentrated under reduced pressure to afford the crude aldehyde 22 (0.97 g), which was used in the next step without further purification. The aldehyde 22 was dissolved in anhydrous THF (5 mL) and the solution was added dropwise to a solution of freshly prepared tetradecyl magnesium bromide (C14H29MgBr, 2.3 mmol) in anhydrous THF (5 mL) at 0°C under N2 over 10 min. The mixture was stirred at 0°C for 30 min and was then stirred for 2 h at room temperature. The reaction was quenched by addition of satd. NH4Cl solution (20 mL) and the mixture was stirred for 10 min. The mixture was extracted with ethyl ether (3 × 50 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude mixture was purified by flash chromatography eluting with ethyl acetate/petroleum ether (polarity increased from 5% to 20%) to get the major isomer 23 (0.36 g, 48% yield) as the first fraction and the minor isomer 24 (72 mg, 10% yield) as the second fraction.
The major isomer 23: M/S: 999 m/z (M++NH4+). HRMS calcd for C60H87NO10, 981.6330, found 981.6322. 1H NMR (500MHz, CDCl3): δ 7.35-7.29 (m, 20H, Ph), 5.26 (d, J=9.1Hz, 1H, NH), 4.76-4.48 (m, 10H, CH2Ph and OCH2O), 3.99 (bs, 2H), 3.96 (m, 1H), 3.86-3.74 (m, 4H), 3.65-3.63 (m, 2H), 3.41 (s, 3H, OCH3), 3.31 (m, 1H), 2.76 (d, J=5.2 Hz, 1H), 1.71-1.66 (m, 4H), 1.57-1.54 (m, 2H), 1.45 (s, 9H, (CH3)3), 1.33-1.19 (br s, 24H, CH2), 0.91 (t, J=6.9 Hz, 3H, CH3). 13CNMR (125MHz, CDCl3): δ 156.4, 138.8, 138.7, 138.6, 128.5, 128.5, 128.4, 128.3, 128.1, 128.1, 128.0, 127.9, 127.9, 127.8, 127.8, 127.7, 97.9, 83.9, 79.3, 77.4, 77.3, 76.8, 76.8, 74.8, 73.5, 73.5, 73.3, 72.2, 67.9, 56.3, 52.1, 34.0, 32.1, 30.0, 29.9, 29.8, 29.6, 28.6, 27.4, 25.8, 22.9 and 14.3.
(3’S,4’S,5’R)-1-{3’-N-tert-butylcarbamate-4’-methoxymethoxy-5’-hydroxynonadecanyl}-(2,3,4,6-tetra-O-benzyl)-α-C-D-galactopyranoside 24
M/S: 999 m/z (M++NH4+). HRMS calcd for C60H87NO10, 981.633, found 981.6320. 1H NMR (500MHz, CDCl3): δ 7.37-7.29 (m, 20H, Ph), 4.89 (d, J=9.4 Hz, 1H, NH), 4.75-4.48 (m, 10H, CH2Ph and OCH2O), 3.99-3.96 (m, 3H), 3.84-3.77 (m, 3H), 3.75 (d, J=5.2 Hz, 1H), 3.61-3.59 (m, 1H), 3.56 (dd, J=10.6, 3.8Hz, 1H), 3.39 (s, 3H, OCH3), 3.34 (t, J=5.0 Hz, 1H), 2.73 (d, J=6.1 Hz, 1H), 1.91 (m, 1H), 1.71 (m, 2H), 1.62 -1.59 (m, 2H), 1.53 (m, 1H), 1.45 (s, 9H, (CH3)3), 1.38-1.27 (m, 24H, CH2), 0.91 (t, J=6.6 Hz, 3H, CH3). 13C NMR (125MHz, CDCl3): δ 155.9, 138.9, 138.7, 138.6, 138.5, 128.6, 128.6, 128.4, 128.3, 128.2, 128.1, 128.1, 128.0, 127.9, 127.8, 127.7, 98.6, 87.5, 79.3, 77.1, 76.9, 76.5, 74.9, 73.6, 73.4, 73.3, 71.8, 68.4, 56.2, 51.8, 33.0, 32.1, 29.9, 29.8, 29.6, 28.7, 27.1, 26.4, 22.9 and 14.3.
(3’S,4’S,5’R)-3’-N-hexacosanoyl-4’,5’-dihydroxynonadecacyl-α-C-D-galactopyranoside 3
To a solution of the minor isomer 24 (45 mg, 0.046 mmol) in MeOH/1,4-dioxane (1 mL/1 mL) was added HCl solution (2 mL, 12 mmol, 6 M in MeOH) and the solution was stirred at room temperature overnight. The solution was concentrated to dryness under reduced pressure. The mixture was dissolved in THF (5 mL) and was neutralized with satd. ammonia hydroxide solution (5 mL). The mixture was extracted with CHCl3 (3 × 20 mL). The combined organic layers were dried over Na2SO4 (anhydrous), filtered and concentrated in vacuo. The crude compound was coevaporated with anhydrous toluene (3 × 10 mL) and was then put under high vacuum pump for 1 h to afford crude amine (40 mg) as yellow oil.
The amine was dissolved in anhydrous THF (2 mL). The activated ester C25H51COOPhNO2 (55 mg, 0.091 mmol) and DMAP (3 mg) were added at room temperature under N2. The mixture was stirred at room temperature overnight. 0.5 g of celite 545 was added and the solvent was removed in vacuo to get powder. Flash chromatography purification eluting with 10% ethyl acetate/ petroleum ether to remove the excess amount of ester and using 20–30% ethyl acetate/petroleum ether to give the amide 25 (0.04 g, 72% yield).
The amide 25 (0.04 g, 0.033 mmol) was dissolved in a solvent of THF/EtOH (3 mL/3 mL, 1:1). Pd(OH)2/C (0.10 g, 20% by weight) was added in one portion. The solution was degassed three times and was stirred under H2 balloon (1 atm) for 24 h. At the end of the reaction, a white precipitate formed. The solution was filtered through a pad of celite, which was washed with 50% CHCl3/MeOH solution (30 mL) followed by pyridine (20 mL). The solvent was removed in vacuo and the solid was dissolved in pyridine (2 mL). Celite 545 (0.5 g) was added and the solvent was removed in vacuo. The powder was put to the top of the column. Flash chromatography separation eluting with CHCl3, 5%, 10% and 20% MeOH/CHCl3 afforded the target C-glycoside 3 (0.016 g, 42%. 30% yield for three steps) as a white solid. Mp 176-179°C; HRMS calcd for C51H101N1O8, 855.7527, found 855.7514. 1H NMR (500MHz, C5D5N): δ 8.49 (d, J=8.9 Hz, 1H, NH), 4.73 (dd, J=8.8, 5.5 Hz, 1H), 4.52 (m, 3H), 4.37 (dd, J=11.2, 4.5 Hz, 1H), 4.25 (m, 4H), 3.97 (s, 1H, impurity), 2.72 (m, 1H), 2.55 (m, 1H), 2.48 (m, 3H), 2.33 (m, 2H), 2.20 (m, 1H), 2.05 (m, 5H, impurity), 1.96 (m, 2H), 1.86 (m, 3H), 1.69 (m, 1H), 1.57 (m, 5H), 1.31 (m, 56H), 1.03 (m, 3H), 0.89 (t, J=6.9 Hz, 6H, CH3). 13C NMR (125MHz, C5D5N): δ 173.9, 78.9, 77.5, 74.3, 73.2, 72.7, 71.1, 70.9, 63.2, 53.2, 37.3, 34.8, 32.4, 30.7, 30.5, 30.3, 30.3, 30.2, 30.2, 30.2, 30.1, 29.9, 29.9, 26.8, 26.7, 23.2, 23.0 and 14.6.
(3’S,4’S,5’S)-3’-N-hexacosanoyl-4’,5’-dihydroxynonadecacyl–α-C-D-galactopyranoside 26
This material was obtained from major isomer 23 (35% overall yield for three steps) following the same procedures as described for processing isomer 24. Mp 170-175°C; HRMS calcd for C51H101N1O8, 855.7527, found 855.7515. 1H NMR (500MHz, C5D5N): δ 6.80 (broad peak, OH), 6.51 (broad peak, OH), 6.33 (broad peak, OH), 6.09 (broad peak, OH), 5.72 (broad peak, OH), 4.77-4.73 (m, 2H), 4.66 (s, 1H), 4.53 (s, 2H), 4.48 (s, 1H), 4.31-4.29 (m, 2H), 4.20 (s, 1H), 3.79 (s, 1H), 2.89 (br s, 1H), 2.63 (bs, 1H), 2.48 (t, J=7.2 Hz, 2H), 2.31-2.24 (m, 3H), 2.22-2.14 (m, 1H), 2.09-1.97 (m, 1H), 1.91-1.73 (m, 7H), 1.71-1.59 (m, 2H), 1.39-1.26 (m, 58H), 0.92-0.87 (m, 6H). 13C NMR (125MHz, C5D5N): δ175.5, 77.3, 74.3, 72.6, 71.4, 70.7, 62.7, 53.4, 37.1, 34.6, 32.4, 37.1, 34.6, 32.4, 30.6, 30.5, 30.3, 30.2, 30.1, 30.1, 29.9, 29.9, 29.1, 27.2, 26.8, 23.3, 23.1 and 14.6.
(3’R,4’S)-1-{3’-azido-4’,5’-dihydroxy-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (27)
Titanium (IV) isopropoxide (0.22 mL, 0.73 mmol) and azido trimethylsilane (0.2 mL, 1.5 mmol) were added to anhydrous benzene (10 mL) and the solution was refluxed at 80°C under N2 for at least 5 h. Epoxide 13 (0.30 g, 0.48 mmol) was dissolved in anhydrous benzene (10 mL) and was added to the solution in one portion. The mixture was stirred for 15–30 min at 80°C and was cooled to room temperature. The solvent was removed under reduced pressure Diethyl ether (20 mL) was added followed by addition of 5% H2SO4 (10 ml, V/V). The solution was stirred at room temperature until two clear phases appeared. The mixture was extracted with CH2Cl2 (3 × 30 mL). The organic phase was dried with Na2SO4, filtered and concentrated in vacuo. Flash chromatography purification eluting with 50% ethyl acetate/petroleum ether afforded 3-azido-1, 2 diol 27 (0.21 g, 64% yield) as yellow oil. M/S: m/z 685.3 (M++NH4+). HRMS calcd for C39H45N3O7, 667.3258, found 667.3254. IR (KBr): 3435.98, 2873.99, 2100.89, 763.72 cm−1. 1H NMR (300MHz, CDCl3): δ 7.30 (m, 20H), 4.8-4.4 (m, 8H), 4.01-3.83 (m, 4H), 3.77-3.69 (m, 2H), 3.60-3.45 (m, 4H), 3.42-3.34 (m, 1H), 2.5-2.4 (br, OH), 2.25-2.15 (br, OH), 1.90-1.75 (m, 1H), and 1.74-1.50 (m, 3H). 13C NMR (75MHz, CDCl3): δ 138.7, 138.6, 138.5, 138.3, 128.5, 128.4, 128.3, 128.1, 128.0, 127.9, 127.8, 127.7, 127.6, 77.4, 76.9, 74.7, 74.0, 73.8, 73.5, 73.5, 73.4, 72.5, 70.5, 68.1, 64.0, 63.5, 26.9 and 23.9.
(3’R,4’S)-1-{3’-N-tert butyl carbamate-4’,5’-dihydroxy-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (28)
The following materials were obtained from azidodiol 27 via the procedures used for azidodiol 13. Yield: 91% yield for two steps from 27. M/S: m/z 742.3 (M++H+). HRMS calcd for C44H55NO9, 741.3877, found 741.3869. 1H NMR (500 MHz, CDCl3): δ 7.35-7.22 (m, 20H, Ph), 4.75-4.50 (m, 8H, CH2Ph), 4.05-3.98 (br, 1H), 3.98-3.95 (br, 2H), 3.93-3.88 (m, 1H), 3.76-3.72 (m, 2H), 3.69-3.63 (m, 1H), 3.62-3.56 (m, 2H), 3.55-3.48 (m, 1H), 3.44-3.39 (m, 1H), 3.10-3.00 (br, 1H, -OH), 2.18-2.10 (br, 1H, -OH), 1.80-1.72 (m, 1H), 1.69-1.62 (m, 1H), 1.62-1.52 (m, 1H), 1.52-1.47 (m, 1H), and 1.46-1.42 (s, 9H, (CH3)3). 13C NMR (125 MHz, CDCl3): δ 157.5, 138.8, 138.6, 138.5, 138.4, 128.6, 128.6, 128.5, 128.3, 128.1, 128.1, 128.0, 127.9, 127.8, 127.7, 80.1, 77.2, 76.8, 74.7, 74.6, 73.5, 73.4, 73.4, 73.3, 72.5, 68.1, 63.6, 51.6, 28.6 and 24.1.
(3’R,4’S)-1-{3’-N-tert butyl carbamate-4’-hydroxy-5’-(tert-Butyl-dimethyl-silanyloxy)-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (29)
Yield: 99%. M/S: m/z 873.3 (M++NH4+). HRMS calcd for C50H69NO9Si, 855.4742, found 855.4736. 1H NMR (500MHz, CDCl3): δ 7.35-7.22 (m, 20H, Ph), 4.75-4.50 (m, 8H, CH2Ph), 4.05-3.95 (m, 3H), 3.85-3.80 (m, 1H), 3.78-3.73 (m, 1H), 3.73-3.70 (m, 1H), 3.65-3.60 (m, 3H), 3.55-3.50 (m, 1H), 3.48-3.45 (m, 1H), 2.65-2.60 (br, 1H, -OH), 1.76-1.65 (m, 2H), 1.60-1.50 (m, 2H), 1.45-1.40 (s, 9H, (CH3)3), 0.92 (s, 9H, CH3), 0.06 (s, 6H, CH3). 13C NMR (125MHz, CDCl3): δ 156.2, 138.8, 138.7, 138.6, 138.5, 128.5, 128.5, 128.4, 128.3, 128.2, 128.1, 128.0, 127.9, 127.9, 127.8, 127.7, 127.7, 127.7, 127.6, 127.6, 79.1, 76.9, 76.8, 74.6, 73.3, 73.2, 73.1, 73.0, 72.4, 67.8, 65.0, 50.9, 29.0, 28.5, 26.0, 18.4, −5.2 and −5.3.
(3’R,4’S)-1-{3’-N-tert butyl carbamate-4’-methoxymethoxy-5’-(tert-Butyl-dimethyl-silanyloxy)-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (30)
Yield: 99%. M/S: m/z 917 (M++NH4+). HRMS calcd for C52H73NO10Si, 899.5004, found 855.4993. 1H NMR (500MHz, CDCl3): δ 7.35-7.22 (m, 20H, Ph), 4.83 (d, J=9.7 Hz, 1H, NH), 4.75-4.50 (m, 10H, 4CH2 and OCH2O-), 4.05-3.96 (m, 3H), 3.80-3.70 (m, 3H), 3.70-3.65 (m, 1H), 3.65-3.55 (m, 4H), 3.29 (s, 3H, OCH3), 1.76-1.74 (m, 1H), 1.70-1.55 (m, 2H), 1.56-1.48 (m, 1H), 1.42 (s, 9H, (CH3)3, Boc), 0.85 (s, 9H, CH3), and 0.05 (s, 6H, CH3). 13C NMR (125MHz, CDCl3): δ 155.9, 138.9, 138.8, 138.7, 138.6, 128.5, 128.5, 128.4, 128.1, 128.0, 127.9, 127.8, 127.7, 127.6, 97.9, 79.5, 78.9, 77.2, 74.7, 73.4, 73.2, 72.4, 67.9, 63.9, 55.9, 50.9, 29.1, 28.6, 26.1, 18.4 and −5.4.
(3’R,4’S)-1-{3’-N-tert butyl carbamate-4’-methoxymethoxy-5’-hydroxy-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside (31)
Yield: 92%. M/S: m/z 803 (M++NH4+). HRMS calcd for C52H73NO10Si, 785.4139, found 785.4133. 1H NMR (500MHz, CDCl3): δ 7.34-7.24 (m, 20H, Ph), 4.73-4.45 (m, 10H, - CH2Ph and OCH2O), 3.97-3.93 (m, 3H), 3.85-3.79 (m, 2H), 3.74-3.3.71 (m, 2H), 3.65-3.57 (m, 3H), 3.51-3.48 (m, 1H), 3.42-3.40 (m, 1H), 3.33-3.31 (s, 3H, OCH3), 1.76-1.71 (m, 1H), 1.68-1.66 (m, 1H), 1.56-1.53 (m, 2H), and 1.42 (s, 9H, -C(CH3)3). 13C NMR (125MHz, CDCl3): δ 156.9, 138.5, 138.4, 138.3, 138.1, 128.4, 128.3, 128.2, 128.2, 128.1, 128.0, 127.9, 127.8, 127.7, 127.6, 127.5, 127.5, 127.4, 97.0, 81.0, 79.5, 77.3, 76.9, 74.4, 73.2, 73.1, 72.9, 72.8, 72.3, 70.5, 67.8, 61.8, 55.6, 50.3, 28.3 and 28.2.
(3’R,4’S,5’R)-1-{3’-N-tert-butyl carbamate-4’-methoxymethoxy-5’-hydroxy-nonadecanyl}-(2,3,4,6-tetra-O-benzyl)-α-C-D-galactopyranoside (33)
Major isomer: yield: 28% for two steps from 31. M/S: m/z 999 (M++NH4+). HRMS calcd for C60H87NO10, 981.6330, found 981.6322. 1H NMR (500MHz, CDCl3): δ 7.35-7.21 (m, 20H, Ph), 4.74-4.49 (m, 10H, CH2Ph and OCH2O), 4.05-3.96 (m, 3H), 3.85-3.74 (m, 3H), 3.74-3.69 (m, 1H), 3.65-3.60 (dd, J=3.9, 10.3 Hz, 1H), 3.55-3.52 (br s, 1H), 3.34 (s, 3H, OCH3), 3.21 (d, J=6.9 Hz, 1H), 3.05 (d, J=2.3 Hz, -OH), 1.76-1.51 (m, 4H), 1.42 (s, 9H, Boc), 1.35-1.22 (m, 26H), and 0.90 (t, J=6.7 Hz, 3H, CH3). 13C NMR (125MHz, CDCl3): δ 155.8, 138.9, 138.8, 138.7, 138.6, 128.6, 128.5, 128.2, 128.1, 128.0, 127.9, 127.9, 127.8, 127.8, 127.7, 99.2, 87.4, 79.3, 77.1, 76.6, 76.4, 76.4, 74.7, 73.5, 73.3, 73.3, 73.1, 71.6, 68.1, 56.3, 50.8, 32.9, 32.2, 30.0, 29.9, 29.9, 29.8, 29.6, 28.6, 25.8, 22.9 and 14.3.
(3’R,4’S,5’S)-1-{3’-N-tert-butyl carbamate-4’-methoxymethoxy-5’-hydroxynonadecanyl}-(2,3,4,6-tetra-O-benzyl)-α-C-D-galactopyranoside (34)
Minor isomer, yield: 22% for two steps from 31. M/S: m/z 999 (M++NH4 +). HRMS calcd for C60H87NO10, 981.6330, found 981.6325. 1H NMR (500 MHz, CDCl3): δ 7.51-7.24 (m, 20H, Ph), 4.76-4.48 (m, 10H, 4CH2 and OCH2O-), 4.05-3.90 (m, 4H), 3.90-3.85 (m, 1H), 3.85-3.74 (m, 2H), 3.74-3.70 (m, 1H), 3.65-3.60 (m, 1H), 3.31 (s, 3H, OCH3), 3.19 (m, 1H), 1.78-1.51 (m, 4H), 1.50-1.45 (m and s, 11H), 1.45-1.21 (m, 24H), and 0.90 (t, 3H, CH3). 13C NMR (125 MHz, CDCl3): δ 157.5, 138.9, 138.7, 138.6, 138.5, 128.7, 128.6, 128.5, 128.4, 128.4, 128.3, 128.2, 128.1, 128.0, 127.9, 127.9, 127.8, 127.8, 127.7, 98.7, 85.2, 80.0, 76.9, 76.6, 74.8, 73.6, 73.5, 73.4, 73.3, 73.2, 70.8, 68.1, 56.5, 50.8, 32.8, 32.1, 30.1, 29.9, 29.6, 29.1, 28.5, 26.1, 22.9 and 14.3.
(3’R,4’S,5’S)-3’-N-hexacosanoyl-4’,5’-dihydroxynonadecacyl-α-C-D-galactopyranoside (36)
Yield: 54% for three steps from 33. Mp 183-185°C; HRMS calcd for C51H101N1O8, 855.7527, found 855.7522. 1H NMR (500MHz, C5D5N): δ 8.05 (d, J=9.2 Hz, 1H, NH), 6.65 (br, OH), 6.40 (br, OH), 6.20 (br, OH), 5.80 (br, OH), 4.76-4.72 (m, 1H), 4.72-4.66 (m, 2H), 4.54-4.48 (m, 1H), 4.45-4.34 (m, 2H), 4.32-4.27 (m, 1H), 4.12-4.06 (m, 1H), 4.01-3.94 (m, 1H), 2.49-2.35 (m, 5H), 2.27-2.23 (m, 1H), 2.16-2.10 (m, 1H), 1.91-1.77 (m, 5H), 1.52-1.21 (m, 66H), and 0.87 (t, 6H, CH3). 13C NMR (125MHz, C5D5N): δ 173.7, 77.5, 75.8, 74.7, 73.0, 72.7, 71.0, 70.7, 62.8, 50.9, 37.3, 32.6, 32.5, 30.8, 30.7, 30.6, 30.5, 30.4, 30.4, 30.3, 30.3, 30.2, 30.0, 30.0, 27.0, 27.0, 23.4 and 14.7.
(3’R,4’S,5’R)-3’-N-hexacosanoyl-4’,5’-dihydroxynonadecacyl–α-C-D-galactopyranoside (38)
Yield: 55% for three steps from 34. Mp 178–182°C; HRMS calcd for C51H101N1O8, 855.7527, found 855.7523. 1H NMR (500MHz, C5D5N): δ 8.65 (d, J=9.0 Hz, 1H, NH), 6.65 (br, OH), 6.40 (br, OH), 6.20 (br, OH), 5.80 (br, OH), 5.02-5.01 (m, 1H), 4.76-4.72 (m, 1H), 4.72-4.66 (br s, 1H), 4.59-4.56 (m, 1H), 4.42 (m, 1H), 4.38 (m, 1H), 4.33 (m, 1H), 4.19 (br, 1H), 3.94 (m, 1H), 3.89 (m, 1H), 3.63 (d, J=4.7 Hz, less than 1H), 2.49- 2.35 (m, 5H), 2.27-2.23 (m, 1H), 2.16-2.10 (m, 1H), 1.91-1.769 (m, 5H), 1.52-1.21 (m, 66H), and 0.87 (m, 6H, CH3). 13C NMR (125MHz, C5D5N): δ 175.2, 77.8, 76.3, 74.5, 72.8, 72.7, 71.0, 70.9, 63.1, 51.5, 37.1, 34.9, 32.7, 32.7, 30.9, 30.8, 30.8, 30.7, 30.6, 30.6, 30.5, 30.5, 30.4, 30.3, 30.2, 30.1, 29.6, 27.3, 27.2, 23.5, 23.3 and 14.8.
(3’S,4’S)-1-{5’-(tert-Butyl-dimethyl-silanyloxy)-2’,2’-dimethyl-4’-propyl-oxazolidine-3’-N-tertbutylcarbamate-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside 49
The TBS ether compound 18 (0.51 g, 0.6 mmol) was dissolved in anhydrous CH2Cl2 (10 mL). 2,2-dimethoxy-propane (1 mL) was added followed by addition of p-TsOH (7 mg) at 0°C under N2. The solution was then stirred at room temperature for 1 h. The solution was washed with satd. NaHCO3 solution (10 mL), extracted with CH2Cl2 (3 × 50 mL). The combined organic layer was dried over Na2SO4 (anhydrous), filtered and concentrated under reduced pressure. The crude mixture was purified by flash chromatography eluting with 10–30% ethyl acetate/petroleum ether to afford the cyclic compound 49 (0.41 g, 75% yield) as yellow oil. M/S: m/z 913 (M++NH4+) (calcd: for C53H73NO9Si, 895). 1H NMR (500MHz, CDCl3): δ 7.32-7.25 (m, 20H, Ph), 4.79-4.37 (m, 8H, CH2Ph), 4.09-4.05 (m, 2H), 3.97-3.94 (br s, 2H), 3.91-3.86 (m, 3H), 3.79-3.75 (m, 1H), 3.73 (d, J=7.5 Hz, 1H), 3.67-3.55 (m, 2H), 1.82-1.79 (m, 2H), 1.66-1.63 (m, 1H), 1.59-1.55 (br s, 4H), 1.51 (s, 3H, CH3), 1.44 (s, 3H, CH3), 1.39 (s, 6H, CH3), 0.87 (s, 9H, CH3), 0.05 (s, 3H, CH3), and 0.03 (s, 3H, CH3). 13C NMR (125MHz, CDCl3): δ 152.7, 152.2, 138.9, 138.9, 138.8, 138.7, 138.6, 138.4, 128.8, 128.8, 128.7, 128.5, 128.4, 128.3, 128.2, 128.1, 127.9, 127.8, 127.7, 127.7, 127.6, 93.4, 92.9, 79.9, 79.7, 77.3, 77.1, 76.6, 74.9, 73.8, 73.6, 73.5, 73.4, 73.1, 71.6, 68.5, 68.2, 61.3, 58.8, 28.7, 28.6, 28.3, 27.5, 27.4, 27.0, 26.1, 25.3, 23.9, 23.7, 18.5, −5.0 and −5.1 (Note: Because the existence of two conformers of amide, the carbon peaks become to two series of peaks).
(3’S,4’S)-1-{2’,2’-dimethyl-4’-propyl-oxazolidine-3’-N-tertbutylcarbamate-5’-hydroxy-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside 50
To a solution of cyclic compound 49 (0.40 g, 0.45 mmol) in anhydrous THF (5 mL) was added tetrabutylammonia fluoride (1.4 mL, 1.4 mmol, 1 M in THF) dropwise at 0°C under N2. The solution was stirred at room temperature for 1 h. The solvent was removed under reduced pressure. The crude mixture was purified by flash chromatography eluting with 10–30% ethyl acetate/petroleum ether to afford the primary alcohol 50 (0.34 g, 95% yield) as yellow oil. M/S: m/z 799 (M++NH4 +) (calcd: for C47H59NO9, 781). 1H NMR (500MHz, CDCl3): δ 7.33-7.23 (m, 20H, Ph), 4.74-4.49 (m, 8H, CH2Ph), 4.13-4.11 (br s, 1H), 3.93-3.83 (m, 5H), 3.82-3.62 (m, 4H), 3.62-3.53 (br s, 1H), 1.79 (br s, 2H), 1.67 (br s, 2H), 1.55 (br s, 6H, CH3), 1.41 (s, 9H, C(CH3)3). 13C NMR (125MHz, CDCl3): δ 152.1, 138.9, 138.8, 138.7, 138.4, 128.6, 128.5, 128.4, 128.2, 128.1, 128.0, 127.8, 127.8, 127.7, 93.6, 93.0, 79.8, 77.9, 76.8, 76.5, 76.3, 74.9, 73.8, 73.6, 73.3, 72.3, 71.9, 69.1, 68.5, 61.1, 58.8, 28.7, 28.3, 27.6, 25.3, 24.3 and 23.9.
(3’S,4’S)-1-{2’,2’-dimethyl-4’-propyl-oxazolidine-3’-N-tertbutylcarbamate-5’-(4-Bromo-benzoyloxymethyl)-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside 51
To a solution of alcohol 50 (0.33 g, 0.42 mmol) was dissolved in anhydrous CH2Cl2 (10 mL) was added anhydrous pyridine (0.5 mL) and para-bromobenzoyl chloride (0.18 g, 0.84 mmol) at 0°C under N2. The solution was stirred at room temperature for 2 h. The solution was washed with satd. NH4Cl solution (20 mL), extracted with CH2Cl2 (3 × 50 mL). The combined organic layer was dried over Na2SO4 (anhydrous), filtered and concentrated under reduced pressure. The crude mixture was purified by flash chromatography eluting with 10–20% ethyl acetate/petroleum ether to afford benzoyl ester 51 (0.34 g, 82% yield) as yellow oil. M/S: m/z 981 (M++NH4 +) (calcd: for C54H62BrNO10, 963). 1H NMR (500MHz, CDCl3): δ 7.88-7.86 (d, J=8.4 Hz, 2H), 7.52-7.51 (d, J=8.4 Hz, 2H), 7.33-7.23 (m, 20H, Ph), 4.72-4.43 (m, 8H, -CH2Ph), 4.31-4.30 (dd, J=11.2, 5.8 Hz, 2H, CH2OCO), 4.17-4.16 (s, 1H, CHO), 4.02-3.90 (m, 4H), 3.73 (br s, 1H), 3.69-3.68 (br s, 1H), 3.65-3.62 (dd, J= 9.9, 4.8 Hz, Hz, 1H), 1.96-1.91 (m, 1H), 1.73-1.54 (br s, 5H), 1.53-1.49 (br s, 4H), 1.49-1.42 (m, 9H, C(CH3)3). 13C NMR (125MHz, CDCl3): δ 165.6, 152.6, 152.0(amide CO), 138.8, 138.6, 138.2, 131.9, 131.5, 128.9, 128.5, 128.4, 128.2, 128.0, 127.9, 127.8, 127.7, 126.8, 126.5, 93.9, 93.3 (OC(CH3)2N), 80.3, 80.0, 77.9, 76.6, 76.4, 74.9, 74.3, 73.8, 73.6, 73.2, 72.2, 71.9, 68.6, 68.5, 63.1, 58.8, 28.6, 28.2, 27.7, 27.6, 27.3, 25.4, 24.2 and 23.9.
(3’R,4’S)-1-{5’-(tert-Butyl-dimethyl-silanyloxy)-2’,2’-dimethyl-4’-propyl-oxazolidine-3’-N-tertbutylcarbamate-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside 52
Yield: 91% from TBS ether compound 29. M/S: m/z 913 (M++NH4 +) (calcd: for C53H73NO9Si, 895). 1H NMR (500MHz, CDCl3): δ 7.33-7.24 (m, 20H, Ph), 4.735-4.43 (m, 8H, CH2Ph), 3.98 (s, 1H), 3.93-3.91 (m, 2H), 3.85-3.71 (m, 4H), 3.69 (dd, J=7.9, 2.5 Hz, 1H), 3.65 (dd, J=10.0, 5.3 Hz, 1H), 3.59 (s, 2H), 1.91-1.89 (m, 1H), 1.57-1.52 (s, 6H, CH3 and CH2), 1.49 (s, 3H, CH3), 1.42 (s, 9H, (CH3)3), 0.88 (s, 9H, CH3), 0.07 (s, 3H, CH3), 0.04 (s, 3H, CH3). 13C NMR (125MHz, CDCl3): δ 152.1, 138.9, 138.8, 138.7, 128.5, 128.4, 128.1, 128.1, 128.0, 127.9, 127.8, 127.7, 127.7, 127.3, 94.7, 94.4, 80.9, 79.8, 79.1, 76.9, 76.6, 76.3, 74.7, 73.5, 73.2, 72.1, 68.0, 64.8, 59.6, 28.7, 27.7, 26.2, 23.7, 18.5, −5.1 and −5.2.
(3’R,4’S)-1-{2’,2’-dimethyl-4’-propyl-oxazolidine-3’-N-tertbutylcarbamate-5’-hydroxy-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside 53
Yield: 90%. M/S: m/z 799 (M++NH4 +) (calcd: for C47H59NO9, 781). 1H NMR (500MHz, CDCl3): δ 7.33-7.24 (m, 20H, Ph), 4.74-4.45 (m, 8H, CH2Ph), 4.05-3.81 (m, 6H), 3.73-3.69 (s, 2H), 3.66-3.55 (m, 2H), 3.47-3.42 (bs, 2H), 1.94-1.85 (m, 1H), 1.67-1.59 (m, 3H), 1.54 (s, 3H, CH3), 1.48 (s, 3H, CH3), and 1.42 (s, 9H, (CH3)3). 13C NMR (125MHz, CDCl3): δ 152.1, 138.8, 138.6, 138.5, 128.6, 128.5, 128.4, 128.4, 128.4, 128.3, 128.2, 128.2, 128.1, 128.0, 127.9, 127.8, 127.7, 127.7, 79.9, 77.4, 77.1, 74.5, 73.7, 73.6, 73.3, 63.9, 58.4 and 28.7 (note: C(CH3)3 peak at 94 ppm was too small).
(3’R,4’S)-1-{2’,2’-dimethyl-4’-propyl-oxazolidine-3’-N-tertbutylcarbamate-5’-(4- bromo-benzoyloxymethyl)-pentanyl}-(2,3,4,6-tetra-O-benzyl-α-C-D-galactopyranoside 54
Yield: 90%. M/S: m/z 981 (M++NH4+). HRMS calcd for C54H62BrNO10, 963.3557, found 963.3550. 1H NMR (500MHz, CDCl3): δ 7.92-7.90 (d, J= 8.42 Hz, 2H), 7.57-7.55 (d, J=8.4 Hz, 2H), 7.37-7.29 (m, 20H, Ph), 4.76-4.47 (m, 8H, CH2), 4.35-4.34 (m, 2H), 4.22-4.19 (m, 1H), 4.01 (m, 4H), 3.82 (br s, 2H), 3.74-3.72 (m, 1H), 3.69-3.66 (dd, J=10.2, 4.6 Hz, 1H), 2.00-1.98 (m, 1H), 1.62 (br s, 5H), 1.56 (br s, 4H), 1.47 (s, 9H). 13C NMR (125MHz, CDCl3): δ 165.7, 152.1, 138.7, 138.6, 138.4, 131.9, 131.4, 128.9, 128.6, 128.5, 128.5, 128.4, 128.2, 128.1, 127.9, 127.8, 127.8, 127.7, 95.3, 94.5, 80.2, 77.4, 76.7, 76.4, 74.6, 73.5, 73.3, 59.7 and 28.7.
Supplementary Material
Acknowledgements
The research was supported by funding from NIH: to RWF for synthesis (GM 60271); by a subcontract from the NIH-supported NIAID Tetramer Facility at Emory University; by the Division of Research Resources to Hunter College for infrastructure support (RCMI-RR 03037). We thank Dr. Clifford E. Soll at Hunter College/CUNY for MS support and Dr. Michael Blumenstein for his excellent assistance in the NMR experiments. Special thanks to Dr. H. Annoura from Daiichi Suntory Pharma Co., Ltd. Japan for supplying a copy of the spectrum of compound 48, the C-3 epimer of the C-glycoside analog of OCH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dedicated to the memory of Professor Giuseppe Capozzi (1941–2008), Dipartimento di Chimica Organica, Universita di Firenze, a friend and colleague.
References
- 1.(a) Natori T, Koezuka Y, Higa T. Tetrahedron Lett. 1993;34:5591–5592. [Google Scholar]; (b) Akimoto K, Natori T, Morita M. Tetrahedron Lett. 1993;34:5593–5596. [Google Scholar]; (c) Natori T, Morita M, Akimoto K, Koezuka Y. Tetrahedron. 1994;50:2771–2784. [Google Scholar]
- 2.(a) Savage PB, Teyton L, Bendelac A. Chem. Soc. Rev. 2006;35:771–779. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]; (b) Tsuji M. Cell Mol. Life Sci. 2006;63:1889–1898. doi: 10.1007/s00018-006-6073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Nat. Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. Nat. Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 4.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey Dl, McCluskey J, Rossjohn J. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 5.For some recent analog work appearing subsequent to the Savage review in ref. 2, seeFujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong C-H. J. Am. Chem. Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z.Lee T, Cho M, Ko S-Y, Youn H-J, Baek DJ, Cho W-J, Kang C-Y, Kim S. J. Med. Chem. 2007;50:585–589. doi: 10.1021/jm061243q.Yu S-H, Park J-J, Chung S-K. Tetrahedron Asym. 2006;17:3030–3036.Fuhshuku K, Hongo N, Tashiro T, Masuda Y, Nakagawa R, Seino K, Taniguchi M, Mori K. Bioorg. Med. Chem. 2008;16:950–964. doi: 10.1016/j.bmc.2007.10.008.Tashiro T, Nakagawa R, Hirokawa T, Inoue S, Watarai H, Taniguchi M, Mori K. Tetrahedron Lett. 2007;48:3343–3347.Li Q, Ndonye RM, Illarionov PA, Yu KOA, Jerud ES, Diaz K, Bricard G, Porcelli SA, Besra GS, Chang Y-T, Howell AR. J. Comb. Chem. 2007;9:1084–1093. doi: 10.1021/cc070057i.Lee T, Cho M, Ko S-Y, Youn H-J, Baek DJ, Cho W-J, Kang C-Y, Kim S. J. Med. Chem. 2007;50:585–589. doi: 10.1021/jm061243q.Ebensen T, Link C, Riese P, Schulze K, Morr M, Guzmàn CA. J. Immunol. 2007;179:2065–2073. doi: 10.4049/jimmunol.179.4.2065.
- 6.Franck RW, Tsuji M. Acc. Chem. Res. 2006;39:692–701. doi: 10.1021/ar050006z. [DOI] [PubMed] [Google Scholar]; Results since the 2006 reviewTeng MWL, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, Porcelli SA, Besra GS, Takeda K, Yagita H, Kershaw MH, Smyth MJ. Cancer Research. 2007;67:7495–7504. doi: 10.1158/0008-5472.CAN-07-0941.
- 7.Motoki K, Kobayashi E, Uchida T, Fukushima H, Koezuka Y. Bioorg. Med. Chem. Lett. 1995;5:705–710. doi: 10.1016/0968-0896(96)00049-1. [DOI] [PubMed] [Google Scholar]
- 8.Ndonye RM, Izmirian DP, Dunn MF, Yu KOA, Porcelli SA, Khurana A, Kronenberg M, Richardson SK, Howell AR. J. Org. Chem. 2005;70:10260–10270. doi: 10.1021/jo051147h. [DOI] [PubMed] [Google Scholar]
- 9.Similarly conceived C-glycoside syntheses in this immunostimulant family can be found in the following workLu X, Song L, Metelitsa LS, Bittman R. Chembiochem. 2006;7:1750–1756. doi: 10.1002/cbic.200600197.Gurjar MK, Reddy R. Carbohydr. Lett. 1997;2:293–298.Dondoni A, Perrone D, Turturici E. J. Org. Chem. 1999;64:5557–5564. doi: 10.1021/jo990398l.Wipf P, Pierce JG. Org. Lett. 2006;8:3375–3378. doi: 10.1021/ol0613057.Toba T, Murata K, Yamamura T, Miyake S, Annoura H. Tetrahedron Lett. 2005;46:5043–5047.
- 10.Palomo C, Oiarbide M, Landa A, Gonzalez-Rego MC, Garcia JM, Gonzalez A, Odriozola JM, Martin-Pastor M, Linden A. J. Am. Chem. Soc. 2002;124:8637–8643. doi: 10.1021/ja026250s. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Hanson RM, Klunder JM, Ko SY, Masamune H, Sharpless KB. J. Am. Chem. Soc. 1987;109:5765–5780. [Google Scholar]
- 12.Behrens CH, Ko SY, Sharpless KB, Walker FJ. J. Org. Chem. 1985;50:5687–5696. [Google Scholar]
- 13.Plettenberg O, Bodmer-Narkevitch V, Wong C-H. J. Org. Chem. 2002;67:4559–4564. doi: 10.1021/jo0201530. [DOI] [PubMed] [Google Scholar]
- 14.SourKouni-Argirusi G, Kirschning A. Org. Lett. 2000;2:3781–3784. doi: 10.1021/ol006483t. [DOI] [PubMed] [Google Scholar]
- 15.(a) Iida H, Yamazaki N, Kibayasi C. Tetrahedron Lett. 1985;26:3255. [Google Scholar]; (b) Iida H, Yamazaki N, Kibayasi C. J. Org. Chem. 1986;51:1069–1073. [Google Scholar]; (c) Iida H, Yamazaki N, Kibayashi C. J. Org. Chem. 1986;51:3769–3771. [Google Scholar]
- 16.(a) Caron M, Carlier PR, Sharpless KB. J. Org. Chem. 1988;53:5185–5187. [Google Scholar]; (b) Ginesta X, Pasto M, Pericas MA, Riera A. Org. Lett. 2003;5:3001–3004. doi: 10.1021/ol034843h. [DOI] [PubMed] [Google Scholar]
- 17.(a) Chen G, Schmieg J, Tsuji M, Franck RW. Org. Lett. 2004;6:4077–4080. doi: 10.1021/ol0482137. [DOI] [PubMed] [Google Scholar]; (b) Chen G, Franck RW. unpublished result. [Google Scholar]
- 18.See ref. 9e; we thank Dr. Annoura for providing an nmr spectrum of 48
- 19.Mootoo DR, Fraser-Reid B. J. Chem. Soc., Chem. Commun. 1986;21:1570–1571. [Google Scholar]
- 20.Moilanen SB, Potuzak JS, Tan DS. J. Am. Chem. Soc. 2006;129:1792–1793. doi: 10.1021/ja057908f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preliminary results,Tsuji M, Li M.New York, NY: ADARC; We are grateful for their permission to disclose these data.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.