Abstract
Aedes albopictus (Skuse) and Aedes japonicus (Theobald) are two of the most recent and widespread invasive mosquito species to have become established in the United States. The two species co-occur in water-filled artificial containers, where crowding and limiting resources are likely to promote inter- or intraspecific larval competition. The performance of northern Virginia populations of Ae. japonicus and Ae. albopictus competing as larvae under field conditions was evaluated. Per capita rates of population increase for each species were estimated, and the effects of species composition and larval density were determined. In water-containing cups provided with oak leaves, Ae. albopictus larvae exhibited a competitive advantage over Ae. japonicus as a consequence of higher survivorship, shorter developmental time, and a significantly higher estimated population growth rate under conditions of interspecific competition. Intraspecific competition constrained population performance of Ae. albopictus significantly more than competition with Ae. japonicus. In the context of the Lotka-Volterra model of competition, these findings suggest competitive exclusion of Ae. japonicus in those habitats where this species co-occurs with Ae. albopictus.
Keywords: invasive, mosquito, containers, competition
Aedes albopictus (Skuse) and Aedes japonicus (Theobald) are two of the most prominent exotic mosquito species to become recently established in the United States. Ae. albopictus was introduced into the United States from Japan in tire shipments (Hawley et al. 1987, Reiter and Sprenger 1987), which led to its establishment in Texas in 1985 (Sprenger and Wuithiranyagool 1986). State and local mosquito surveillance records indicate that it has since spread rapidly, becoming established across much of the eastern United States from southern Florida to New Jersey, Illinois, Indiana, Ohio, and Missouri (Moore 1999). The westward spread of this invader has been much slower; presumably because of the drier summers and cool, wet winters on the West Coast (Washburn and Hartmann 1992). Ae. albopictus has been intercepted and eradicated in California (Linthicum et al. 2003) and Washington (Craven et al. 1988). The invasion success and rapid spread of Ae. albopictus in the United States has been attributed to its generalized habitat and food requirements, ability to live in human-dominated habitats(Hawley 1988), desiccation-resistant eggs (Focks et al. 1994, Juliano et al. 2002), and larval competitive superiority to resident species (Livdahl and Willey 1991, Juliano 1998).
The introduction of Ae. japonicus into the United States was initially reported by Peyton et al. (1999) from light trap collections from New York and New Jersey in August and September 1998. However, an archival search by Andreadis et al. (2001) showed that this species was actually first detected 1 mo earlier in Connecticut. Ae. japonicus has since been detected along the East Coast, with reports as far south as Alabama (Mullen 2005), north to Maine (Foss and Dearborn 2001), and west to Missouri (Gallitano et al. 2006), from what seem to be multiple introductions from Japan (Fonseca et al. 2001). Ae. japonicus seems to have become established on the West Coast in Washington (Roppo et al. 2004) and has recently been detected in Mississippi, Nevada (Moore 2005), and Hawaii (Larish and Savage 2005). The used tire trade is the suspected mode of introduction of Ae. japonicus into the United States as it was for Ae. albopictus (Peyton et al. 1999, Lounibos 2002).
The current U.S. distributions of these two species overlap considerably, although Ae. japonicus seems to be more cold tolerant (Tanaka et al. 1979) than Ae. albopictus, as evidenced by the establishment of the former species in eastern Canada (Thielman and Hunter 2006). It has been predicted that the American range of Ae. albopictus may eventually expand northward as far as the −5°C isotherm, as it does in Asia; however, at such latitudes, populations would likely not overwinter (Nawrocki and Hawley 1987). Furthermore, both species are container-inhabiting mosquitoes commonly found in water-filled artificial container habitats such as automobile tires, bird baths, and flower pot saucers; however, rock pools seem to be the favored habitat of Ae. japonicus in its native range (Tanaka et al. 1979). The aquatic larvae of both species feed on microorganisms and particulate matter in the water column as well as on leaves and other organic detritus ( Merritt et al. 1992).
Severe crowding and limiting resources are frequent in these habitats; thus, it is likely that larval competition, inter- or intraspecific, may have important effects on the growth, survivorship, and reproductive success of these species (Juliano and Lounibos 2005). Therefore, larval conditions may have a significant impact on overall population growth. Those species that can maintain positive population growth under interspecific conditions of greater density or lower resource availability than a competitor are considered to have a competitive advantage. Such a competitive advantage is even greater if one species can maintain positive population growth under conditions that result in negative population growth for a competitor.
The role of interspecific competition in structuring communities of container-dwelling mosquitoes has been well documented, perhaps best so for Ae. albopictus and Ae. aegypti in the southeastern United States (Barrera 1996, Juliano 1998, Juliano et al. 2004), where interspecific larval competition was the probable cause of the decline in range and abundance of the latter species throughout most of this area (O’Meara et al. 1995, Juliano et al. 2004, Juliano and Lounibos 2005). Ae. albopictus has also been shown experimentally to be a superior larval competitor to the U.S. resident mosquito species Aedes triseriatus (Say) (Livdahl and Willey 1991), Aedes sierrensis (Ludlow) (Washburn and Hartmann 1992), and Culex pipiens L. (Costanzo et al. 2005). Although the invasion of an introduced species may negatively impact resident species as a result of interspecific larval competition, the effects of other interactions such as predation, habitat alteration, or apparent competition mediated by shared enemies should also be considered when assessing invasion outcomes (Juliano and Lounibos 2005).
Understanding the invasion dynamics of Ae. albopictus and Ae. japonicus is important not only because of the ecological consequences resulting from their interactions with native container-inhabiting mosquitoes, but also because these species may transmit arboviruses that cause human and animal disease. In its native range, Ae. albopictus is a known vector of dengue virus, which has been isolated from wild-caught individuals of this species in its invasive range in the Americas (Ibañez-Bernal et al. 1997, Méndez et al. 2006). This species was also implicated as the vector in the 2001 outbreak of dengue in Hawaii (Effler et al. 2005). Invasive Ae. albopictus have also been recovered infected with eastern equine encephalitis (Mitchell et al. 1992) and LaCrosse encephalitis viruses (Gerhardt et al. 2001) in the United States and chikungunya virus in Italy (Enserink 2007).
Although Ae. japonicus is not considered an important disease vector in its native range in Asia, it is capable of transmitting Japanese encephalitis virus (Takashima and Rosen 1989) and has also been indicated as a competent experimental vector of eastern equine (Sardelis et al. 2002a), LaCrosse (Sardelis et al. 2002b), and St. Louis encephalitis viruses (Sardelis et al. 2003). Both Ae. albopictus and Ae. japonicus have been shown to be competent vectors of West Nile virus in the laboratory (Turell et al. 2001), and wild-caught adults of both species have been recovered infected with this virus (Holick et al. 2002, Scott 2003, Godsey et al. 2005).
Although considerable data exist regarding the interspecific interactions and competitive outcomes involving Ae. albopictus and other container-inhabiting mosquito species, there are no comparable reports on its interactions with Ae. japonicus. Because Ae. albopictus and Ae. japonicus co-occur frequently in containers where their ranges overlap, an investigation of larval competitive interactions between these two species was proposed. An experiment designed similar to those of Juliano (1998) and Braks et al. (2004), with the exception that only a single resource level was implemented, was conducted to test for competitive advantage between Virginia populations of these species in competition under field conditions in Fairfax, VA. Comparisons were made between species for intra- and interspecific effects of larval density on survivorship, development time, body size, and population growth.
Materials and Methods
The experiment was conducted in forest surrounding a streambed located directly behind the Fairfax County Department of Health in Fairfax, VA (latitude 38°50′ N, longitude 77°19′W) from August to October 2006. Routine surveillance data collected by the Department of Health indicated that both Ae. albopictus and Ae. japonicus were commonly detected on this property as both larvae in artificial containers and as adults in CO2-baited CDC light traps and gravid traps. The Ae. albopictus and Ae. japonicus used in this experiment were the progeny of females caught in Reiter-Cummings modified gravid traps (Bioquip, Rancho Dominquez, CA) in Fairfax, VA.
Inter- and intraspecific larval competition was studied by monitoring the survivorship and development of larvae in 400-ml black polypropylene cups (10.5 cm in height, 6.5 cm base diameter). Field surveys of habitats in the area (unpublished data) showed that these species co-occurred in approximately one fifth of containers sampled, the majority of which contained <1 liter of water and were shaded. Mean number of Ae. albopictus per container was 42.4 ± 12.8 (SE), with a range of 1–250, whereas an average of 17.3 ± 4.1 Ae. japonicus was collected per container, with a range of 1–77.Based on these data, three density-composition combinations for each species (Ae. albopictus:Ae. japonicus: 10:0, 50:0, 0:10, 0:50, and 25:25) were used in a randomized block design. One replicate of each density-composition combination was placed at each of five experimental sites, spaced ≈30 m apart, for a total of five replicates per treatment and 25 cups. Although both species have been found locally in containers with varying sun exposure (i.e., none, partial, and full exposure), all five sites used were heavily shaded.
On 10 August, each cup was randomly labeled with a unique number and letter corresponding to one of the five treatments and sites, where they were secured to plastic stakes to prevent toppling. A larval resource base was provided by fallen pin oak leaves (Quercus palustris) that had been collected, washed with distilled water, and dried at room temperature for 1 wk before quartering, weighing, and sorting. To allow for the leaves to soak and be colonized by microorganisms, 4 d before the start of the experiment, 1 g was added to each of the 25 cups containing 200 ml of distilled water. Each container was covered with fiberglass screen (0.5-mm mesh) and secured with a rubber band to prevent entry of other macrofauna and detritus. In the laboratory, eggs of Ae. albopictus and Ae. japonicus were synchronously hatched (Novak and Shroyer 1978), and 24 h later, first-instar larvae were counted into aliquots of 10, 25, and 50. Within 1 h after counting, the larvae were distributed into appropriate cups.
Each container was monitored daily for the presence of pupae, which were collected and housed singly in sealed 50-ml vials containing water from their respective field cup. Each vial was labeled with the appropriate site and treatment identifier before being secured with a rubber band to a plastic stake at the field site. On emergence, adults were killed by freezing before scoring by container, species, sex, and day of emergence. The experiment ended on 11 October when the final adult emerged. Ambient temperature was monitored hourly for the duration of the experiment with three Onset HOBO data loggers located in the middle and at either end of the experimental area. The average ambient temperature during the experiment was 18.77 ± 0.06°C, with a range of 7.76–29.73°C (n = 4,197 hours). For 2 d (31 August and 1 September), the area was subjected to intense wind and rain because of a hurricane off the Atlantic Coast. To prevent damage to experiments and loss of data, the cups at each site were successfully covered and secured with a tarpaulin during this time
Population Growth Correlates
To quantify the effects of inter- and intraspecific competition on Ae. albopictus and Ae. japonicus, the mean survivorship, median development time of males and females, and median body size at adulthood of females per replicate were analyzed by one-factor, randomized complete block analysis of variance (ANOVA; SAS Institute 1989, PROC GLM), with treatment as effect and sites as blocks. Median development times and body size were used to meet assumptions of homogeneous variance and normality. Significant treatment effects were further analyzed using pairwise comparisons among treatment means with a sequential Bonferroni adjustment for the number of tests done (experimentwise α = 0.05). Survivorship was calculated as the proportion of adults that emerged from the initial cohort of first instar larvae. Development time was calculated as the number of days from hatching to adult emergence. Adult body size was estimated from the length of one wing, which was removed from each female and measured under a dissecting microscope with an ocular micrometer (Packer and Corbet 1989).
Composite Index of Population Performance
Survivorship, female development time, and wing length were used to estimate the per capita rate of population change using a composite index of population performance for each species per replicate, r′ (Livdahl and Sugihara 1984):
where N0 is the initial number of females (assumed to be 50% of the cohort), AX is the number of females eclosing on day x, wX is the mean wing length of females eclosing on day x, and f(wX) is a function relating egg production to wing length. D is the time from adult eclosion to reproduction, taken as 14 d for Ae. albopictus (Livdahl and Willey 1991) and 12 d for Ae. japonicus (unpublished data). A regression relating female wing length to fecundity for Ae. albopictus was obtained from Lounibos et al. (2002):
where wX is the wing length in millimeters on day x. Because no suitable equation could be derived from the literature, a regression for Ae. japonicus was determined experimentally. The mean estimated per capita rates of change (r′) for Ae. albopictus and Ae. japonicus were analyzed by one-factor, randomized complete block ANOVA (SAS Institute 1989, PROC GLM), with treatment as effect and sites as blocks. Significant treatment effects were further analyzed using pairwise comparisons among treatment means with a sequential Bonferroni adjustment for the number of tests done (experimentwise α = 0.05).
Estimation of Ae. japonicus Fecundity from Wing Length
A regression for Ae. japonicus was determined experimentally under controlled conditions of 26°C and 12 h:12 h light:dark in an insectary at the Florida Medical Entomology Laboratory in Vero Beach, FL. Ae. japonicus eggs used for this experiment were obtained from a colony maintained at the Headlee Research Laboratory Mosquito Research and Control Unit at Rutgers University in New Brunswick, NJ. This colony originated from larval collections from a horse farm in New Egypt, Ocean County, NJ, in 2001.
Eggs were hatched simultaneously using a medium of Brewer's yeast and lactalbumen, and 24 h later, larvae were transferred into enamel pans containing ≈1 liter of distilled water at a density of 100 per pan, where they were reared under conditions of varying food availability. Food consisted of pin oak leaves (Q. palustris) from Fairfax, VA, that were collected and dried at room temperature before quartering, weighing, and sorting into 0.5-, 1.5-, and 3-g allotments. These varying food levels were used to generate adult females with a large range of body size. Four days before the start of the experiment, three allotments of each leaf size were added to the enamel pans. This period allowed for the leaves to soak and be colonized by microorganisms.
On pupation, mosquitoes were transferred to plastic cups containing distilled water and placed in 0.028-m3 (30.5 by 30.5 by 30.5 cm) rearing cages. Each cage was checked daily for adult emergence, after which any remaining pupae were removed to maintain separate adult cohorts by date of emergence. Cotton soaked in a 20% sucrose solution was provided as a source of carbohydrates for adults at all times. Beginning 2 d after emergence, adult females of each cohort were offered a blood-meal from a restrained chicken placed within the cage daily for 1 h. It should be noted that chickens were also used by Lounibos et al. (2002) to determine the fecundity of Ae. albopictus from wing length. On visual inspection immediately after the bloodfeeding opportunity, those females that appeared to have fed to repletion were removed from the cage using a mouth aspirator and placed singly in 12-dram plastic vials containing a 2.5 by 7.6-cm strip of wet seed germination paper (Anchor Paper, St. Paul, MN) to serve as an oviposition substrate (Steinly et al. 1991). Those females that did not feed to repletion on the first bloodfeeding opportunity were excluded from the study. Laid eggs were counted, and females were subsequently frozen and killed.
One wing was removed from each female and measured under a dissecting microscope with an ocular micrometer (Packer and Corbet 1989) to provide an estimate of body size for each female. The terminal abdominal segment of each female was subsequently removed, and the ovaries were teased out with the aid of a dissecting microscope to count any retained (fully formed and chitinous) eggs. This experiment was limited to the first gonotrophic cycle for each species because the majority of female mosquitoes will most likely complete only one cycle (Packer and Corbet 1989).
Results
Survivorship to Adulthood
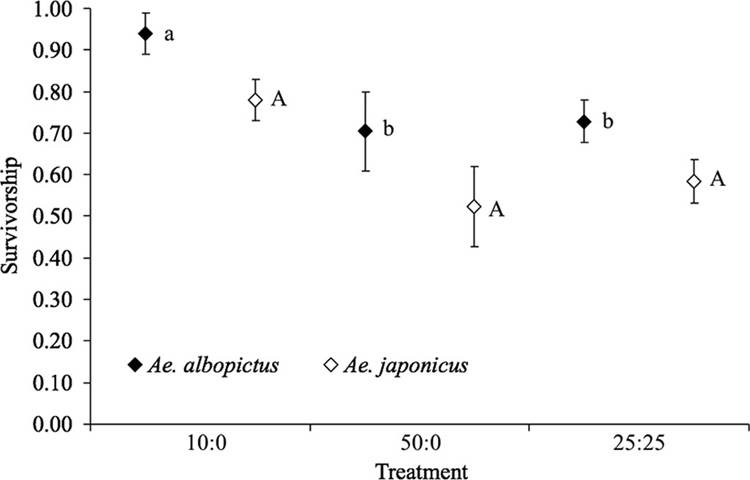
Mean survivorship to adulthood of Ae. albopictus was affected by treatment (F2,8 = 7.16, P = 0.017) but that of Ae. japonicus was not (F2,8 = 2.89, P = 0.113). Mean survivorship of Ae. albopictus was significantly higher in the 10:0 treatment than the 50:0 or 25:25 treatments, which were not different from one another (Fig. 1). Within density treatments, mean survivorship of Ae. albopictus was consistently higher than that of Ae. japonicus (Fig. 1). Mean survivorship to adulthood for neither Ae. albopictus (F4,8 = 0.89, P = 0.514) nor Ae. japonicus (F4,8 = 0.30, P = 0.871) was affected by site.
Fig. 1.
Mean survivorship (proportion of the original number of larvae surviving to adulthood) of Ae. albopictus and Ae. japonicus (±SE). Lowercase and uppercase letters indicate significant differences among competition treatments resulting from pairwise comparisons (P< 0.05) for Ae. albopictus and Ae. japonicus, respectively. ANOVA did not indicate a significant difference in survivorship among treatments for Ae. japonicus.
Developmental Time
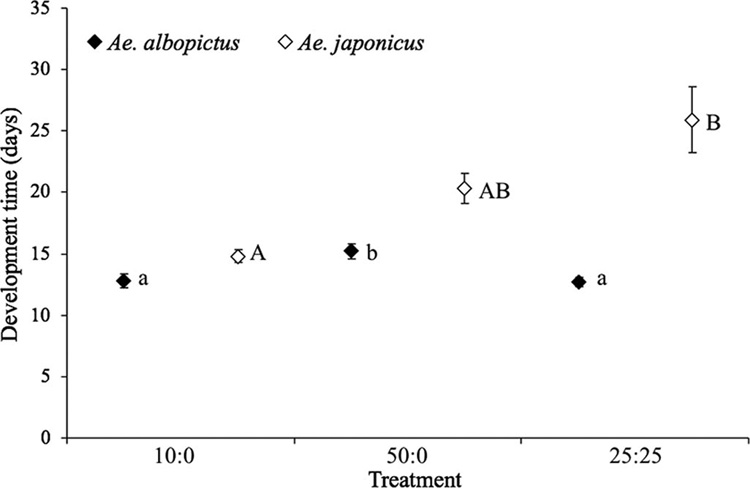
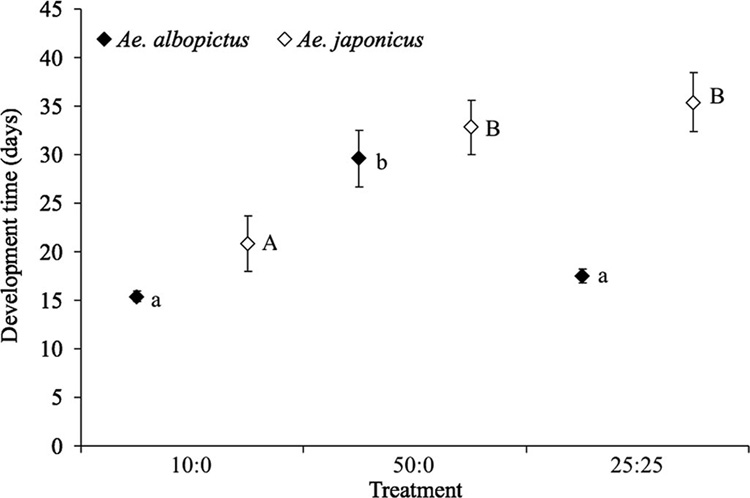
The average median time from hatch to adulthood was significantly affected by density treatment for female (F2,8 = 22.90,P<0.001) but not male (F2,8 = 2.92, P = 0.112) Ae. albopictus. Comparisons of means for Ae. albopictus showed that development time was significantly shorter for females in the 10:0 and 25:25 density-composition treatments than in the 50:0 treatment (Fig. 2 and Fig 3). The mean median development time of both male (F2,8 = 9.46, P = 0.008) and female (F2,8 = 6.02, P = 0.025) Ae. japonicus was significantly affected by treatments; however, the pattern of significant differences in pairwise comparisons differed between the sexes in this species (Fig. 2 and Fig 3). Within any individual density-composition treatment, mean median development times of both male and female Ae. albopictus were consistently lower than those of Ae. japonicus (Fig. 2 and Fig 3). Development time of males and females, respectively, was unaffected by site for Ae. albopictus (F4,8 = 0.87, P = 0.521 and F4,8 = 1.35, P = 0.332) or Ae. japonicus (F4,8 = 0.92, P = 0.496 and F4,8 = 0.58, P = 0.687).
Fig. 2.
Means of median time to adulthood for male Ae. albopictus and Ae. japonicus (±SE). Lowercase and uppercase letters indicate significant differences among competition treatments resulting from pairwise comparisons (P < 0.05) for Ae. albopictus and Ae. japonicus, respectively.
Fig. 3.
Means of median time to adulthood for female Ae. albopictus and Ae. japonicus (±SE). Lowercase and uppercase letters indicate significant differences among competition treatments resulting from pairwise comparisons (P < 0.05) for Ae. albopictus and Ae. japonicus, respectively.
Female Wing Length
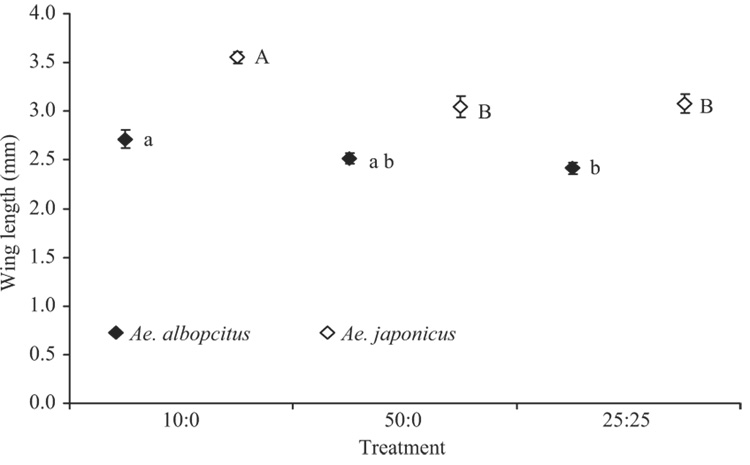
Mean median wing lengths of Ae. japonicus females were significantly affected by density treatments (F2,8 = 23.12, P<0.001), but those of Ae. albopictus were not (F2,8 = 4.03, P = 0.062). For Ae. japonicus, the low density mean median wing length was significantly greater than those from either high density treatment (Fig. 4). The average median wing length of Ae. albopictus was not affected by site (F4,8 = 0.50, P = 0.5737); however, that of Ae. japonicus was affected (F4,8 = 5.24, P = 0.023).
Fig. 4.
Means of median wing lengths of Ae. albopictus and Ae. japonicus adult females (±SE). Lowercase and uppercase letters indicate significant differences among competition treatments resulting from pairwise comparisons (P< 0.05) for Ae. albopictus and Ae. japonicus, respectively.
Estimation of Ae. japonicus Fecundity from Wing Length
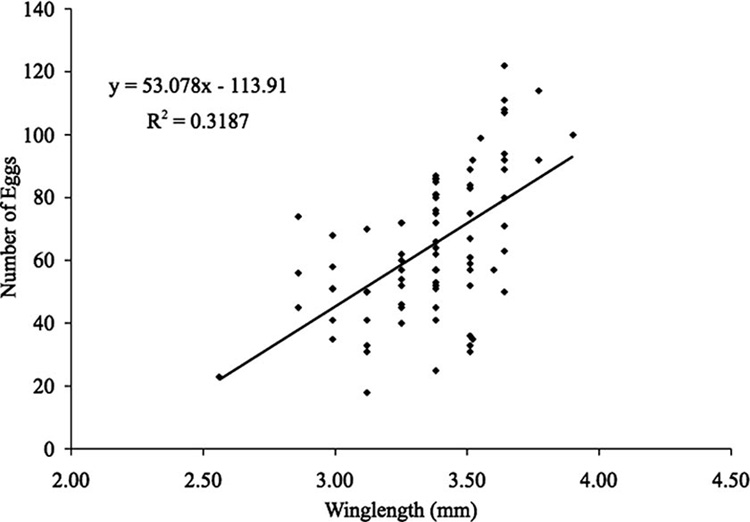
A total of 79 Ae. japonicus females oviposited one or more eggs, and total egg production per female ranged from 18 to 122 eggs. The mean total eggs produced per female was 64.5 ± 23.0 (SD). The average wing length of females was 3.4 ± 0.2 mm, with a range of 2.6–3.9 mm. A single combined linear regression (SAS Institute 1989) was obtained for all three larval diet regimens, and the relationship of fecundity to wing length was determined for Ae. japonicus (Fig. 5). There was a positive regression of total number of eggs produced per female on wing length, yielding the following regression equation for Ae. japonicus:
where wX is the wing length in millimeters on day x.
Fig. 5.
Relationship between wing length (mm) and total eggs produced for Ae. japonicus.
Composite Index of Population Performance (r′)
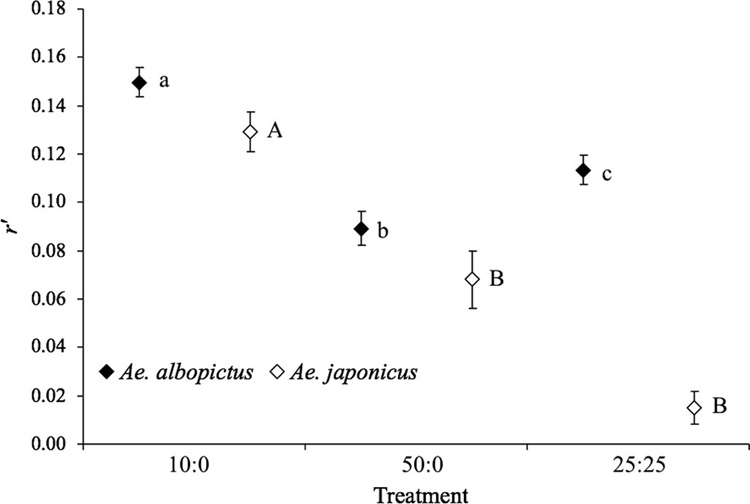
The mean estimated per capita rate of increase was significantly affected by density treatments for both Ae. albopictus (F2,8 = 21.31, P < 0.001) and Ae. japonicus (F2,8 = 12.56, P = 0.003; Fig. 6). Comparisons of means showed that r′ of both Ae. albopictus and Ae. japonicus was significantly higher for the low-density treatment than either of the high-density treatments, which in turn did not differ (Fig. 6). Within any individual density-composition treatment, mean r′ of Ae. albopictus was consistently higher than that of Ae. japonicus. Mean per capita rate of population increase for neither Ae. albopictus (F4,8 = 0.73, P = 0.597) nor Ae. japonicus (F4,8 = 0.46, P = 0.762) was affected by site.
Fig. 6.
Mean estimates (±SE) of population performance (r′, the per capita rate of population increase for the cohort) for female Ae. albopictus and Ae. japonicus adults. Lowercase and uppercase letters indicate significant differences among competition treatments resulting from pairwise comparisons (P < 0.05) for Ae. albopictus and Ae. japonicus, respectively.
Discussion
Invasion success and spread of non-native species can be enhanced by superiority in interspecific competition, particularly when similar species and limited resources are encountered (Williamson 1996). Inter-specific larval resource competition plays an important role in structuring the mosquito communities of artificial container habitats that are subject to invasion (Juliano and Lounibos 2005). In the context of this experiment, although both species were able to maintain positive population growth under interspecific conditions, it seems that Ae. albopictus may have a competitive advantage over Ae. japonicus. This is supported by the consistently higher survivorship, shorter development time, and higher per capita rate of population increase of Ae. albopictus compared with Ae. japonicus across density treatments.
The Lotka-Volterra model of two-species competition (Lotka 1925, Volterra 1926, 1931) provides a useful frame of reference for interpreting these findings. This model accounts for the effect that the presence of one species will have on the population of another species, relative to the competitive effect that two members of the same species would have on each other. In this respect, the Lotka-Volterra competition equations may be used to predict the potential outcome when two species are in competition for the same resources. Consider that Ae. albopictus and Ae. japonicus, occurring at densities Na and Nj, respectively, have the carrying capacities Ka and Kj, at which each reaches an equilibrium population size (dN/dt = 0), and instantaneous rates of increase, ra and rj, in the absence of one another. The simultaneous growth of the two competing species occurring together can be described by the following pair of differential logistic equations:
where α and β are competition coefficients; α is the effect of Ae. albopictus on Ae. japonicus and β is the effect of Ae. japonicus and Ae. albopictus. The outcome of competition depends on the relative values of Ka, Kj, α, and β
Stable coexistence can occur only when both Kj/α > Ka and Ka/β > Kj; that is, each population must inhibit its own growth more than that of the other species. This is most likely to occur when the carrying capacities of the two species are equivalent and the competition coefficients are less than one. The equal inter- and intraspecific effects on Ae. japonicus imply that α ≈ 1, whereas the lesser interspecific effect on Ae. albopictus, compared with intraspecific effect, suggests that β < 1. Similar results have been reported from experiments with Ae. albopictus in competition with Ae. sierrensis (Washburn and Hartmann 1992) and Ae. triseriatus (Teng and Apperson 2000).
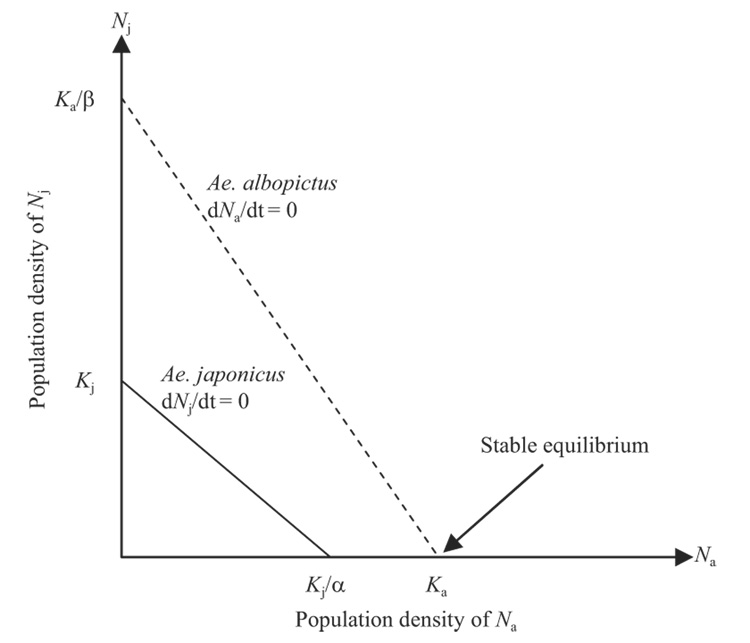
Although no estimates of carrying capacities were obtained for either species in this experiment, because r′ for Ae. japonicus at high density was closer to zero, indicating little or no growth, the dNj/dt is presumably closer to zero at high density and Ae. japonicus is closer to Kj than Ae. albopictus is to Ka. Therefore, it is likely that Kj < Ka. When isoclines of both species are superimposed on one another, the Na isocline lies above the Nj isocline, and the elimination of Ae. japonicus by competitive exclusion is predicted (Fig. 7), because the only point of stable equilibrium under this scenario occurs when Na = Ka and Nj = 0.
Fig. 7.
Isoclines of Ae. albopictus and Ae. japonicus, based on a Lotka-Volterra model (see text), with stable equilibrium occurring at Na = Ka and Nj = 0, suggesting competitive exclusion of the latter species.
Although the shorter development times of Ae. albopictus may simply reflect intrinsic metabolic differences between the species, when coupled with the higher mean per capita rate of increase, it may suggest that Ae. albopictus is able to forage and acquire re-sources more efficiently or uses different feeding behaviors that are more effective in this type of larval habitat. A recent comparison of foraging behaviors found Ae. japonicus to be the more active of the two species in various media that included leaves and tire water (O'Donnell and Armbruster 2007), which suggests that activity per se does not necessarily mirror competitive ability. Ho et al. (1973) suggested that perhaps the higher content of proteinases in the gut of Ae. albopictus facilitates a more efficient feeding style, which ultimately allows this species to develop faster than other container-inhabiting mosquitoes.
These results imply that Ae. albopictus has a slight competitive advantage over Ae. japonicus; however, these two species may coexist in areas where they overlap despite this observation. Although this experiment was conducted in the field under manipulated but ecologically realistic conditions, it is important to consider that variations in resource level, type, or frequency (Braks et al. 2004), temperature (Lounibos et al. 2002), sun exposure, container type (Livdahl and Willey 1991), larval density, and season (Teng and Apperson 2000) may influence larval competition differently among mosquito species. Furthermore, these findings should be viewed in context with field observations of co-occurrences, as well as seasonal distributions, habitat preferences, and overwintering behaviors, because these factors may ultimately influence niche segregation and the community structure of the artificial containers in which these species coexist. Similarly, although interspecific larval competition is likely an important factor influencing the survivorship, growth, reproductive success, and population performance of mosquitoes in container environments with limited resources, other factors such as predation (Griswold and Lounibos 2005), 2006, intraguild predation (Edgerly et al. 1999), apparent competition mediated by shared enemies (Juliano 1998), habitat alteration (Bertness 1984), and differences in foraging behavior (Yee et al. 2004, O’Donnell and Armbruster 2007) may also be important and warrant further research with respect to interactions between these two species. Finally, it should be noted that noncompetitive characteristics such as adult survival and mating and bloodfeeding success might ultimately influence the relative success of larger mosquito populations.
In addition to these ecological consequences, these findings may potentially have epidemiological implications, particularly with respect to LaCrosse encephalitis virus, in which both Ae. albopictus and Ae. japonicus are competent vectors. Their continued coexistence in containers in LaCrosse endemic regions may be important in epizootic, or potentially even epidemic, transmission of the disease, although this will require further study. Because larval competition has been linked to greater infection and dissemination rates of dengue and Sindbis viruses for Ae. albopictus (Alto et al. 2005, 2008), similar effects are possible with respect to arboviruses circulating in areas in which this species is sympatric with Ae. japonicus.
Acknowledgments
We thank the City of Fairfax and the Fairfax Department of Health for permission to conduct field work on their land; J. Frescholtz and J. vander Voort for support in the field; R. Escher for assistance in determining the body size fecundity relationship for Ae. japonicus; L. McCuiston for providing Ae. japonicus eggs used for part of this research; and G. O’Meara and S. A. Juliano for reviewing an early draft of this manuscript. This work was supported by funds from National Institutes of Health Grant AI-044793.
References Cited
- Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arbovirus infections in Aedes mosquitoes. Ecology. 2005;86:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc. Biol. Sci. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera: Culicidae) in Connecticut, USA. J. Med. Entomol. 2001;38:774–779. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol. Entomol. 1996;21:117–127. [Google Scholar]
- Bertness MD. Habitat and community modification by an introduced herbivorous snail. Ecology. 1984;65:370–381. [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourenço-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes in Brazil. Ann. Entomol. Soc. Am. 2004;97:130–139. [Google Scholar]
- Costanzo KS, Mormann K, Juliano SA. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae) J. Med. Entomol. 2005;42:559–572. doi: 10.1093/jmedent/42.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RB, Eliason DA, Francy DB, Reiter P, Campos EG, Jakob WL, Smith GC, Bozzik CJ, Moore GG, Maupin GO, Monath TP. Importation of Aedes albopictus and other exotic mosquito species into the United States in used tires from Asia. J. Am. Mosq. Control Assoc. 1988;4:138–142. [PubMed] [Google Scholar]
- Edgerly JS, Willey MS, Livdahl T. Intraguild predation among treehole mosquitoes Aedes albopictus, Ae. aegypti, and Ae. triseriatus (Diptera: Culicidae), in laboratory microcosms. J. Med. Entomol. 1999;36:394–399. doi: 10.1093/jmedent/36.3.394. [DOI] [PubMed] [Google Scholar]
- Effler PW, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, Elm J, Tom T, Reiter P, Rigau-Perez JG, Hayes JM, Mills K, Napier M, Clark GG, Gubler DJ for the Hawaii Dengue Outbreak Investigation Team. Dengue Fever, Hawaii, 2001–2002. Emerg. Infect. Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink M. Tropical disease follows mosquitoes to Europe. Science. 2007;317:1485. doi: 10.1126/science.317.5844.1485a. [DOI] [PubMed] [Google Scholar]
- Focks DA, Linda SB, Craig GB, Hawley WA, Pumpuni CB. Aedes albopictus (Diptera: Culi-cidae)—a statistical model of the role of temperature, photoperiod, and geography in the induction of egg diapause. J. Med. Entomol. 1994;31:278–286. doi: 10.1093/jmedent/31.2.278. [DOI] [PubMed] [Google Scholar]
- Fonseca DMS, Campbell WJ, Crans M, Mogi I, Miyagi T, Toma M, Bullians TG, Andreadis RL, Berry B, Pagac MR, Sardelis, Wilkerson RC. Aedes (Finlaya) japonicus (Diptera: Culicidae), a newly recognized mosquito in the United States: analyses of genetic variation in the United States and putative source populations. J. Med. Entomol. 2001;38:135–146. doi: 10.1603/0022-2585-38.2.135. [DOI] [PubMed] [Google Scholar]
- Foss KA, Dearborn RG. Preliminary faunistic survey of mosquito species (Diptera: Culicidae) with a focus on population densities and potential breeding sites in greater Portland, Maine. Augusta, ME: Maine Department of Conservation, Maine Forest Service, Forest Health and Monitoring Division; 2001. [Google Scholar]
- Gallitano S, Blaustein L, Vonesh J. First occurrence of Ochlerotatus japonicus in Missouri. J. Vector Ecol. 2006;30:347–348. [PubMed] [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of LaCrosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 2001;7:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsey MS, Blackmore MS, Panella NA, Burkhalter K, Gottfried K, Halsey LA, Rutledge R, Langevin SA, Gates R, Lamonte KM, Lambert AM, Lanciotti RS, Blackmore CGM, Loyless T, Stark L, Oliveri R, Conti L, Komar N. West Nile virus epizootiology in the southeastern United States, 2001. Vector-Borne Zoonot. Dis. 2005;5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol. Entomol. 2005;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2006;87:987–995. doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 1988;4 Suppl:1–40. [PubMed] [Google Scholar]
- Hawley WA, Pumpuni CB, Brady RH, Craig GB. Aedes albopictus in North America: probable introduction in used tires from Northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Ho BC, Chan KL, Chan YC. The biology and bionomics of Aedes albopictus (Skuse) In: Chan YC, Chan KL, Ho BC, editors. Vector Control in Southeast Asia. Singapore: Southeast Asian Ministers for Education Organization Workshop; 1973. pp. 125–143. [Google Scholar]
- Holick J, Kyle A, Ferraro W, Delaney RR, Iwaseczko M. Discovery of Aedes albopictus infected with West Nile virus in southeastern Pennsylvania. J. Am. Mosq. Control Assoc. 2002;18:131. [PubMed] [Google Scholar]
- Ibañez-Bernal S, Briseño B, Mutebi J-P, Argot E, Rodriguez G, Martinez-Camposc C, Paz R, de la Fuente-San Roman P, Tapia-Conyer R, Flisser A. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med. Vet. Entomol. 1997;11:305–309. doi: 10.1111/j.1365-2915.1997.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol. Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia (Berl.) 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in south Florida: differences between sites of coexistence and exclusion? Oecologia (Berl.) 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larish LB, Savage HM. Introduction and establishment of Aedes (Finlaya) japonicus japonicus (Theobald) on the island of Hawaii: Implications for arbovirus transmission. J. Am. Mosq. Control Assoc. 2005;21:318–321. doi: 10.2987/8756-971X(2005)21[318:IAEOAF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Linthicum KJ, Kramer VL, Madon MB, Fujioka K the Surveillance-Control Team. Introduction and potential establishment of Aedes albopictus in California in 2001. J. Am. Mosq. Control Assoc. 2003;19:301–308. [PubMed] [Google Scholar]
- Livdahl TP, Sugihara G. Non-linear interactions of populations and the importance of estimating per capita rates of change. J. Anim. Ecol. 1984;53:573–580. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lotka AJ. Elements of physical biology. Baltimore, MD: Williams and Wilkins; 1925. [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Suárez S, Menédez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J. Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- Méndez F, Barreto M, Arias JF, Renfigo G, Muñoz J, Burbano ME, Parra B. Humanand mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am. J. Trop. Med. Hyg. 2006;74:678–683. [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, Niebylski ML, Karabatsos N, Martin D, Mutebi J-P, Craig GB, Mahler MJ. Isolation of eastern equine encephalitis from Aedes albopictus in Florida. Science. 1992;257:526–527. doi: 10.1126/science.1321985. [DOI] [PubMed] [Google Scholar]
- Moore CG. Aedes albopictus in the United States: current status and prospects for further spread. J. Am. Mosq. Control Assoc. 1999;15:221–227. [PubMed] [Google Scholar]
- Moore CG. Arthropod-borne and Infectious Diseases Laboratory and Department of Environmental and Radiological Health Sciences. Fort Collins, CO: Colorado State University; 2005. Exotic and invasive vectors of public health importance in the United States. [Google Scholar]
- Mullen GR. First report of Ochlerotatus japonicus in Alabama. Ala. Vector Manage. Soc. Newsl. 2005;15:2. [Google Scholar]
- Nawrocki SJ, Hawley WA. Estimation of the Northern limits of the distribution of Aedes albopictus in North America. J. Am. Mosq. Control Assoc. 1987;3:314–317. [PubMed] [Google Scholar]
- Novak RJ, Shroyer DA. Eggs of Aedes triseriatus and A. hendersoni: a method to stimulate optimal hatch. Mosq. News. 1978;38:515–521. [Google Scholar]
- O’Donnell DL, Armbruster P. Comparison of foraging behavior of Aedes albopictus and Aedes japonicus (Diptera: Culicidae) J. Med. Entomol. 2007;44:984–989. doi: 10.1603/0022-2585(2007)44[984:colfbo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Packer MJ, Corbet PS. Size variation and reproductive success of female Aedes punctor (Diptera: Culicidae) Ecol. Entomol. 1989;14:297–309. [Google Scholar]
- Peyton EL, Campbell SR, Candeletti TM, Romanowski M, Crans WJ. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. J. Am. Mosq. Control Assoc. 1999;15:238–241. [PubMed] [Google Scholar]
- Reiter P, Sprenger D. The used tire trade: a mechanism for the worldwide dispersal of container breeding mosquitoes. J. Am. Mosq. Control Assoc. 1987;3:494–501. [PubMed] [Google Scholar]
- Roppo MR, Lilya JL, Maloney FA, Sames WJ. First occurrence of Ochlerotatus japonicus in the state of Washington. J. Am. Mosq. Control Assoc. 2004;20:83–84. [PubMed] [Google Scholar]
- Sardelis MR, Dohm JD, Pagac B, Andre RG, Turell MJ. Experimental transmission of eastern equine encephalitis virus by Ochlerotatus j. japonicus (Diptera: Culicidae) J. Med. Entomol. 2002a;39:480–484. doi: 10.1603/0022-2585-39.3.480. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Andre RG. Laboratory transmission of LaCrosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae) J. Med. Entomol. 2002b;39:635–639. doi: 10.1603/0022-2585-39.4.635. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Andre RG. Experimental transmission of St. Louis encephalitis virus by Ochlerotatus j. japonicus. J. Am. Mosq. Control Assoc. 2003;19:159–162. [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide. Version 6. 4th ed. Volume 2. Cary, NC: SAS Institute; 1989. [Google Scholar]
- Scott JJ. PhD dissertation. New Brunswick, NJ: Rutgers, The State University of New Jersey; 2003. The ecology of the exotic mosquito Ochlerotatus (Finlaya) japonicus japonicus (Theobald 1901) (Diptera: Culicidae) and an examination of its role in the West Nile virus cycle in New Jersey. [Google Scholar]
- Sprenger D, Wuithiranyagool T. The discovery and distribution of Aedes albopictus in Harris County, Texas, USA. J. Am. Mosq. Control Assoc. 1986;2:217–219. [PubMed] [Google Scholar]
- Steinly BA, Novak RJ, Webb DW. A new method for monitoring mosquito oviposition in artificial and natural containers. J. Am. Mosq. Control Assoc. 1991;7:649–650. [PubMed] [Google Scholar]
- Takashima I, Rosen L. Horizontal and vertical transmission of Japanese encephalitis virus by Aedes japonicus (Diptera: Culicidae) J. Med. Entomol. 1989;26:454–458. doi: 10.1093/jmedent/26.5.454. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Mizusawa K, Saugstad ES. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae) Contrib. Am. Entomol. Inst. 1979;16(ii–vii):1–987. [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperatures. J. Med. Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Thielman A, Hunter FF. Establishment of Ochlerotatus japonicus (Diptera: Culicidae) in Ontario, Canada. J. Med. Entomol. 2006;43:138–142. doi: 10.1603/0022-2585(2006)043[0138:eoojdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Volterra V. Fluctuations in the abundance of a species considered mathematically. Nature (Lond.) 1926;118:558–560. [Google Scholar]
- Volterra V. Animal ecology. New York: Chapman; 1931. [Google Scholar]
- Washburn JO, Hartmann EU. Could Aedes albopictus (Diptera: Culicidae) become established in California tree holes? J. Med. Entomol. 1992;29:995–1005. doi: 10.1093/jmedent/29.6.995. [DOI] [PubMed] [Google Scholar]
- Williamson M. Biological invasions. New York: Chapman & Hall; 1996. [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Interspecific differences in feeding behavior and survival under food-limited conditions for larval Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2004;97:720–728. doi: 10.1603/0013-8746(2004)097[0720:IDIFBA]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]