Abstract
Background/Aims
The liver is comparatively rich in plasmacytoid (p) dendritic cells (DC),- innate immune effector cells that are also thought to play key roles in the induction and regulation of adaptive immunity.
Methods
Liver and spleen pDC were purified from fms-like tyrosine kinase ligand-reated control or lipopolysaccharide-injected C57BL/10 mice. Flow cytometric and molecular biologic assays were used to characterize their function and interaction with naturally-occurring regulatory T cells (Treg).
Results
While IL-10 production was greater for freshly-isolated liver compared with splenic pDC, the former produced less bioactive IL-12p70. Moreover, liver pDC expressed a low Delta4/Jagged1 Notch ligand ratio, skewed towards T helper 2 cell differentiation/cytokine production, and promoted allogeneic CD4+ T cell apoptosis. T cell proliferation in response to liver pDC was, however, enhanced by blocking IL-10 function at the initiation of cultures. In the absence of naturally occurring CD4+CD25+ regulatory T cells, similar levels of T cell proliferation were induced by liver and spleen pDC and the pro-apoptotic activity of liver pDC was reversed.
Conclusion
The inferior T cell allostimulatory activity of in vivo-stimulated liver pDC may depend on the presence and function of Treg, a property that may contribute to inherent liver tolerogenicity.
Keywords: Dendritic cells, Liver, T cells, Mouse, Endotoxin, Toll-like receptor, Tolerance
1. Introduction
It is generally accepted that the liver has inherent tolerogenic properties (1, 2), as evidenced by its role in oral and portal venous tolerance and the comparative ease of acceptance of hepatic allografts in animals (3–5) and humans (6–8). Dendritic cells (DC) are highly-specialised initiators and regulators of innate and adaptive immunity, that have been implicated in hepatic tolerogenicity (2, 9, 10). Impairment of their function also contributes to immune suppression during sepsis (11). While interstitial DC are rare in normal liver, making them difficult to isolate (12), their numbers are increased following partial hepatectomy (13) and enhanced by endogenous hematopoietic growth factors, in particular fms-like tyrosine kinase-3 ligand (Flt3L) (14, 15).
Several DC subsets are present in normal mouse liver (16), including conventional myeloid (m)DC (CD11c+CD11b+CD8α−), CD8α+ DC (CD11cloCD11b−), natural killer (NK) DC and plasmacytoid (p)DC (CD11c−CD11b−NK1.1−B220+) (17–20). pDC are more abundant in the liver compared with secondary lymphoid tissue, such as spleen (19). They are the major source of type-1 interferons (IFNs) in the body (21) and important in anti-viral responses, although their numbers and function are reduced in hepatitis C virus-infected livers (22). While mDC tend to prime T helper (Th)1 responses, pDC can induce Th1, Th2 or T regulatory cell (Treg) responses, depending on the nature of the antigen (Ag) and the costimulatory signals they transmit to T cells (23, 24). Both mDC and pDC subsets, particularly immature DC, can exhibit tolerogenic properties and expand or induce regulatory T cells (Treg) (25). Recently, pDC have been implicated in transplant tolerance and the induction of Ag-specific Treg in allograft recipients (26, 27).
Since the liver is located downstream from the gut, interstitial DC and other hepatic APC are exposed continually to microbe-derived ‘danger’ signals, recognized by pattern-recognition receptors, in particular Toll-like receptors (TLR) (28, 29). Refractory responses of freshly-isolated murine liver DC (bulk DC or mDC) to TLR stimulation (‘endotoxin tolerance’) (30–32) may represent an adaptive response to prevent chronic liver inflammation. The mechanistic basis of endotoxin tolerance has been studied largely in macrophages, and negative regulators of TLR signaling have been implicated in promotion of this acquired hyporesponsiveness (33, 34). Little work has been conducted on DC, in particular liver DC, although recently, IL-6 and signal transducer and activator of transcription 3 activity have been shown to down-regulate responses of murine liver DC to endotoxin (32).
DC that are phenotypically immature, such as freshly-isolated liver DC (17, 19, 30), and mDC that are maturation-resistant (35), are poor T cell stimulators and can regulate alloimmune reactivity, in vitro and in vivo. On the other hand, TLR4 engagement on hepatic Ag-presenting cells (APC) is involved in liver inflammation (36, 37). Since mouse pDC [that constitutively express low levels of TLR4 (30, 38–40)] are well-represented in the liver, the influence of TLR4 ligation on their function and on the outcome of their interactions with T cells may impact significantly on hepatic immune reactivity and alloimmune responses. Indeed, intrahepatic lipopolysaccharide (LPS) levels may be elevated intraoperatively in liver transplantation (41). In this study, we examined the functional biology of liver pDC from control and LPS-stimulated mice. We also investigated mechanisms underlying differences between liver and splenic pDC in allogeneic T cell stimulatory ability, including the role of naturally-occurring Treg. The findings confirm that, compared with spleen pDC, liver pDC are inferior allostimulators and resistant to LPS stimulation in vivo. Moreover, their depressed ability to stimulate T cell proliferation, and their capacity to promote T cell apoptosis appear to be dependent on the presence of Treg.
2. Materials and methods
2.1 Mice
Male C57BL/10 (B10) (H2b) and BALB/c (H2d) mice (8–12 wks old) were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in the specific pathogen-free facility of University of Pittsburgh School of Medicine. Experiments were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and under an Institutional Animal Care and Use Committee-approved protocol. Mice received Purina rodent chow (Ralston Purina, St. Louis, MO) and tap water ad libitum.
2.2 Reagents
RPMI-1640 complete medium was used for cell culture (31). Chinese hamster ovary cell-derived recombinant human fms-like tyrosine kinase 3 ligand (Flt3L) was provided by Amgen (Seattle, WA). LPS (ultra pure; E.coli K12) was purchased from Invivogen (San Diego, CA). Mice received LPS (100 µg) or PBS i.v. via the lateral tail vein. Neutralizing rat anti-mouse IL-10 mAb (JES5-2A5; azide-free) was from BD PharMingen (San Diego, CA). Recombinant mouse IL-12 was from Sigma (St. Louis, Mo).
2.3 Isolation of DC
CD11c+ cells were isolated from livers and spleens of normal animals or mice given the DC poietin Flt3L (10 µg/mouse/day i.p., for 10 days). Bulk DC were enriched by density centrifugation using Nycodenz (Sigma). For pDC purification (>95%), mPDCA1+ cells were positively selected from the DC-enriched fraction using immunomagnetic beads (Miltenyi Biotec, Auburn, CA). For mDC purification, cells harvested after density centrifugation were incubated with biotin-conjugated rat anti-mouse B220/CD45R and CD49b and depleted by negative selection. CD11c+ cells were then positively selected using MACS® (Miltenyi Biotec). The purity of mDC (CD11c+B220−DX-5−) was consistently >95 % (35).
2.4 Flow cytometry (cell surface staining)
Cells were treated with FcγR-blocking Ab (rat anti-mouse CD16/32 mAb) (2.4G2) to avoid non-specific Ab binding. For cell surface staining, they were then incubated for 30 min with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, PE-Cy5, or PE-Cy7-conjugated mAbs to detect expression of CD11c (clone HL3), B220/CD45R (RA3-6B2), IAb β-chain (25-9-17), CD86 (GL1), TLR4 (UT41) (eBioscience, San Diego, CA) or FasL (CD95L) (MFL3) (eBioscience). These mAbs, and appropriate Ig isotype controls, were obtained from BD PharMingen, unless specified. Flow analysis was performed using a LSR II flow cytometer (BD Bioscience, San Jose, CA) and results expressed as % positive cells and mean fluorescence intensity (MFI).
2.5 Real-time RT-PCR
Messenger RNAs (mRNAs) for TLR4, Delta4, Jagged1 and β actin were quantified in duplicate by SYBR Green two-step, real-time RT-PCR. After generating first-strand cDNA from purified DCs, the PCR reaction mixture was prepared using SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA), with primers designed according to published sequences (42, 43). Thermal cycling conditions were 10 min at 95°C to activate DNA polymerase, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min on an ABI PRISM 7000 Sequence Detection System (PE Applied Biosystems). Using the manufacturer’s software, real-time PCR data were plotted as the ΔRn fluorescence signal versus the cycle number. The threshold cycle was defined as the cycle number at which the ΔRn crossed this threshold. The expression of each gene was normalized to β actin mRNA content and calculated relative to control using the comparative cycle threshold method.
2.6 T cell purification
CD4+ T cells were purified from spleen cell suspensions using Dynal® Mouse CD4 Negative Isolation kits (Dynal Biotech, Oslo, Norway). In some experiments, the resultant CD4+ population was then incubated with biotin-conjugated anti-CD25 mAb (PC61-5; eBioscience) to remove naturally-occurring Treg, and CD4+CD25− T cells isolated by negative selection using anti-biotin microbeads and LD separation columns (Miltenyi Biotec) (35, 44). Purity was consistently >97%.
2.7 Mixed leukocyte reaction (MLR)
MLRs were performed using graded numbers of B10 DC as stimulators of purified allogeneic BALB/c T cells (2× 105/well) in 72 h MLR using 96-well, round-bottom plates, as described (45). In some experiments, neutralizing anti-IL-10 mAb, isotype control Ig or IL-12 was added at the start of cultures.
2.8 CFSE-T cell proliferation assay
Purified CD4+ T cells were CFSE (carboxyfluorescein succinimidyl ester)-labeled using the Vibrant CFDA SE Cell Tracer Kit (Invitrogen) according to the manufacturer’s instructions. BALB/c bulk CD4+ or CD4+CD25− T cells (2×105) were co-cultured with 1× 104 allogeneic (B10) DC for 4 days in 96-well, round-bottom plates, then analyzed by flow cytometry.
2.9 Analysis of T cell apoptosis
CD4+ or CD4+CD25− T cells stimulated with DC in MLR at a 20:1 ratio were harvested at 72h and labeled with FITC-conjugated anti-CD4 mAb and PE-Cy5-conjugated anti-CD11c mAb. The incidences of viable cells and early and late apoptotic T cells were determined using Annexin V-PE apoptosis detection kits (BD PharMingen). After staining of externalized phosphatidylserine with Annexin-V-PE and incubation in the vital dye 7-amino-actinomycin D (7-AAD), data were acquired immediately and analyzed as described above.
2.10 Intracellular cytokine staining
Purified DC were treated with brefeldin A (GolgiPlug™; 1 µl/ml, BD PharMingen) for 16 h, then labeled with FITC- or PE-Cy5-conjugated mAb and fixed in 1% paraformaldehyde. The cells were permeabilized with 0.1% saponin, then incubated with anti-IL-12p40/p70 (C15.6) and anti-IL-10 (JES5-16E3), or rat IgG (all BD PharMingen) for 30 min. T cells recovered from 96 h MLR were restimulated with plate-bound anti-CD3ε (10 µg/ml; clone 17A2) and 2 µg/ml soluble anti-CD28 mAb (37.51) (each from BD PharMingen) for 5 h at 37°C, in the presence of brefeldin A. After cell surface staining with PE-Cy7-CD4 mAb, the cells were fixed, permeabilized, then stained with anti-IFNγ (XMG1.2), anti-IL-4 (BVD4-1D11) or isotype control Ig (all from BD PharMingen).
2.11 ELISA
Levels of IL-12p70, IFN , IL-4, or IL-10 in culture supernatants were determined by ELISA, using commercial kits (Biolegend Inc., San Diego, CA) and following the manufacturer’s instructions.
Statistical analyses
Data are expressed as means ± 1 SD. Significances of differences between means were determined by unpaired Student’s ‘t’-test. A ‘p’ value < 0.05 was considered significant.
3. Results
3.1 Freshly-isolated liver pDC from control and LPS-injected mice induce less allogeneic T cell proliferation than splenic pDC
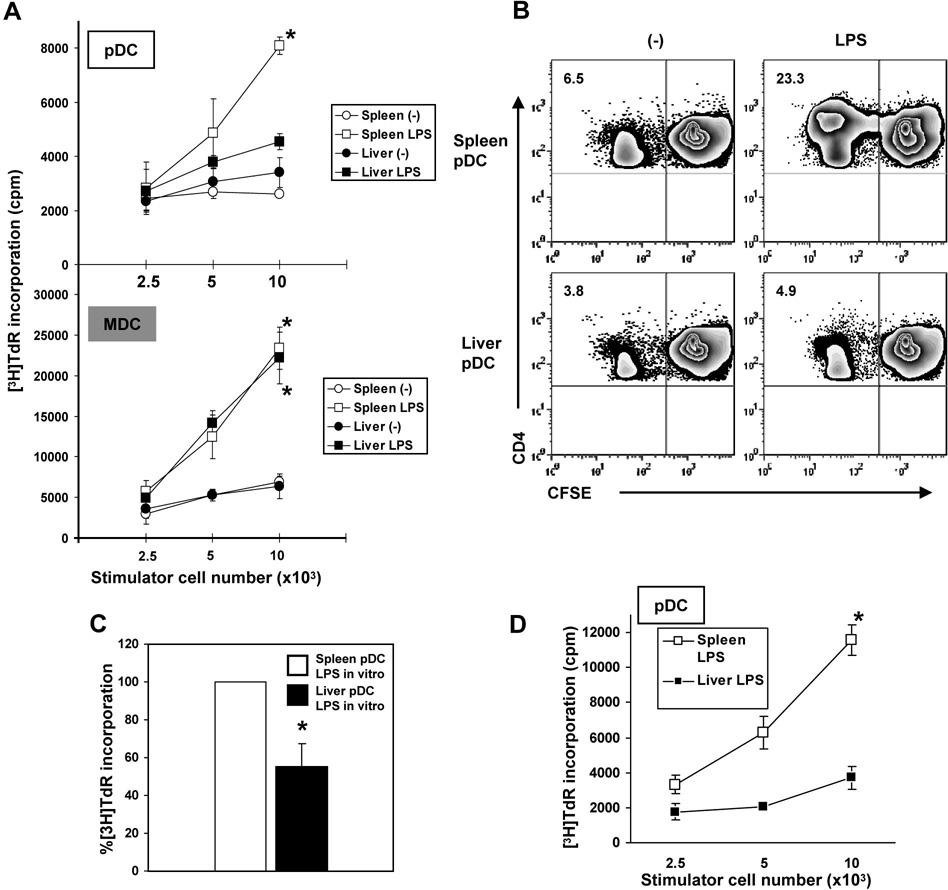
To examine their allostimulatory activity, freshly-isolated pDC (and for comparison, mDC) from livers or spleens of control (B10) mice or animals injected 2 hr earlier with LPS, were co-cultured in MLR with normal, allogeneic (BALB/c) CD4+ T cells. T cell proliferation was quantified by both [3H]TdR incorporation and CFSE dilution analysis, as described in the Materials and methods. pDC elicited lower levels of T cell proliferation than mDC (Fig. 1A), especially after in vivo LPS administration. Compared with spleen pDC, liver pDC from LPS-injected mice were poor stimulators of CD4+ T cell proliferation (Fig. 1A, B). To determine whether the difference in allostimulatory capacity between liver and spleen pDC was due to a direct LPS effect, mPDCA-1+ cells from both tissues were cultured with LPS (1 µg/ml) overnight (16h). LPS-stimulated liver pDC induced significantly less T cell proliferation than LPS-stimulated spleen pDC of Flt3L-treated animals (Fig. 1C). Further, liver pDC from LPS-injected normal (non-Flt3L-treated) mice displayed similar, significantly reduced ability to induce proliferation of allogeneic CD4+ T cells compared with normal LPS-activated spleen pDC (Fig. 1D).
Fig. 1.
Liver pDC (immunobead-purified; mPDCA-1+) from LPS-injected C57BL10 (B10) mice induce significantly lower proliferation of allogeneic CD4+ T cells in 72h MLR, as determined by (A), [3H]TdR incorporation, and (B), CFSE dilution analysis compared with spleen pDC (*p<0.01). The numbers in the upper left quadrants indicate percent positive CD4+ T cells. A minimum of 20,000 CD4+ gated cells were analyzed. In comparison, LPS-stimulated mDC from spleen or liver induced much higher T cell proliferation compared with untreated liver mDC (A; *p<0.01). (C), pDC from liver or spleen were cultured overnight (16h) with LPS (1µg/ml), then used as stimulators in MLR. Results are expressed as relative [3H]TdR incorporation induced by spleen pDC as stimulators (*p<0.01). (D), Allostimulatory capacity of liver and spleen pDC from LPS-injected normal (non-Flt3L-mobilized) B10 mice (*, p<0.01). Data are representative of 5 (A and B) or 2 independent experiments (D), or means ± 1SD of 3 experiments (C).
3.2 MHC class II, CD86 and TLR4 expression do not differ significantly between liver and spleen pDC
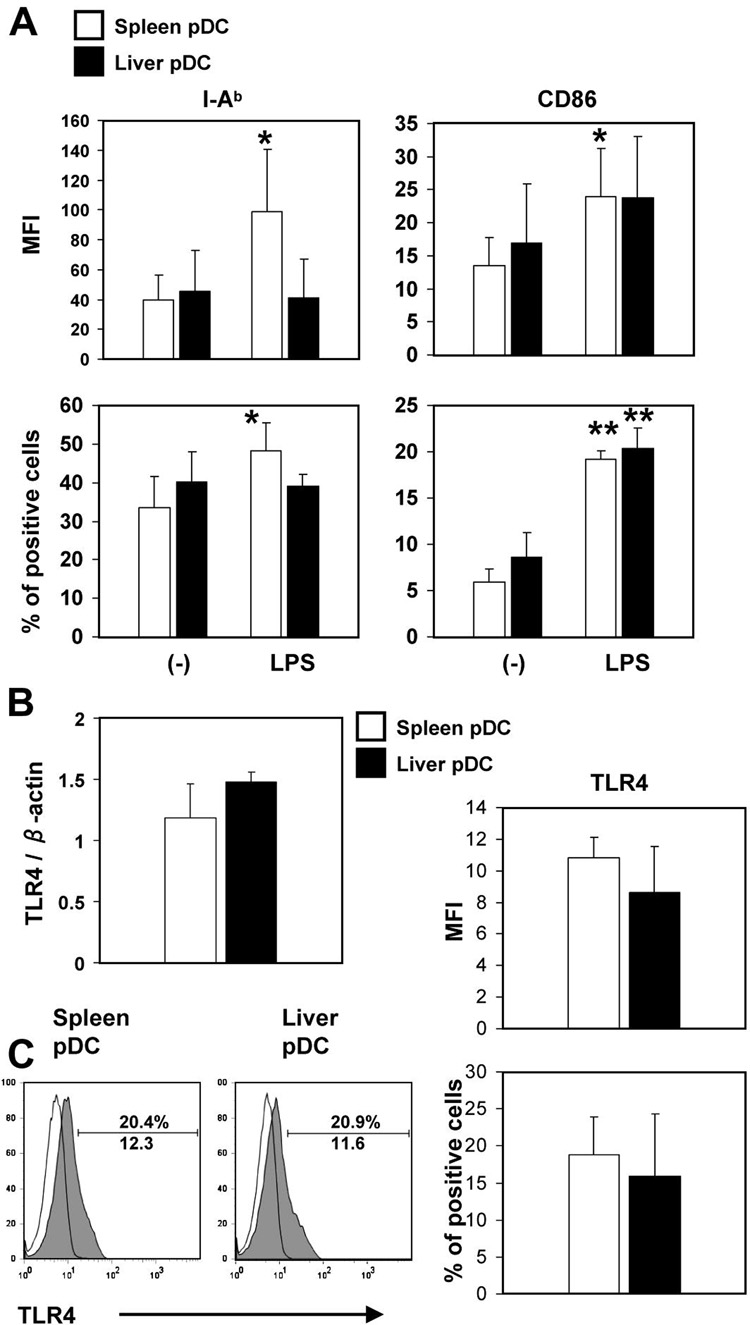
To determine whether liver pDC were refractory to LPS-induced maturation, we analyzed MHC class II and costimulatory molecule (CD86) expression on freshly-isolated liver and spleen pDC from LPS-infused B10 mice by flow cytometry. Liver and spleen pDC from control mice expressed similar, low to intermediate levels of surface MHC class II (IAb) and CD86. Following LPS administration, MHC class II and CD86 were upregulated significantly on spleen pDC, but only the incidence of CD86+ cells was upregulated significantly for liver pDC (Fig.2A). TLR4 message and cell surface expression of TLR4 did not differ significantly between freshly-isolated, immunobead-purified liver and spleen pDC (mPDCA-1+), as determined by quantitative RT-PCR and flow cytometry, respectively (Fig 2B & C).
Fig. 2.
(A), Freshly-isolated liver and spleen pDC from control mice (no LPS administration) display similar surface levels of MHC class II (IAb) and costimulatory (CD86) molecules. Following LPS administration, MHC class II and CD86 were upregulated on spleen pDC, but only CD86 (incidence of positive cells) was upregulated on liver pDC (*p<0.05, **p<0.01, compared with control mice) (B), The expression of TLR4 does not different significantly between freshly-isolated liver and spleen pDC from control mice, as determined by quantitative real-time RT-PCR. RNA was isolated from freshly-isolated, spleen or liver mPDCA1+ cells and RT-PCR for TLR4 and β actin performed. Results are expressed as relative TLR4 mRNA expression normalized to β actin RNA. (C), Cell surface TLR4 staining confirmed similar levels on freshly-isolated liver and spleen pDC. Staining shown is for the mPDCA1+ pDC population. Open histograms represent appropriate Ig isotype control staining. A minimum of 20,000 CD11c+ B220+ gated cells were analyzed. Data are representative of 3 independent experiments (C), or means ± 1SD of 4 (A and B) or 3 experiments (C).
3.3 LPS-stimulated liver pDC express lower levels of IL-12, but higher levels of IL-10 that regulates T cell proliferation
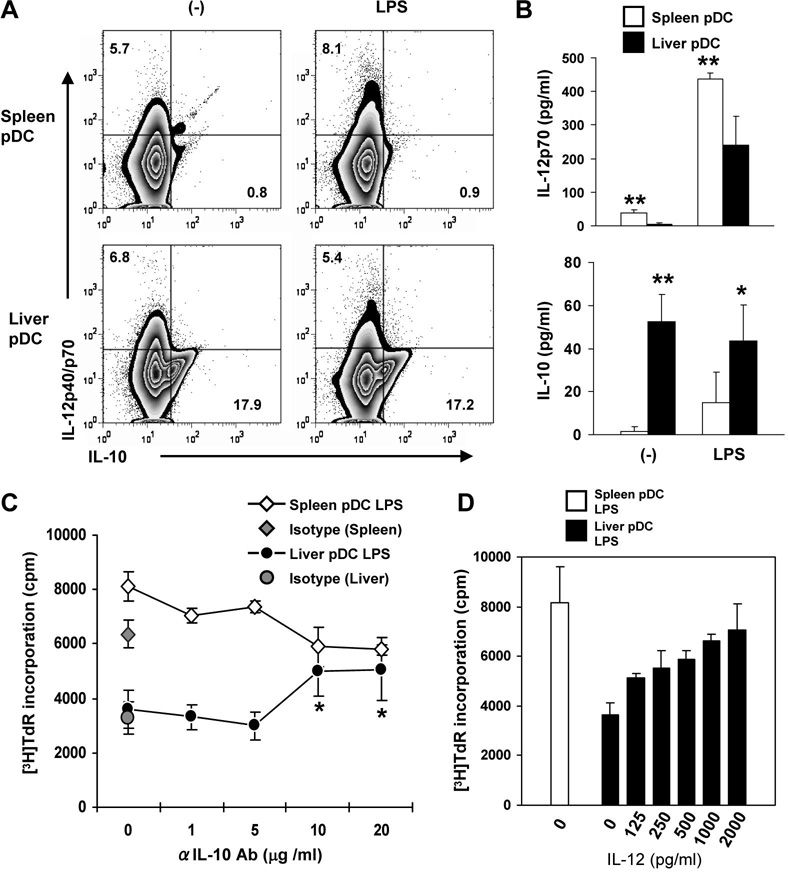
To gain further insight into the functional differences between liver and spleen pDC, we next examined the expression of specific immunomodulatory cytokines by these cells, using flow cytometry and ELISA. As shown in Fig. 3A, flow analysis of intracellular cytokine levels revealed similar low IL-12 (p40/p70), but much higher IL-10 levels in control and LPS-stimulated liver compared with spleen pDC. IL-12 (IL-12p70) secretion by in vivo-stimulated liver pDC was significantly lower than that of splenic pDC. On the other hand, IL-10 secretion by liver pDC was significantly higher than that of splenic pDC, with or without LPS administration (Fig. 3B). Thus, the inferior T cell allostimulatory ability of in vivo LPS-stimulated liver pDC compared with spleen pDC is consistent with their differential regulation of pro-(IL-12) and anti-inflammatory cytokine (IL-10) production. In keeping with this conclusion, addition of neutralizing anti-IL-10 mAb or exogenous IL-12 at the start of LPS-stimulated liver pDC:allogeneic T cell MLR, significantly enhanced the proliferative response to a level similar to that achieved with splenic pDC (Fig C & D).
Fig. 3.
Unstimulated and LPS-stimulated liver pDC produce higher levels of IL-10 than splenic pDC. IL-12 and IL-10 were quantified by (A), intracellular staining, and (B), ELISA, as described in the Materials and Methods. Flow cytometric analysis of intracellular cytokine production and ELISA confirmed similar low levels of IL-12, but higher levels of IL-10 production by unstimulated and LPS-stimulated liver pDC compared with spleen pDC. Thus, the inferior T cell allostimulatory ability of LPS-stimulated liver pDC is associated with their differential regulation of pro-(IL-12) and anti-inflammatory (IL-10) cytokine production. The numbers indicate percent positive CD11c+ B220+ cells. A minimum of 20,000 CD11c+ B220+ gated cells were analyzed. (C), Addition of neutralizing anti-IL-10 mAb at the start of 72hr MLR cultures enhanced T cell proliferative responses induced by LPS-stimulated liver pDC, but not spleen pDC. *p<0.05 compared with untreated cultures, isotype control cultures, or mAb concs ≤ 5µg/ml. (D), Addition of exogenous recombinant mouse IL-12 at the start of 72hr MLR cultures enhanced T cell proliferative responses induced by LPS-stimulated liver pDC. Data are representative of at least 3 (A and C) or 2 independent experiments (D), or means ± 1SD of 4 experiments (B). *, p<0.05; **, p<0.01.
3.4 LPS-stimulated liver pDC skew allogeneic T cells towards Th2 differentiation, associated with a low Delta 4/Jagged 1 ratio
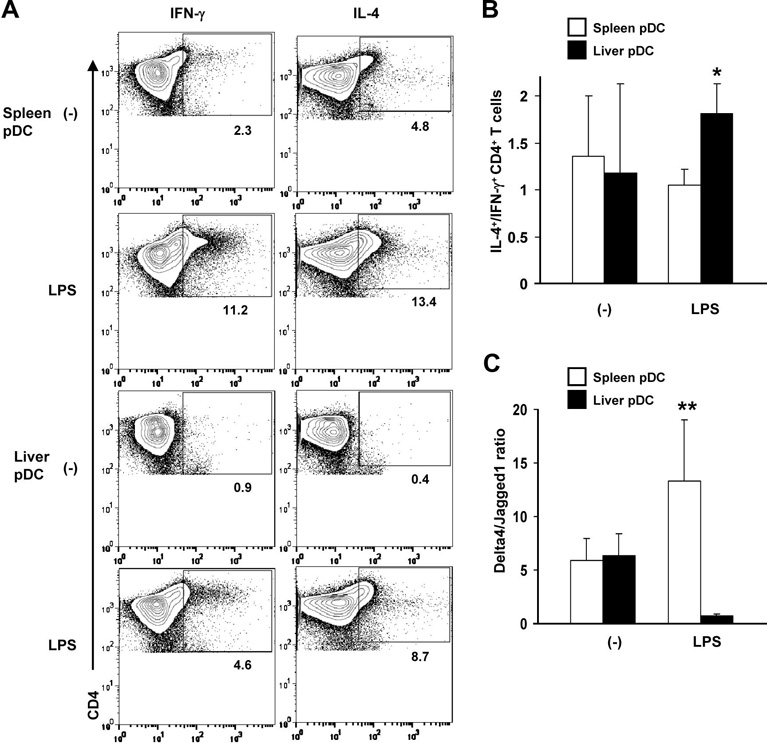
We next compared intracellular Th1 (IFNγ) and Th2 cytokine (IL-4) production by normal, allogeneic CD4+ T cells stimulated with liver or spleen pDC. As shown in Fig. 4A, the incidences of IFNγ- and IL-4-producing CD4+ T cells induced by control or LPS-activated pDC in MLR were lower for liver compared with splenic pDC. This resulted in a significantly higher IL-4+/IFNγ+ CD4+ T cell ratio for liver pDC compared with spleen pDC (Fig 4B). The levels of secreted IFNγ were substantially and significantly lower in MLR cultures stimulated with liver pDC, whereas secreted levels of IL-4 did not differ significantly (data not shown).
Fig. 4.
(A), The incidence of IFNγ- and IL-4-producing allogeneic CD4+ T cells stimulated by LPS-activated liver pDC in MLR was lower than that induced by LPS-activated splenic pDC. The numbers indicate percent positive CD4+ T cells. A minimum of 20,000 CD4+ gated cells were analyzed. (B), Ratio of IL-4/IFNγ-producing CD4+ T cells in 96 hr MLR cultures stimulated with LPS-stimulated liver pDC compared with spleen pDC. (C), The Delta4/ Jagged1 (Notch ligand) mRNA ratio expressed by freshly-isolated, LPS-activated liver pDC was much lower than that of activated spleen pDC. Data are representative of 3 experiments (A), or means ± 1SD of 3 (B) or 4 experiments (C). *, p<0.05; **, p<0.01.
Previous studies (46) have suggested that the balance of expression of different Notch ligands on APC may direct CD4+ T cells to differentiate into either Th1 or Th2 cells. According to this paradigm, elevated expression of Delta 4 induces Th1 cells, while elevated expression of Jagged1 induces Th2 cells. Thus, the ratio of Delta 4 to Jagged1 mRNA expression may predict a Th1 response. As shown in Fig. 4C, the expression of Jagged1 relative to Delta 4 mRNA was lower on pDC from liver compared with spleen pDC of LPS-injected mice, consistent with comparatively poor Th1-polarizing function of the liver pDC, and skewing towards Th2.
3.5 The inferior T cell allostimulatory activity of liver pDC is dependent on naturally-occurring CD4+ CD25+ Treg
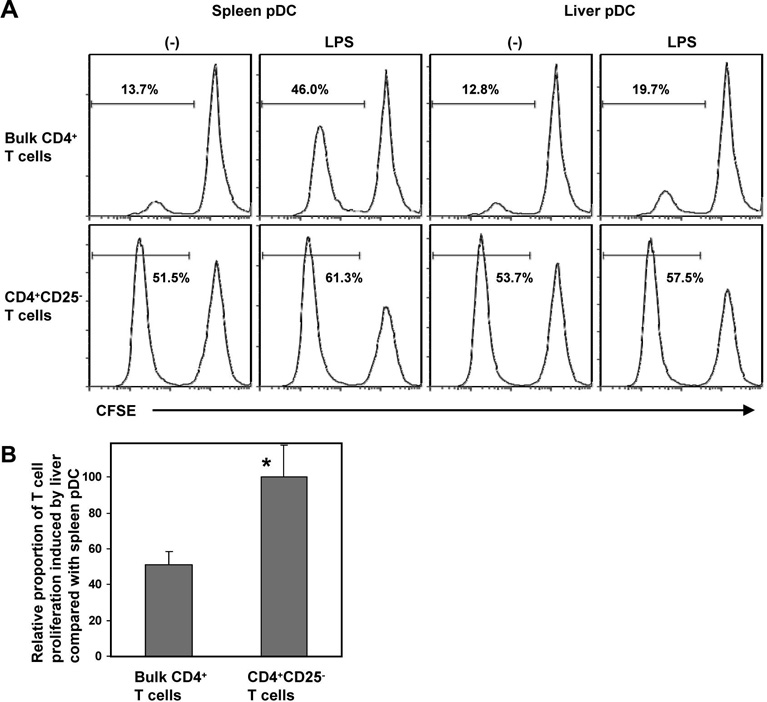
Phenotypically immature DC with weak allostimulatory capacity can expand or enrich for Treg (35, 47). We therefore examined whether the poor T cell stimulatory properties of liver pDC might correlate with interactions with naturally-occurring CD4+CD25+ (Foxp3+) Treg. Although CD25 is not a definitive marker of functional T reg, naturally-arising CD25+ CD4+ T cells play an indispensable role in self tolerance and negative control of immune responses (48). When naturally-occurring CD4+ CD25+ cells were removed from the normal responder T cell population, immediately before the start of MLR, similar extents of CFSE-labeled T cell proliferation were observed when liver or spleen pDC from LPS-treated mice were used as stimulators (Fig. 5A & B), reversing the inferior allostimulatory capacity of the liver pDC significantly. These data suggest that Treg are rendered more effective at inhibiting effector T cell proliferation in the presence of liver than spleen pDC, and that naturally-occurring Treg may suppress the differentiation/function of liver pDC.
Fig. 5.
Naturally-occurring Treg (CD4+CD25+) contribute to the poor allostimulatory activity of liver pDC. (A), When CD4+CD25+ Treg were removed from the responder T cell population at the start of MLR, similar extents of CFSE-labeled T cell proliferation were observed when liver or spleen pDC were used as stimulators, reversing the inferior T cell allostimulatory activity of either unstimulated or LPS-stimulated liver pDC. The numbers indicate percent proliferating CD4+ T cells. A minimum of 20,000 CD4+ gated cells were analyzed. (B), confirms the statistical significance of the observed effect for pDC from LPS-treated mice. Data are representative of 3 independent experiments (A), or means ± 1SD of 3 experiments (B); *p<0.05.
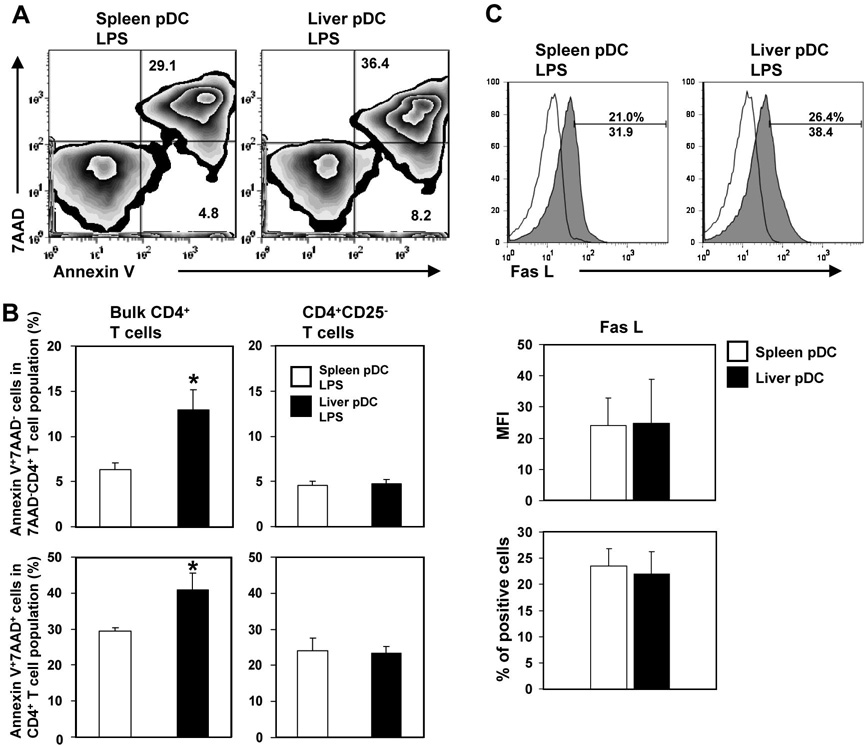
3.6 LPS-stimulated liver pDC induce a higher incidence of early and late apoptosis in allogeneic CD4+ T cells than splenic pDC: dependence on naturally-occurring CD4+CD25+ Treg
To further investigate mechanisms that might underlie the poor T cell proliferative response induced by liver pDC, we next examined the influence of pDC on apoptosis of normal allogeneic responder bulk CD4+ T cells stimulated with liver or splenic pDC from LPS-injected mice. As shown in Fig. 6A and B (left side), the incidence of early (annexin V+ 7AAD−) and late (annexin V+/7-AAD+) apoptotic CD4+ T cells was enhanced significantly in MLR cultures stimulated with liver compared with spleen pDC. However, when naturally-occurring CD4+ CD25+ Treg were removed from the responder T cell population, immediately before the start of the MLR, the pro-apoptotic effect of liver pDC was reversed (Fig. 6B; right side). Similar levels of cell surface death ligand FasL (CD95L) were expressed by the pDC populations from the 2 organs (Fig. 6C), making it very unlikely that signaling via the FasL-Fas pathway contributed to the greater proapoptotic activity of liver pDC.
Fig. 6.
(A, and B - left side), LPS-activated liver pDC induce higher incidences of early (Annexin V+ 7AAD−) and late apoptotic cells (Annexin V+/7-AAD+) in responder allogeneic CD4+ T cell populations in 72h MLR compared with splenic pDC. The numbers indicate percent positive CD4+ T cells. (B - right side), Removal of CD4+ CD25+ Treg immediately before the start of the MLR reversed the greater proapoptotic effect of liver pDC. (C), Freshly-isolated liver and spleen pDC from LPS-injected mice display similar surface levels of Fas Ligand (CD95L). Open histograms represent Ig isotype control staining. A minimum of 20,000 CD4+ (A and B) or CD11c+ (C) gated cells were analyzed. Data are representative of 3 independent experiments (A and C), or are means ± 1SD of 3 experiments (B and C);*P<0.05.
4. Discussion
DCs are rare, uniquely well-equipped bone-marrow-derived professional APCs, found ubiquitously in lymphoid and non-lymphoid tissues. pDCs constitute a major subset of DCs in mice and humans (49, 50) and play crucial roles in innate and adaptive immunity (51). Compared with the spleen, relatively high proportions of pDC relative to conventional mDC are found in normal mouse liver, or in livers in which DC have been expanded by systemic administration of the DC poietin Flt3L (19, 39). In the recent study by Shu et al (39), a ratio of pDC to mDC in normal liver about 5 times higher than that in the spleen was reported, whereas a 4 times higher incidence of pDC relative to mDC in normal mouse liver was reported by Pillarisetty et al (19). Due to their overall paucity in normal liver, we used Flt3L as in our and others’ previous studies (15, 17, 30, 31, 52, 53), to expand liver DC, in particular pDC. It has been reported that Flt3L treatment (adenoviral gene delivery) for 10 days (the standard dosing schedule) increases liver pDC 200-fold, and spleen pDC 28-fold, thus enabling the isolation and purification of adequate numbers of pDC for analysis. Use of Flt3L protein to mobilize DC in this study avoided the possibility of DC activation associated with adenoviral delivery of the Flt3L gene (54).
Previous reports have shown that bulk liver DC are less mature and have weaker T cell stimulatory ability than DC from secondary lymphoid tissues (15, 19, 30, 39). We found that both freshly-isolated normal mouse liver and spleen pDC were phenotypically immature and weak allogeneic T cell stimulators. However, compared with splenic pDC from LPS-injected mice, liver pDC were much inferior allostimulators. Moreover, liver pDC produced the anti-inflammatory cytokine IL-10, and were deficient (compared with spleen pDC) in their capacity to elicit IFNγ production by allogeneic CD4+ T cells, and skewed towards Th2 differentiation. This was consistent with the low Delta 4/Jagged 1 ratio (46) observed in LPS-stimulated liver compared with spleen pDC. In addition, liver pDC promoted higher incidences of apoptotic CD4+ T cells in MLR than spleen pDC. The functional deficiency of liver pDC as T cell stimulators and their proapoptotic activity was reversed by removal of CD4+CD25+ cells at the start of cultures, indicating that the deficiency was dependent on naturally-occurring Treg. Our preliminary findings also reveal that, in the presence of liver pDC compared with spleen pDC, the production of the cytotoxic effector molecule perforin by Treg is enhanced (unpublished observations), suggesting a mechanism (55) that may underlie the poor allogeneic effector T cell responses observed with liver pDC.
There have been few previous accounts of the phenotype and function of pure populations of pDC isolated from mouse or human liver. Recently, Shu et al (39) reported that liver pDC from normal mice exhibited efficient endocytotic activity, a characteristic of immature DC, and produced significant levels of IL-12p40, IL-6 and tumor necrosis factor α (IL-10 was not examined) in response to TLR agonists. However, as reported herein, these DC were much inferior inducers of allogeneic T cell proliferation than splenic pDCs. As in the present study, Kingham et al (15) used Flt3L to expand hepatic pDC; they reported that the in vivo-expanded liver pDC (induced by adenovirus encoding murine Flt3L cDNA) secreted similar levels of IL-12, IL-6 and IL-10, but comparatively high levels of IFNα following in vitro TLR9 ligation. However, CpG stimulation failed to enhance their poor inherent T cell allostimulatory ability. Despite differences in the models used, and in the precise nature of the investigations conducted in these and the current studies, the findings endorse overall the view that pDC, relatively enriched in the liver environment, are inferior stimulators of adaptive T cell responses.
Evidence has accumulated that pDC play an important role in regulation of T cell responses and in the promotion of tolerance (25), including the induction of Treg (24, 56–58). Our finding that liver pDC have greater ability than their splenic counterparts to enhance the apoptotic death of allogeneic T cells is in keeping with similar findings on immature/maturation-resistant or regulatory DC (35, 59, 60). It is also consistent with the apoptotic death of graft-infiltrating T cells, that correlates with murine liver allograft survival in the absence of immunosuppressive therapy (61). Other studies have shown that pDC (in vivo-mobilized, as in this study) can promote allogeneic stem cell engraftment and skin graft survival (27), and inhibit organ allograft rejection (62). In addition, pDC in secondary lymphoid tissue appear to mediate tolerance to organ grafts by inducing alloAg-specific Treg (26). To our knowledge, there have been no previous reports of interactions between liver pDC and Treg. pDC are comparatively abundant in the liver (19, 39), and there is also a substantial population of naturally-occurring Treg in this organ (63). Our novel finding that naturally-occurring Treg down-modulate the ability of in vivo-stimulated liver pDC to drive allogeneic T proliferation to a greater extent than splenic pDC, suggests a mechanism that may underlie the deficient stimulatory function of this comparatively enriched DC subset in the liver.
It has been proposed for some time that, in the context of allograft tolerance, hepatic DC can induce T cell apoptosis and exert regulatory/suppressive functions (9, 64, 65). In this regard, it may be significant that T cells activated in the liver are functionally defective, with shortened half-life (66). The new data presented herein suggest that, in concert with naturally-occurring Treg, hepatic pDC, that are comparatively abundant in the liver, may play a key role in regulation of alloimmune reactivity, with implications for the outcome of hepatic allografts and liver transplant tolerance.
Acknowledgements
The work was supported by National Institutes of Health (NIH) grants R01 DK 49745 and R01 AI60994 (AWT). DT was in receipt of a fellowship from the Uehara Foundation and The Hillman Pediatric Transplant Center, Children’s Hospital of Pittsburgh. TLS was supported non concurrently by NIH T32 CA82084, the Roger Jenkins fellowship of the American Liver Foundation and an American Society of Transplantation Basic Science Fellowship. GR held a fellowship from The Transplantation Society.
The authors thank Dr. David Kaczorowski and Dr. Hidetaka Hara for technical advice, Dr. Heth R. Turnquist for valuable discussion, and Ms. Miriam Freeman for excellent administrative support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: DC, dendritic cells; Flt3L, fms-like tyrosine kinase 3 ligand; mDC, myeloid DC; pDC plasmacytoid DC; Treg, regulatory T cells; Foxp3, forkhead winged helix protein-3
References
- 1.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 2.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:194–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 3.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 4.Kamada N. Transfer of specific immunosuppression of graft rejection using lymph from tolerant liver-grafted rats. Immunology. 1985;55:241–247. [PMC free article] [PubMed] [Google Scholar]
- 5.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 7.Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243–249. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–921. [PubMed] [Google Scholar]
- 9.Thomson AW, Lu L. Are dendritic cells the key to liver transplant tolerance? Immunol Today. 1999;20:27–32. doi: 10.1016/s0167-5699(98)01378-4. [DOI] [PubMed] [Google Scholar]
- 10.Lau AH, de Creus A, Lu L, Thomson AW. Liver tolerance mediated by antigen presenting cells: fact or fiction? Gut. 2003;52:1075–1078. doi: 10.1136/gut.52.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Venet F, Chung CS, Lomas-Neira J, Ayala A. Changes in dendritic cell function in the immune response to sepsis. Expert Opin Biol Ther. 2007;7:929–938. doi: 10.1517/14712598.7.7.929. [DOI] [PubMed] [Google Scholar]
- 12.Woo J, Lu L, Rao AS, Li Y, Subbotin V, Starzl TE, et al. Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells. Transplantation. 1994;58:484–491. doi: 10.1097/00007890-199408270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellaneta A, Di Leo A, Francavilla R, Margiotta M, Barone M, Amoruso A, et al. Functional modification of CD11c+ liver dendritic cells during liver regeneration after partial hepatectomy in mice. Hepatology. 2006;43:807–816. doi: 10.1002/hep.21098. [DOI] [PubMed] [Google Scholar]
- 14.Steptoe RJ, Fu F, Li W, Drakes ML, Lu L, Demetris AJ, et al. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J Immunol. 1997;159:5483–5491. [PubMed] [Google Scholar]
- 15.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445–454. doi: 10.1002/hep.21457. [DOI] [PubMed] [Google Scholar]
- 16.Sumpter TL, Abe M, Tokita D, Thomson AW. Dendritic cells, the liver, and transplantation. Hepatology. 2007;46:2021–2031. doi: 10.1002/hep.21974. [DOI] [PubMed] [Google Scholar]
- 17.O'Connell PJ, Morelli AE, Logar AJ, Thomson AW. Phenotypic and functional characterization of mouse hepatic CD8 alpha+ lymphoid-related dendritic cells. J Immunol. 2000;165:795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 18.Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–2330. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 19.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 20.Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, et al. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–365. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 21.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 22.Lai WK, Curbishley SM, Goddard S, Alabraba E, Shaw J, Youster J, et al. Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol. 2007;47:338–347. doi: 10.1016/j.jhep.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 26.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 27.Fugier-Vivier IJ, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, et al. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201:373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 29.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 30.De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174:2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 31.Abe M, Tokita D, Raimondi G, Thomson AW. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct Th1-type responses. Eur J Immunol. 2006;36:2483–2493. doi: 10.1002/eji.200535767. [DOI] [PubMed] [Google Scholar]
- 32.Lunz JG, 3rd, Specht SM, Murase N, Isse K, Demetris AJ. Gut-derived commensal bacterial products inhibit liver dendritic cell maturation by stimulating hepatic interleukin-6/signal transducer and activator of transcription 3 activity. Hepatology. 2007;46:1946–1959. doi: 10.1002/hep.21906. [DOI] [PubMed] [Google Scholar]
- 33.Rauh MJ, Ho V, Pereira C, Sham A, Sly LM, Lam V, et al. SHIP represses the generation of alternatively activated macrophages. Immunity. 2005;23:361–374. doi: 10.1016/j.immuni.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of Toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 35.Turnquist H, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 36.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 37.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 38.Angelov GS, Tomkowiak M, Marcais A, Leverrier Y, Marvel J. Flt3 ligand-generated murine plasmacytoid and conventional dendritic cells differ in their capacity to prime naive CD8 T cells and to generate memory cells in vivo. J Immunol. 2005;175:189–195. doi: 10.4049/jimmunol.175.1.189. [DOI] [PubMed] [Google Scholar]
- 39.Shu SA, Lian ZX, Chuang YH, Yang GX, Moritoki Y, Comstock SS, et al. The role of CD11c(+) hepatic dendritic cells in the induction of innate immune responses. Clin Exp Immunol. 2007;149:335–343. doi: 10.1111/j.1365-2249.2007.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Fugger R, Hamilton G, Steininger R, Mirza D, Schulz F, Muhlbacher F. Intraoperative estimation of endotoxin, TNF alpha, and IL-6 in orthotopic liver transplantation and their relation to rejection and postoperative infection. Transplantation. 1991;52:302–306. doi: 10.1097/00007890-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Soboll G, Schaefer TM, Wira CR. Effect of toll-like receptor (TLR) agonists on TLR and microbicide expression in uterine and vaginal tissues of the mouse. Am J Reprod Immunol. 2006;55:434–446. doi: 10.1111/j.1600-0897.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 43.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 44.Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol. 2006;176:2808–2816. doi: 10.4049/jimmunol.176.5.2808. [DOI] [PubMed] [Google Scholar]
- 45.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 46.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 50.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 51.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 52.Morelli AE, O'Connell PJ, Khanna A, Logar AJ, Lu L, Thomson AW. Preferential induction of Th1 responses by functionally mature hepatic (CD8a− and CD8a+) dendritic cells: association with conversion from liver transplant tolerance to acute rejection. Transplantation. 2000;69:2647–2657. doi: 10.1097/00007890-200006270-00027. [DOI] [PubMed] [Google Scholar]
- 53.Abe M, Zahorchak AF, Colvin BL, Thomson AW. Migratory responses of murine hepatic myeloid, lymphoid-related, and plasmacytoid dendritic cells to CC chemokines. Transplantation. 2004;78:762–765. doi: 10.1097/01.tp.0000130450.61215.3b. [DOI] [PubMed] [Google Scholar]
- 54.Morelli AE, Larregina AT, Ganster RW, Zahorchak AF, Plowey JM, Takayama T, et al. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Martin P, Del Hoyo GM, Anjuere F, Arias CF, Vargas HH, Fernandez LA, et al. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 57.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 59.Lu L, Li W, Zhong C, Qian S, Fung JJ, Thomson AW, et al. Increased apoptosis of immunoreactive host cells and augmented donor leukocyte chimerism, not sustained inhibition of B7 molecule expression are associated with prolonged cardiac allograft survival in mice preconditioned with immature donor dendritic cells plus anti-CD40L mAb. Transplantation. 1999;68:747–757. doi: 10.1097/00007890-199909270-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lan A, Wang Z, Raimondi G, Wu W, Colvin BL, DeCreus A, et al. 'Alternatively-activated’ dendritic cells preferentially secrete IL-10, expand Foxp3+ CD4+ T cells and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868–5877. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 61.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 62.Abe M, Wang Z, De Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808–1819. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 63.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells (Dayt) 1995;13:622–639. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharland A, Yan Y, Wang C, Bowen DG, Sun J, Sheil AG, et al. Evidence that apoptosis of activated T cells occurs in spontaneous tolerance of liver allografts and is blocked by manipulations which break tolerance. Transplantation. 1999;68:1736–1745. doi: 10.1097/00007890-199912150-00018. [DOI] [PubMed] [Google Scholar]
- 66.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–712. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]