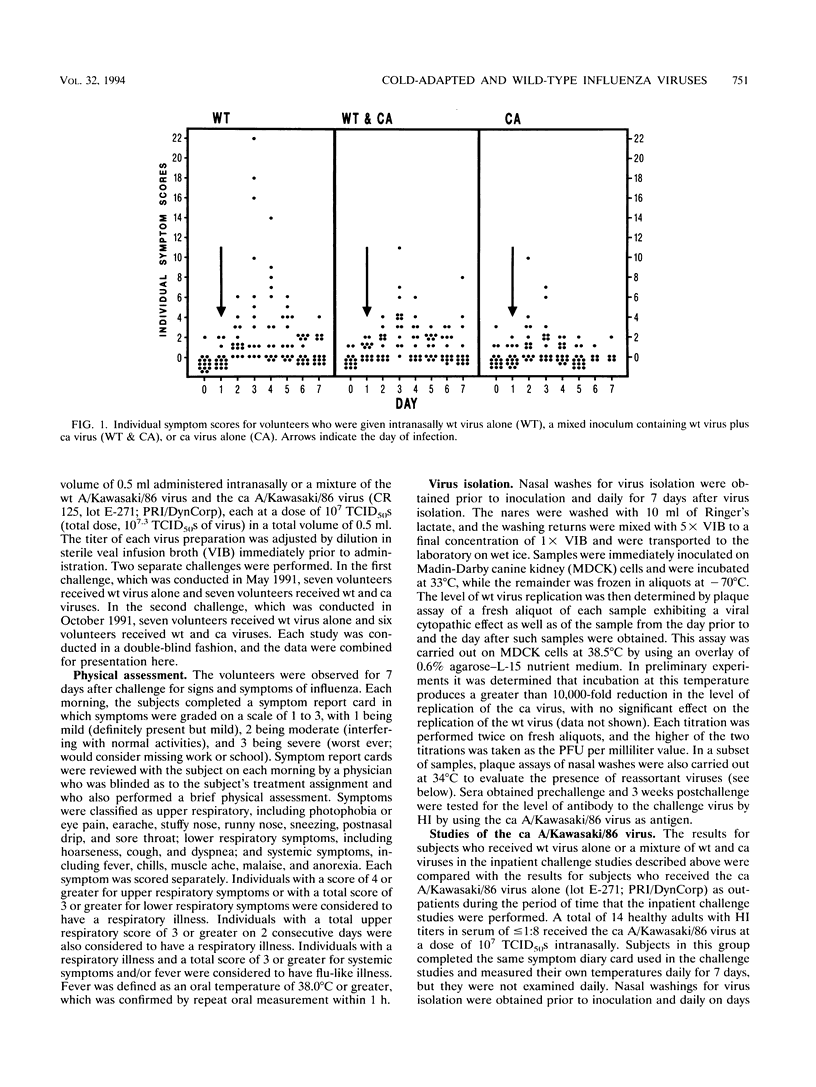
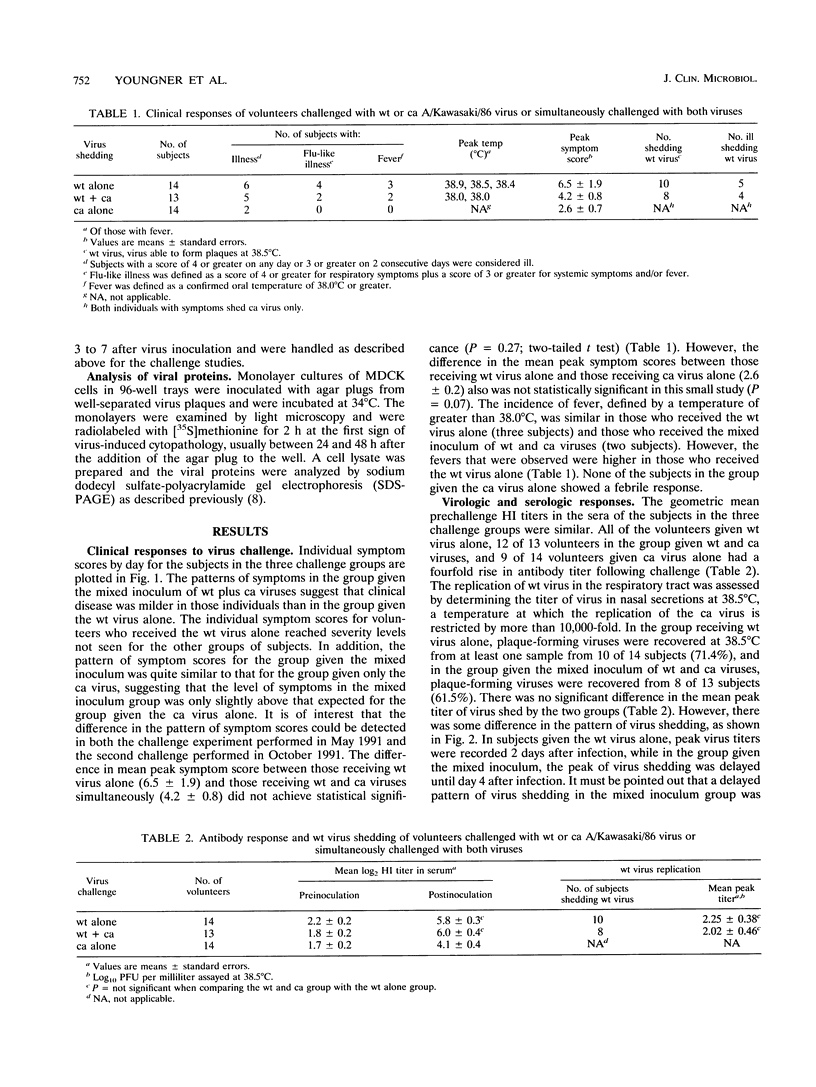
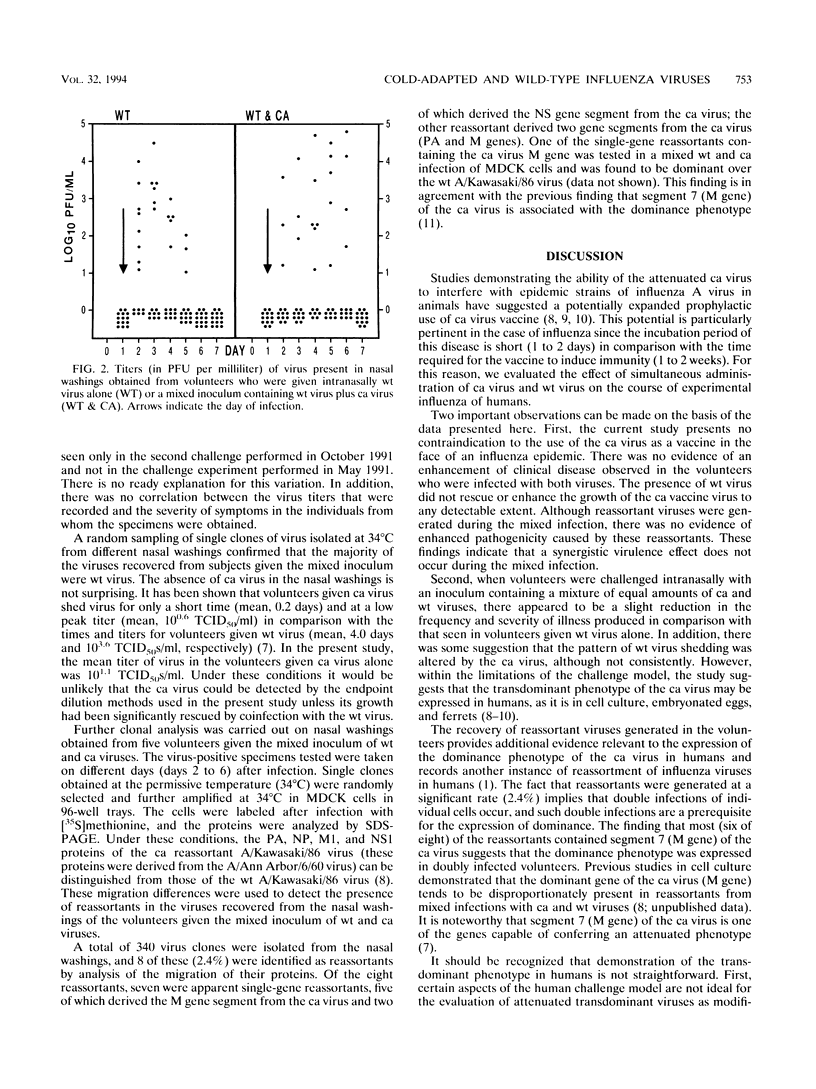
Abstract
On the basis of the ability of the attenuated cold-adapted strain of influenza A virus to suppress disease production in ferrets simultaneously infected with epidemic influenza virus (P. Whitaker-Dowling, H.F. Maassab, and J.S. Youngner, J. Infect. Dis. 164:1200-1202, 1991), an evaluation of the ability of the cold-adapted virus to modify clinical disease in humans was made. Adult volunteers with prechallenge serum hemagglutination-inhibition titers to the influenza A/Kawasaki/86 (H1N1) virus of < or = 1:8 received either 10(7) 50% tissue culture infective doses of the wild-type A/Kawasaki virus or a mixture of 10(7) 50% tissue culture infective doses of each of the wild-type virus and a cold-adapted A/Kawasaki reassortant virus by intranasal drops in a randomized, double-blind fashion. Symptoms and wild-type virus shedding were assessed daily for 6 days following challenge. Results were compared with those derived from another group of volunteers who received only cold-adapted virus. Volunteers who received the mixed inoculum of cold-adapted and wild-type viruses had lower symptom scores than those who received wild-type virus alone, suggesting that coinfection with the cold-adapted virus may modify wild-type virus infection, but the differences were not statistically significant in this small study. The data demonstrate that administration of cold-adapted influenza A virus to humans at the time of wild-type virus infection is a safe procedure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox N. J., Bai Z. S., Kendal A. P. Laboratory-based surveillance of influenza A(H1N1) and A(H3N2) viruses in 1980-81: antigenic and genomic analyses. Bull World Health Organ. 1983;61(1):143–152. [PMC free article] [PubMed] [Google Scholar]
- Maassab H. F. Adaptation and growth characteristics of influenza virus at 25 degrees c. Nature. 1967 Feb 11;213(5076):612–614. doi: 10.1038/213612a0. [DOI] [PubMed] [Google Scholar]
- Maassab H. F., Kendal A. P., Abrams G. D., Monto A. S. Evaluation of a cold-recombinant influenza virus vaccine in ferrets. J Infect Dis. 1982 Dec;146(6):780–790. doi: 10.1093/infdis/146.6.780. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Clements M. L., Madore H. P., Steinberg J., O'Donnell S., Betts R., Demico D., Reichman R. C., Dolin R., Maassab H. F. Dose response of cold-adapted, reassortant influenza A/California/10/78 virus (H1N1) in adult volunteers. J Infect Dis. 1984 May;149(5):816–816. doi: 10.1093/infdis/149.5.816. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Rennels M. B., Douglas R. G., Jr, Betts R. F., Couch R. B., Cate T. R., Jr, Chanock R. M., Kendal A. P., Maassab H. F., Suwanagool S. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect Immun. 1980 Aug;29(2):348–355. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. H., Betts R. F., DeBorde D., Tierney E. L., Clements M. L., Herrington D., Sears S. D., Dolin R., Maassab H. F., Murphy B. R. Four viral genes independently contribute to attenuation of live influenza A/Ann Arbor/6/60 (H2N2) cold-adapted reassortant virus vaccines. J Virol. 1988 Feb;62(2):488–495. doi: 10.1128/jvi.62.2.488-495.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. H., Clements M. L., De Borde D., Maassab H. F., Murphy B. R. Attenuation of wild-type human influenza A virus by acquisition of the PA polymerase and matrix protein genes of influenza A/Ann Arbor/6/60 cold-adapted donor virus. J Clin Microbiol. 1985 Nov;22(5):719–725. doi: 10.1128/jcm.22.5.719-725.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Dowling P., Lucas W., Youngner J. S. Cold-adapted vaccine strains of influenza A virus act as dominant negative mutants in mixed infections with wild-type influenza A virus. Virology. 1990 Apr;175(2):358–364. doi: 10.1016/0042-6822(90)90420-v. [DOI] [PubMed] [Google Scholar]
- Whitaker-Dowling P., Maassab H. F., Youngner J. S. Dominant-negative mutants as antiviral agents: simultaneous infection with the cold-adapted live-virus vaccine for influenza A protects ferrets from disease produced by wild-type influenza A. J Infect Dis. 1991 Dec;164(6):1200–1202. doi: 10.1093/infdis/164.6.1200. [DOI] [PubMed] [Google Scholar]
- Whitaker-Dowling P., Zvolenski R., Youngner J. S. The genes associated with trans-dominance of the influenza A cold-adapted live virus vaccine. Virology. 1991 Jan;180(1):81–87. doi: 10.1016/0042-6822(91)90011-y. [DOI] [PubMed] [Google Scholar]


