Summary
Drugs activating 5-hydroxytryptamine 2C receptors (5-HT2CRs) potently suppress appetite, but the underlying mechanisms for these effects are not fully understood. To tackle this issue, we generated mice with global 5-HT2CR deficiency (2C null) and mice with 5-HT2CRs re-expression only in pro-opiomelanocortin (POMC) neurons (2C/POMC mice). We show that 2C null mice predictably developed hyperphagia, hyperactivity, and obesity and showed attenuated responses to anorexigenic 5-HT drugs. Remarkably, all these deficiencies were normalized in 2C/POMC mice. These results demonstrate that 5-HT2CR expression solely in POMC neurons is sufficient to mediate effects of serotoninergic compounds on food intake. The findings also highlight the physiological relevance of the 5-HT2CR-melanocortin circuitry in the long-term regulation of energy balance.
Keywords: HUMDISEASE, MOLNEURO, SIGNALING
Introduction
The central 5-hydroxytryptamine (5-HT) system, including the 5-HT2C receptors (5-HT2CRs) plays critical roles in the regulation of energy homeostasis. This is best demonstrated by the hyperphagia and obesity in mice with global 5-HT2CR deficiency (Nonogaki et al., 1998; Tecott et al., 1995). 5-HT2CRs also contribute to the anorexigenic effects of d-fenfluramine (d-Fen) (Vickers et al., 1999), a drug that was widely prescribed and was clinically effective to combat obesity in the 1990s. However, the drug was withdrawn from clinical use due to adverse cardiopulmonary events (Connolly et al., 1997). Nonetheless, the efficacy of this drug regimen in humans underscores the importance of the central 5-HT system in regulating energy balance and the need to understand the mechanisms underlying its effects. More recently, commonly used atypical antipsychotic drugs (AAPDs) have been reported to cause serious weight gain, which may be associated with their 5-HT2CR antagonist properties and with polymorphisms in the 5-HT2CR gene (Reynolds et al., 2002; Templeman et al., 2005). Furthermore, a splicing variant of 5-HT2CRs with impaired function has been suggested to contribute to hyperphagia and obesity in patients with Prader-Willi syndrome (Kishore and Stamm, 2006). Collectively, these observations strongly suggest that an improved understanding of the mechanisms by which 5-HT2CRs regulate feeding behavior and body weight homeostasis may not only lead to the development of antiobesity drugs with fewer side effects but also may facilitate countering the metabolic deficits commonly seen in AAPD consumers or patients with Prader-Willi syndrome.
Pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of hypothalamus (ARC) produce α-melanocyte-stimulating hormone (α-MSH), an endogenous agonist of melanocortin 4 receptors (MC4Rs) (Cone, 2005; Elmquist et al., 1999; Williams and Schwartz, 2005). The central melanocortin system is required to maintain food intake, body weight, and glucose homeostasis (Cone, 2005; Elmquist et al., 1999; Williams and Schwartz, 2005; Yeo et al., 2000).
The melanocortin pathway has been hypothesized to be downstream of 5-HT2CRs and mediate the effects of 5-HT2CRs on feeding behavior. Particularly, POMC neurons in the ARC coexpress 5-HT2CRs (Heisler et al., 2002) and receive inputs from 5-HT-immunoreactive nerve terminals (Kiss et al., 1984). This anatomical evidence indicates that central 5-HT is positioned to directly act on POMC neurons via 5-HT2CRs. The potential role of 5-HT2CRs in POMC neurons is supported by electrophysiological studies showing that 5-HT drugs, including d-Fen, activate POMC neurons via 5-HT2CR-mediated mechanisms (Heisler et al., 2002; Qiu et al., 2007). In addition, 5-HT2CR agonists stimulate POMC expression in the ARC (Lam et al., 2008; Zhou et al., 2007). Finally, we have previously shown that the anorexigenic effect of d-Fen is blunted in mice lacking MC4Rs (Heisler et al., 2006). Collectively, these findings suggest that the intact central melanocortin pathway is required for the acute actions of 5-HT2CRs to regulate energy balance. However, whether direct 5-HT action only on POMC neurons is sufficient to mediate potent anorexigenic effects of 5-HT compounds is unknown. In addition, the physiological significance of the interaction of the central melanocortin system and 5-HT2CRs in regulating long-term energy balance remains to be established.
To directly address these questions, we generated mice with global deficiency of 5-HT2CR (2C null) and mice with 5-HT2CRs re-expressed specifically and only in POMC neurons (2C/POMC). Taking advantage of these unique genetic models, we directly tested the hypotheses that 5-HT2CRs in POMC neurons mediate the anorexigenic effects of 5-HT agents and that the re-establishment of the 5-HT2CR-melanocortin circuitry is sufficient to rescue the obese phenotypes caused by global 5-HT2CR deficiency.
Results and Discussion
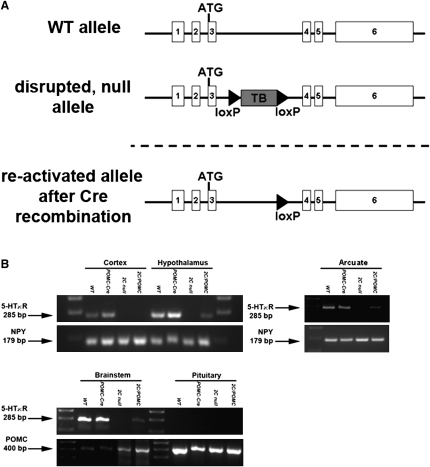
2C null (loxTB 5-HT2CR) mice were generated by inserting a loxP-flanked transcription blocker (loxTB) (Balthasar et al., 2005; Zigman et al., 2005) into the 5-HT2CR gene to globally disrupt its expression. Crossing 2C null mice with POMC-Cre mice (Balthasar et al., 2004) produced 2C/POMC mice, in which expression of endogenous 5-HT2CRs was reactivated selectively in POMC neurons by Cre-recombinase (Figure 1A).
Figure 1.
Generation of loxTB 5-HT2CR Mice
(A) A disrupted 5-HT2CR allele was generated by inserting a loxP-flanked transcriptional blocker (loxTB) between exons 3 and exon 4 of the 5-HT2CR gene. Expression of Cre-recombinase removes the transcriptional blocker and allows 5-HT2CR expression.
(B) Messenger RNAs of 5-HT2CR and neuropeptide Y (NPY) or POMC were detected with RT-PCR in the cerebral cortex, whole hypothalamus, ARC, brainstem, and pituitary of WT, POMC-Cre, 2C null, and 2C/POMC mice.
Using PCR primers specific for 5-HT2CR mRNA, we found that expression of 5-HT2CR mRNA was disrupted in the cerebral cortex, whole hypothalamus, ARC, and brainstem of 2C null mice. In 2C/POMC mice, 5-HT2CR mRNA was re-expressed only in samples of the whole hypothalamus or brainstem. Further, we found specific re-expression in samples of microdissected arcuate nucleus, where POMC neurons are located (Bronstein et al., 1992; Elias et al., 1999). We found no re-expression in the cerebral cortex (Figure 1B). Thus, 5-HT2CR mRNA was re-expressed in a POMC-specific manner. Although POMC mRNA is expressed in the anterior pituitary gland, we found no endogenous 5-HT2CR mRNA expressed in the pituitary of wild-type (WT) mice. It should be noted that POMC is also expressed by a small population of neurons in the nucleus of solitary tract (NTS) in the brainstem (Fan et al., 2004; Huo et al., 2006), a brain region regulating feeding and satiety. Thus, in addition to 5-HT2CRs expressed by ARC POMC neurons, we cannot rule out that the small number of POMC neurons in the NTS may contribute to the responses outlined below. We were not able to histologically validate re-expression of 5-HT2CRs specifically in POMC neurons due to the lack of highly selective reagents such as 5-HT2CR antibodies or ligands specific for 5-HT2CR binding.
To further confirm that our 2C null mice lack 5-HT2CRs, we assessed the acute effects on food intake to anorexigenic 5-HT agents in 2C null and WT mice. We found that while d-Fen significantly reduced 1 hr food intake in WT mice, this acute anorexigenic effect was attenuated in 2C null mice (Figure 2A). Similarly, an agonist of 5-HT2CRs and 5-HT 1B receptors, meta-chlorophenylpiperazine (mCPP) (Hewitt et al., 2002), decreased food intake in WT mice, an effect that was blunted in 2C null mice (Figure 2B). These findings are consistent with the results obtained in conventional 5-HT2CR knockout mice that showed attenuated anorexigenic effects in response to both d-Fen and mCPP (Tecott et al., 1995; Vickers et al., 1999).
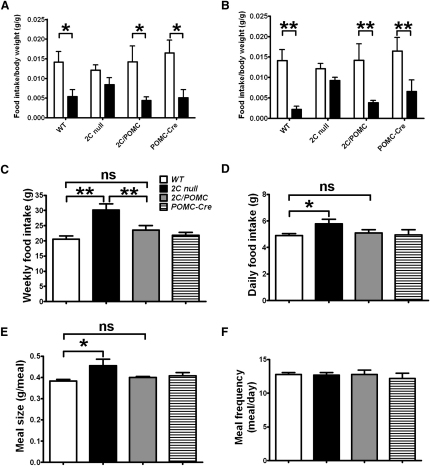
Figure 2.
5-HT2CRs in POMC Neurons Rescue the Attenuated Anorexigenic Effects of 5-HT Agents, Hyperphagia and Decreased Satiety in 2C Null Mice
Four-month-old body-weight-matched HFD-fed mice (n = 6–9 per genotype) were fasted overnight, and then saline, d-Fen (3 mg/kg) (A), or mCPP (5 mg/kg) (B) were intraperitoneally injected 30 min prior to presentation of HFD. Food intake in the following hour was measured and normalized by their body weight. (C) Weekly food intake was measured in singly housed HFD-fed mice (n = 5–7 per genotype) at 11 weeks. 2C null mice consumed significantly more diet than WT, 2C/POMC, and POMC-Cre mice, and there was no significant difference in food intake of WT, 2C/POMC, and POMC-Cre mice. Daily food intake (D), meal size (E), and meal frequency (F) of 3-month-old chow-fed mice (n = 5–11 per genotype) were measured with the TSE system. 2C null mice had significantly increased food intake and meal size than WT, and these parameters were not significantly different among WT, 2C/POMC, and POMC-Cre mice. There was no significant difference in meal frequency among the four genotypes. Data are presented as mean ± SEM, and ∗p < 0.05 and ∗∗p < 0.01 in one-way ANOVA analysis with Student-Newman-Keuls post hoc comparison.
To determine whether selective re-expression of 5-HT2CRs in POMC neurons is sufficient to restore the anorexigenic responses to 5-HT drugs, we assessed 2C/POMC mice, whose 5-HT2CRs were re-expressed selectively in POMC neurons. Notably, the anorexigenic effects induced by both d-Fen and mCPP were restored to levels that were indistinguishable from those in WT mice (Figures 2A and 2B). Thus, in line with our previous finding that the anorexigenic effect of d-Fen is blunted in mice lacking MC4Rs (Heisler et al., 2006), the current results indicate that 5-HT2CRs expressed by POMC neurons are sufficient to mediate the acute anorexigenic effects of 5-HT compounds.
5-HT2CRs are also physiological regulators of feeding, as 5-HT2CR knockout mice are hyperphagic (Nonogaki et al., 1998; Tecott et al., 1995). Consistent with these observations, our 2C null mice showed a hyperphagic phenotype. Specifically, a 50% increase in weekly food intake was observed in high fat diet (HFD)-fed 2C null mice compared to their WT littermates at 11 weeks (Figures 2C). Similarly, 12-week-old chow-fed 2C null mice were hyperphagic (Figure 2D). Remarkably, food intake of 2C/POMC mice on HFD or chow was comparable to that of WT mice (Figures 2C and 2D). These findings demonstrate that 5-HT2CRs expressed by POMC neurons are sufficient to normalize the hyperphagia characteristic of mice with 5-HT2CR deficiency.
Previously, it has been reported that mCPP-induced suppression in food intake in mice is associated with an early termination of feeding behavior, an effect that was partially reversed by a selective 5-HT2CR antagonist (Hewitt et al., 2002). Similarly, d-Fen produces an advanced satiation in WT mice, and this response is attenuated in mice with 5-HT2CR deficiency (Vickers et al., 1999). These findings suggest that activation of 5-HT2CRs enhances satiety signals to terminate food intake. Consistent with this notion, here we show that meal size of 2C null mice was significantly bigger than that of WT littermates, while their meal frequency was comparable to that of WT mice (Figures 2E and 2F). However, meal size of 2C/POMC mice was not statistically different from that of WT mice, which indicates that 5-HT2CRs re-expressed in POMC neurons are sufficient to advance the satiation process and therefore suppress feeding. Interestingly, a proportion of POMC neurons in the ARC have been shown to project to the NTS in the brainstem to potentiate satiety signals (Zheng et al., 2005). Thus, it is plausible that endogenous 5-HT, by acting on 5-HT2CRs in the ARC POMC neurons, activates POMC neurons innervating the NTS to increase satiety and suppress food intake. However, another possibility that we cannot exclude is that the 5-HT2CR-induced satiation is, at least partly, mediated by POMC neurons in the NTS, since 5-HT2CRs are also reactivated in NTS POMC neurons in 2C/POMC mice.
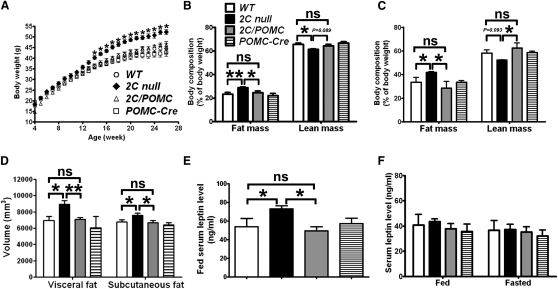
To test the significance of the 5-HT2CR-melanocortin circuitry in the long-term regulation of energy homeostasis, we measured the body weight and body composition of WT, 2C null, and 2C/POMC mice. Global deletion of 5-HT2CRs leads to late-onset obesity in chow-fed mice, a phenotype that can be accelerated by HFD-feeding (Nonogaki et al., 1998). In agreement, we found that our 2C null mice fed with HFD showed significant differences in body weight compared to their WT littermates starting at 14 weeks of age, and by week 26, 2C null mice weighed 8 g more than WT mice (Figure 3A). Deletion of 5-HT2CRs also results in alterations in adiposity. We found a significant increase in fat mass in 2C null mice as early as 13 weeks of age, which was accompanied by a decrease in lean mass (Figure 3B). At week 28, 2C null mice accumulated significantly more body fat mass than WT mice, and their body lean mass tended to decrease (Figure 3C). We used CT scans to further confirm that 2C null mice had significantly increased adiposity in both visceral and subcutaneous depots (Figure 3D). Notably, we found that selective re-expression of 5-HT2CRs in POMC neurons was sufficient to rescue the obese phenotype observed in 2C null mice. Particularly, HFD-fed 2C/POMC mice had comparable body weight as WT mice (Figure 3A). Both young and old 2C/POMC mice had similar levels of adiposity as age-matched WT littermates (Figures 3B and 3C). In addition, visceral and subcutaneous fat distributions in old 2C/POMC mice were similar to WT mice (Figure 3D). Thus, these findings demonstrate that 5-HT2CRs re-expressed in POMC neurons are sufficient to rescue obesity caused by global 5-HT2CR deficiency.
Figure 3.
5-HT2CRs in POMC Neurons Rescue Obesity, Hyperadiposity, and Hyperleptinemia in 2C Null Mice
(A) Weekly body weight was measured in group-housed mice fed with HFD (n = 20–39 per genotype). Body weight of 2C null mice started to be significantly higher than that of WT, 2C/POMC, and POMC-Cre mice from 14 weeks of age, and there was no significant difference in body weight of WT, 2C/POMC, and POMC-Cre mice. 2C null mice fed with HFD accumulated significantly higher fat mass than WT, 2C/POMC, and POMC-Cre littermates at week 13 (B) and at week 28 (C), and lean mass was significantly decreased in young 2C null mice and tended to decrease in old 2C null mice; there was no significant difference in fat mass and lean mass among WT, 2C/POMC, and POMC-Cre mice. N = 6–10 per genotype in (B) and (C). (D) 23-week-old HFD-fed 2C null mice showed significantly increased visceral and subcutaneous fat storage than WT, 2C/POMC, and POMC-Cre mice, and there was no significant difference in fat distribution in WT, 2C/POMC, and POMC-Cre mice. N = 4–5 per genotype in (D). (E) Eight-month-old HFD-fed 2C null mice had significantly higher leptin level than WT, 2C/POMC, and POMC-Cre mice, and there was no significant difference in leptin levels of WT, 2C/POMC, and POMC-Cre mice. N = 4–5 per genotype in (E). (F) There is no significant difference in fed and fasted serum leptin levels in 4-month-old HFD-fed mice (n = 5–6 per genotype). Data are presented as mean ± SEM, and ∗p < 0.05 and ∗∗p < 0.01 in one-way ANOVA analysis with Student-Newman-Keuls post hoc comparison.
Secondary to obesity and increased fat mass, mice lacking 5-HT2CRs have been shown to develop late-onset hyperleptinemia and leptin insensitivity (Nonogaki et al., 1998). Consistently, we detected significantly increased serum leptin in old HFD-fed 2C null mice (Figure 3E). Young 2C null mice had similar serum leptin levels as WT littermates (Figure 3F). Re-expression of 5-HT2CRs in POMC neurons normalized serum leptin level in old HFD-fed 2C/POMC mice (Figure 3E).
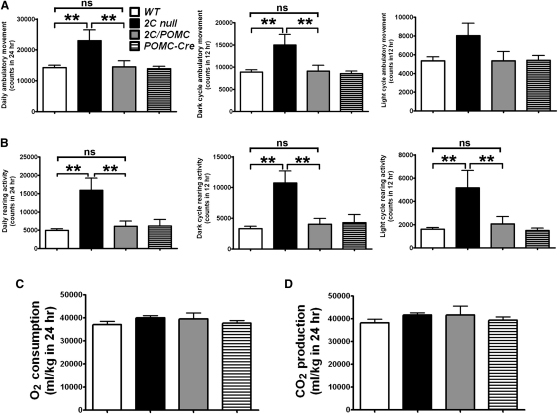
It has been reported that young pre-obese 5-HT2CR knockout mice also display elevated physical activity (Nonogaki et al., 2003). However, young mutant mice display normal energy expenditure (Nonogaki et al., 2003). Consistent with these observations, we found that ambulatory movements and rearing activities of young chow-fed 2C null mice were significantly increased compared to WT littermates (Figures 4A and 4B). We found no difference in the energy expenditure as measured by O2 consumption and CO2 production (Figures 4C and 4D). Notably, the hyperactivity of 2C null mice was completely normalized in 2C/POMC mice (Figures 4A and 4B). These data indicate that selective re-expression of 5-HT2CRs in POMC neurons is sufficient to normalize the hyperactivity in mice lacking 5-HT2CRs.
Figure 4.
5-HT2CRs in POMC Neurons Rescue Hyperactivity in 2C Null Mice
(A) Three-month-old chow-fed 2C null mice showed significantly more ambulatory movement than WT, 2C/POMC, and POMC-Cre mice over the entire 24 hr (left panel) and 12 hr dark cycle (middle panel), but no significant difference was observed over the 12 hr light cycle (right panel). (B) 2C null mice showed significantly more rearing activity than WT, 2C/POMC, and POMC-Cre mice over the entire 24 hr (left panel), 12 hr dark cycle (middle panel), and 12 hr light cycle (right panel). There was no significant difference in ambulatory movement and rearing activity of WT, 2C/POMC, and POMC-Cre mice. O2 consumption (C) and CO2 production (D) were not significantly different among four genotypes. N = 5–11 per genotype. Data are presented as mean ± SEM, and ∗∗p < 0.01 in one-way ANOVA analysis with Student-Newman-Keuls post hoc comparison.
In addition to hyperphagia and obesity, another characteristic phenotype of mice with 5-HT2CR deficiency is spontaneous seizures, which result in a high mortality rate in relatively young mice (Tecott et al., 1995). Although we did not quantitatively analyze the epileptic phenotype in our mice, we anecdotally observed several episodes of tonic-clonic seizures in both 2C null and 2C/POMC mice. In addition, similar to 5-HT2CR knockout mice (Tecott et al., 1995), our 2C null mice displayed a significant decreased survival rate compared to WT mice (see the Supplemental Data available online). Interestingly, the survival rate of HFD-fed 2C null mice is significantly higher than that of 2C null mice on chow (see Supplemental Data). This is interesting given the fact that epileptic patients can benefit from ketogenic diets (Kim do and Rho, 2008). Notably, 2C/POMC mice showed a similar survival rate to their 2C null littermates, regardless whether they were fed with HFD or chow (see Supplemental Data). Therefore, these results suggest that re-expression of 5-HT2CRs in POMC neurons is not sufficient to rescue the epileptic phenotype characteristic of 5-HT2CR knockout mice. One may argue that the seizures and the associated high mortality rate may confound the interpretation of the metabolic measurements in 2C null and 2C/POMC mice. However, given the likely metabolic expense of seizures, we would suggest that the seizure phenotype would mask the observed obese phenotype in 5-HT2CR knockout mice. Finally, it is important to note that 2C null and 2C/POMC mice displayed distinct metabolic profiles despite the fact that they suffered almost identical mortality rate (presumably due to similar epileptic status).
Serotoninergic neurons have broad projections that innervate the entire central nervous system and regulate diverse behaviors including feeding. For years, research focused on the mechanisms of the central 5-HT system, including 5-HT2CRs, has been one of the priorities in the obesity field because of the potent effects of serotoninergic agents on feeding. Indeed, drugs generally influencing 5-HT bioavailability, such as sibutramine, are among the few approved current pharmacotherapies for obesity. However, due to the widespread distribution of 5-HT2CRs in the brain (Molineaux et al., 1989) and the lack of commercially available 5-HT2CR-selective drugs, discerning mechanisms underlying functions of 5-HT2CRs has proven particularly difficult. Here, taking advantage of unique genetic mouse models, we have provided direct evidence that POMC neurons are physiologically important targets of potent anorexigenic 5-HT compounds such as d-Fen to suppress food intake. In addition, our results highlight the importance of the central 5-HT2CRs expressed by POMC neurons in maintaining normal feeding behavior and body weight homeostasis. However, our findings do not demonstrate that POMC neurons are the only site where 5-HT2CRs regulate energy balance, as our results do not exclude the possibility that other redundant neuronal populations expressing 5-HT2CRs may also be sufficient to mediate similar effects. For example, in addition to the ARC, 5-HT2CRs are also found in other brain regions implicated in the regulation of body weight homeostasis, including the paraventricular nucleus, ventromedial nucleus, dorsomedial nucleus, lateral hypothalamic area, and parabrachial nucleus (Hoffman and Mezey, 1989; Pasqualetti et al., 1999; Wright et al., 1995). These regions all receive serotoninergic projections (Petrov et al., 1992; Steinbusch and Nieuwenhuys, 1981). The physiological relevance of 5-HT2CR expression in these sites is yet to be characterized. However, our unique mouse model will allow us to directly address the importance of 5-HT2CRs in any site in the central nervous system in which specific expression of Cre-recombinase can be directed.
Experimental Procedures
Generation of 2C Null and 2C/POMC Mice
2C null (LoxTB 5-HT2CR) mice were created by inserting a loxP-flanked transcriptional blocking cassette (loxTB) (Balthasar et al., 2005; Zigman et al., 2005) between the exons 3 and 4 of the X-linked 5-HT2CR gene (Figure 1A). The targeting construct was generated using ET cloning and related technologies within EL250 cells and the BAC that contains the 5-HT2CR gene (Mouse RPCI.22 BAC clone 32 B 10) (Invitrogen, Carlsbad, CA). The loxTB 5-HT2CR gene was placed in a pGEM-T plasmid using the following PCR template (left homology arm [TAATTATAAACACTATTATACACAGAGATTTCCAATTTATTAACTAAAATTACTTTCAAAGTCATGCCTTACCGG TCCAACGCGTTGGATGCATAG] and right homology arm [GAGCTTAAAACATTAGCAATCAGCAGCAAAGATGCA AATATTCCTCAACCGTGTCAGTACTATAGACTAAACCGGTGTATTTTCTCCTTACGCATC]) and then linearized. The final targeting construct, which consisted of the 5-HT2CR-loxTB flanked by 4 kb 5-HT2CR homology arms, was electroporated into ES cells, and correct targeting was confirmed by Southern blot analyses. For Southern blot analysis, ES cells were digested with NCO1 for 4 hr at 37°C. Primers were labeled with Megaprime DNA labeling kit. The right probe was 202 bp and amplified using TCACAATTGAAGACATTTCCTG and TGTTGGGTTTCTTTGTGGTTCTC. Detection of a nontargeted ES cell would produce an 11,095 bp band, and a target ES cell would produce a 6718 bp band. The left probe was 265 bp and amplified using TGTGAGAAATGCTGCAGGAATAAA and TTGGGAAGTTTTGTTTTTGTGGA. Detection of a nontargeted ES cell would produce an 11,095 bp band, while a targeted ES cell would produce a 6570 bp band. Targeted ES cells were injected into blastocysts, and, after germline transmission was established, the chimera carrying the recombinant allele was crossed onto a C57BL/6J background.
Female loxTB 5-HT2CR heterozygous mice (backcrossed to the C57BL/6J background for eight generations) were crossed with male POMC-Cre hemizygous mice (backcrossed to the C57BL/6J background for five generations), and only male offspring were collected for the experiments described below. These male mice carry one of the following genotypes: wild-type (WT), hemizygous loxTB 5-HT2CR (2C null), hemizygous loxTB 5-HT2CR and hemizygous POMC-Cre (2C/POMC), as well as hemizygous POMC-Cre (POMC-Cre). All mice were housed in their home cages with food and water available ad libitum in a temperature-controlled room with 12 hr light, 12 hr dark cycle in the animal facility of University of Texas Southwestern Medical Center at Dallas.
Food Intake and Body Weight
For the HFD-feeding study, mice were group housed (two to five mice per cage) and provided with a 42% fat diet (TD.88137, Harlan Teklad) from 5 weeks of age, and body weight was measured weekly. Another HFD cohort was individually housed, and a similar growth pattern was observed as in the group-housed cohort. Food intake was measured weekly from mice that were individually housed.
RT-PCR
The cerebral cortex, whole hypothalamus, ARC, brainstem, and pituitary were taken from WT, 2C null, 2C/POMC, and POMC-Cre mice. Total RNA was extracted with the QIAzol Lysis Reagent (QIAGEN, Valencia, CA) and reverse-transcribed to cDNA using SuperScript II First-Strand cDNA Synthesis kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Expression of 5-HT2CR was detected in all the tissues with primers that span exon 3 and 4 of 5-HT2CR gene (forward, CTCACTCCTTGTGCACCT; reverse, CCCACCAGCATATCAGCAATG). As positive controls, expression of NPY was detected in the cerebral cortex, whole hypothalamus, and ARC with NPY primers (forward, TGCTCGTGTGTTTGGGCATTCTGG; reverse, GAGACACTGATTTCAGACCTC); POMC was detected in the brainstem and pituitary using POMC primers (forward, CAGACCTCCATAGATGTGTGGAGC; reverse, CTCAGCAACGTTGGGGTACAC). Due to the low level of 5-HT2CR mRNA in the ARC of 2C/POMC mice, the first PCR products of ARC tissues from all mice were purified and subjected to the second PCR amplification using the same primers. 5-HT2CR PCR amplicons from the hypothalami of WT, 2C/POMC, and POMC-Cre mice were gel purified and sequenced in the local core facility, and we confirmed that 5-HT2CR mRNA re-expressed in 2C/POMC hypothalamus was identical to those of WT and POMC-Cre hypothalami.
Body Composition and Fat Distribution
Body composition was measured with the Bruker minispec mq10 MRS system. For fat distribution, mice were anesthetized with 1% isoflurane inhalation and then the trunk (from base of the skull as the spinal canal begins to widen and the distal end of the tibia) of each mouse was scanned at an isotropic voxel size of 93 μm (80 kV, 450 μA, and 100 ms integration time) using the eXplore Locus micro-CT scanner (GE Health Care). Three-dimensional images were reconstructed from two-dimensional grayscale image slices and visualized using Microview Software (GE Medical System). Density values for soft tissue and bone were calibrated from a phantom (GE Health Care) containing air bubble, water, and hydroxyl apatite rod. The separation of fat regions was obtained from the appropriate grayscale value (upper threshold, −165; lower threshold, −360). The abdominal muscular wall was used as the differentiation line to separate visceral adipose tissue from subcutaneous adipose tissue. The contour lines were drawn around the viscera and three-dimensional ROI was generated. The visceral fat was determined from the histogram of these segmented viscera using the same thresholds. Subcutaneous fat was obtained by subtracting visceral fat from the total body fat.
Acute Anorexigenic Responses to 5-HT Compounds
To assess whether 5-HT2CRs in POMC neurons are sufficient to mediate anorexigenic effects of 5-HT compounds, 4-month-old HFD-fed mice were individually housed and weight matched. After overnight fasting, the mice received intraperitoneal injections of saline, d-Fen (3 mg/kg), or mCPP (5 mg/kg). HFD was represented to their cages 30 min after the injections. Food intake in the next hour was measured and normalized by their body weight. Each mouse was tested with all three treatments (d-Fen, mCPP, and saline), administered in a counterbalanced order, with a minimum of 5 days between the treatments. Data were presented as mean ± SEM. Differences among genotypes were determined by two-way ANOVA analysis, followed by the post hoc Student-Newman-Keuls test.
Serum Leptin
Fed and/or fasted serum leptin levels were measured in HFD-fed mice at 4 or 8 months of age. At month 4, food was removed from the home cages for 2 hr (fed condition) or for overnight (fasted condition), and blood was collected from the saphenous vein. At month 8, trunk blood was collected 2 hr after mice were deprived of food. Leptin levels were measured using the leptin ELISA kit (Crystal Chem Inc., Downers Grove, IL) according to the manufacturer's instruction.
Physical Activity, Energy Expenditure, and Meal Patterns
Physical activity, energy expenditure, and meal patterns were monitored using a combined indirect calorimetry system (TSE Systems GmbH, Bad Homburg, Germany) (Pfluger et al., 2008). After adaptation for 6 days, physical activity was determined for 4 days using a multidimensional infrared light beam system with beams installed on cage bottom and cage top levels. Ambulatory movement was defined as breaks of any two different light beams at cage bottom level, while rearing was recorded once the mouse broke any light beam at the top level. Simultaneously, O2 consumption and CO2 production were measured to determine the energy expenditure. In addition, meal patterns were determined continuously by integration of weighing sensors fixed at the top of the cage from which the food containers have been suspended into the sealed cage environment. Meals were defined as food intake events with a minimum duration of 60 s and a break of 300 s between food intake events.
Statistical Analysis
Data were presented as mean ± SEM. Statistical analyses were carried out with SigmaStat software. Unless otherwise mentioned, all data were analyzed by one-way ANOVA analysis, followed by the post hoc Student-Newman-Keuls test when the ANOVA analysis indicated significant differences. p < 0.05 indicated statistical significance.
Acknowledgments
We would like to thank the Dr. Aktar Ali and Ms. Laura Brule and the Mouse Metabolic Phenotyping Core at University of Texas Southwestern Medical Center at Dallas (supported by PL1 DK081182-01). Data presented in this paper were also supported by the following: Y.X. (Canadian Institute of Health Research), J.E.J. (F32 DK63853-01), L.K.H. (DK065171 and Wellcome Trust), J.M.Z. (K08DK068069-01A2), B.B.L. (PO1 DK56116, project 3), and J.K.E. (R01DK53301, R01MH61583, and RL1DK081185) and support from the American Diabetes Association and a Smith Family Foundation Pinnacle Program Project Award.
Published: November 25, 2008
Footnotes
The Supplemental Data include one figure and can be found with this article online at http://www.neuron.org/supplemental/S0896-6273(08)00801-5.
Supplemental Data
References
- Balthasar N., Coppari R., McMinn J., Liu S.M., Lee C.E., Tang V., Kenny C.D., McGovern R.A., Chua S.C., Jr., Elmquist J.K., Lowell B.B. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T., Ferreira M., Tang V., McGovern R.A., Kenny C.D. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bronstein D.M., Schafer M.K., Watson S.J., Akil H. Evidence that beta-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res. 1992;587:269–275. doi: 10.1016/0006-8993(92)91007-2. [DOI] [PubMed] [Google Scholar]
- Cone R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Connolly H.M., Crary J.L., McGoon M.D., Hensrud D.D., Edwards B.S., Edwards W.D., Schaff H.V. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Elias C.F., Aschkenasi C., Lee C., Kelly J., Ahima R.S., Bjorbaek C., Flier J.S., Saper C.B., Elmquist J.K. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elmquist J.K., Elias C.F., Saper C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Fan W., Ellacott K.L., Halatchev I.G., Takahashi K., Yu P., Cone R.D. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat. Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- Heisler L.K., Cowley M.A., Tecott L.H., Fan W., Low M.J., Smart J.L., Rubinstein M., Tatro J.B., Marcus J.N., Holstege H. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Heisler L.K., Jobst E.E., Sutton G.M., Zhou L., Borok E., Thornton-Jones Z., Liu H.Y., Zigman J.M., Balthasar N., Kishi T. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hewitt K.N., Lee M.D., Dourish C.T., Clifton P.G. Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol. Biochem. Behav. 2002;71:691–700. doi: 10.1016/s0091-3057(01)00709-2. [DOI] [PubMed] [Google Scholar]
- Hoffman B.J., Mezey E. Distribution of serotonin 5-HT1C receptor mRNA in adult rat brain. FEBS Lett. 1989;247:453–462. doi: 10.1016/0014-5793(89)81390-0. [DOI] [PubMed] [Google Scholar]
- Huo L., Grill H.J., Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- Kim do Y., Rho J.M. The ketogenic diet and epilepsy. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:113–120. doi: 10.1097/MCO.0b013e3282f44c06. [DOI] [PubMed] [Google Scholar]
- Kishore S., Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311:230–232. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- Kiss J., Leranth C., Halasz B. Serotoninergic endings on VIP-neurons in the suprachiasmatic nucleus and on ACTH-neurons in the arcuate nucleus of the rat hypothalamus. A combination of high resolution autoradiography and electron microscopic immunocytochemistry. Neurosci. Lett. 1984;44:119–124. doi: 10.1016/0304-3940(84)90068-5. [DOI] [PubMed] [Google Scholar]
- Lam D.D., Przydzial M.J., Ridley S.H., Yeo G.S., Rochford J.J., O'Rahilly S., Heisler L.K. Serotonin 5–HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineaux S.M., Jessell T.M., Axel R., Julius D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc. Natl. Acad. Sci. USA. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki K., Strack A.M., Dallman M.F., Tecott L.H. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat. Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- Nonogaki K., Abdallah L., Goulding E.H., Bonasera S.J., Tecott L.H. Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT(2C) receptor mutant mice. Diabetes. 2003;52:315–320. doi: 10.2337/diabetes.52.2.315. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M., Ori M., Castagna M., Marazziti D., Cassano G.B., Nardi I. Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in human brain. Neuroscience. 1999;92:601–611. doi: 10.1016/s0306-4522(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Petrov T., Krukoff T.L., Jhamandas J.H. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J. Comp. Neurol. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- Pfluger P.T., Kirchner H., Gunnel S., Schrott B., Perez-Tilve D., Fu S., Benoit S.C., Horvath T., Joost H.G., Wortley K.E. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- Qiu J., Xue C., Bosch M.A., Murphy J.G., Fan W., Ronnekleiv O.K., Kelly M.J. Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Mol. Pharmacol. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- Reynolds G.P., Zhang Z.J., Zhang X.B. Association of antipsychotic drug-induced weight gain with a 5–HT2C receptor gene polymorphism. Lancet. 2002;359:2086–2087. doi: 10.1016/S0140-6736(02)08913-4. [DOI] [PubMed] [Google Scholar]
- Steinbusch H.W., Nieuwenhuys R. Localization of serotonin-like immunoreactivity in the central nervous system and pituitary of the rat, with special references to the innervation of the hypothalamus. Adv. Exp. Med. Biol. 1981;133:7–35. doi: 10.1007/978-1-4684-3860-4_1. [DOI] [PubMed] [Google Scholar]
- Tecott L.H., Sun L.M., Akana S.F., Strack A.M., Lowenstein D.H., Dallman M.F., Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Templeman L.A., Reynolds G.P., Arranz B., San L. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet. Genomics. 2005;15:195–200. doi: 10.1097/01213011-200504000-00002. [DOI] [PubMed] [Google Scholar]
- Vickers S.P., Clifton P.G., Dourish C.T., Tecott L.H. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl.) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Williams D.L., Schwartz M.W. The melanocortin system as a central integrator of direct and indirect controls of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R2–R3. doi: 10.1152/ajpregu.00226.2005. [DOI] [PubMed] [Google Scholar]
- Wright D.E., Seroogy K.B., Lundgren K.H., Davis B.M., Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Yeo G.S., Farooqi I.S., Challis B.G., Jackson R.S., O'Rahilly S. The role of melanocortin signalling in the control of body weight: evidence from human and murine genetic models. QJM. 2000;93:7–14. doi: 10.1093/qjmed/93.1.7. [DOI] [PubMed] [Google Scholar]
- Zheng H., Patterson L.M., Phifer C.B., Berthoud H.R. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R247–R258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- Zhou L., Sutton G.M., Rochford J.J., Semple R.K., Lam D.D., Oksanen L.J., Thornton-Jones Z.D., Clifton P.G., Yueh C.Y., Evans M.L. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman J.M., Nakano Y., Coppari R., Balthasar N., Marcus J.N., Lee C.E., Jones J.E., Deysher A.E., Waxman A.R., White R.D. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.