Abstract
Store-operated Ca2+ influx represents a major route by which cytosolic Ca2+ can be elevated during platelet activation, yet its molecular identity in this cell type remains highly controversial. Using quantitative RT-PCR analysis of candidate receptor-operated cation entry pathways in human platelets, we show a >30-fold higher expression of message for the recently discovered Orai1 store-operated Ca2+ channel, and also the store Ca2+ sensor STIM1, when compared to the non-selective cation channels TRPC1, TRPC6 and TRPM2. Orai1 and STIM1 gene transcripts were also detected at higher levels than TRPC1, TRPC6 and TRPM2 in primary murine megakaryocytes and human megakaryocytic cell lines. In direct electrophysiological recordings from murine megakaryocytes, Ca2+ ionophore-induced store depletion stimulated CRAC currents, which are known to require Orai1, and these overlapped with TRPC6-like currents following P2Y receptor activation. Together with recent transgenic studies, these data provide evidence for STIM1:Orai1 as a primary pathway for agonist-evoked Ca2+ influx in the platelet and megakaryocyte.
Keywords: Platelets, calcium, Orai1, CRAC, STIM1, megakaryocyte, store-operated Ca2+ entry
Introduction
Ca2+ mobilisation pathways are potentially important anti-thrombotic targets due to the key role of receptor-mediated cytosolic Ca2+ increases ([Ca2+]i) in platelet activation [1]. It is well established that IP3-dependent Ca2+ release represents a central pathway for mobilising Ca2+ downstream of both G-protein-coupled and tyrosine kinase-linked surface receptors [2]. In addition, parallel activation of plasma membrane cation channels can sustain and amplify agonist-evoked [Ca2+]i responses due to the comparatively infinite content of the extracellular versus intracellular Ca2+ pools. The platelet expresses one ligand-gated non-selective cation channel, the P2X1 receptor, which can contribute to Ca2+ entry following stimulation by ATP released from damaged vasculature or secreted from platelet granules [3]. However, the molecular nature of other receptor-operated Ca2+ entry pathways, particularly that activated by depletion of internal stores [4;5], remains highly controversial [4;6]. Members of the canonical transient receptor potential family of ion channels (TRPC) represent prime candidates for Ca2+ influx pathways stimulated by products of phospholipase-C, and most groups agree that platelets and megakaryocytes express TRPC1 and TRPC6 [6-8]. In addition, megakaryocytes possess TRPM2, a member of the melanostatin family of TRP channels, which may contribute to Ca2+ influx during haemostasis and thrombosis since activated platelets are known to generate superoxide, an endogenous ligand of the channel [9;10].
Recent seminal work by several groups has shown that store-dependent Ca2+ influx in various non-excitable cell types occurs by coupling of the intracellular store Ca2+ sensor STIM1 (stromal interaction molecule 1) [11-13] to plasma membrane CRAC channels, of which Orai1 (also known as CRACM1) forms an essential component [14-16]. Emerging evidence indicates an important role for STIM1 in platelet function as heterozygote mice carrying an activating point mutation in the EF-hand motif of STIM1 display premature platelet activation, leading to accelerated platelet consumption and macrothrombocytopenia [17]. To date, TRPC1 is the only ion channel suggested to couple STIM1 to store-dependent Ca2+ entry in the platelet [18]. Surprisingly, however, TRPC1-deficient mice show no platelet-related phenotype [19]. We have therefore used quantitative RT-PCR to examine the relative expression of STIM1, Orai1 and TRP channels that may contribute to receptor-operated Ca2+ influx in the platelet. Evidence from human platelets, primary murine megakaryocytes and human megakaryocytic cell lines strongly support the hypothesis that STIM1-coupled Orai1 channels contribute to store-operated Ca2+ entry in this cell type.
Methods
Cell preparation
Human platelets were isolated as described elsewhere [20]. Briefly, 100 ml blood was collected from each donor by self-propagated flow through a Venflon™ IV cannula (diameter: 1.2 mm; BD, NJ, USA) into 10 ml Falcon tubes containing 1.5 ml citrate-dextrose solution (Sigma product no C3821), 2 mM EDTA, 0.1 μM PGE1 and 0.3 mM aspirin (all from Sigma, MO, USA). The project was approved by the Ethics Committee of Lund University, Sweden and all blood donors gave written informed consent to participate in the study. Platelet rich plasma (PRP) was obtained by centrifugation for 20 min at 200 g min followed by 10 min at 200 g and passed through a Pall AutoStop™ Leukocyte removal filter (Pall Inc., NY, USA). The platelet filtrates were leukocyte- and erythrocyte-depleted using anti-CD235a and anti-CD45-coated magnetic Dynabeads (BD Inc. NJ, USA and Dynal, Oslo, Norway) according to the manufacturer’s instructions. The purified platelets were collected by centrifugation (10 min, 800 g), dissolved in 4 ml TRIzol® (Invitrogen, CA, USA) and stored at -85 °C until RNA isolation. The megakaryocytic cell lines HEL and MEG-01 were obtained from the European Collection of Cell Cultures (ECACC, Porton Down, Salisbury, UK) and cultured in RPMI 1640, supplemented with 10% fetal calf serum, 100 units ml-1 penicillin and 100 μg ml-1 streptomycin in a humidified atmosphere at 37°C with 5% CO2. Primary murine megakaryocytes from the tibial and femoral marrow of adult C57/Bl6 mice were prepared for electrophysiological experiments and selected individually using glass micropipettes for RT-PCR studies as previously described [8]. 35 individual megakaryocytes were combined for each reverse transcription reaction, which was subsequently diluted for PCR reactions.
Reagents and solutions
The standard external saline used for isolating megakaryocytes for electrophysiological experiments contained (in mM) 145 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose and 10 HEPES, pH 7.35 (NaOH). For electrophysiological recordings, salines were designed to eliminate K+-selective membrane currents; thus, KCl in the external saline was replaced with an equal concentration of NaCl and the K+ channel blocker 4-aminopyridine (2 mM) was present throughout. For normal intracellular Ca2+ buffering conditions, the pipette saline contained (in mM) 150 CsCl, 2 MgCl2, 0.05 Na2GTP, 0.05 K5Fura-2, 10 Hepes, 0.1 EGTA, pH 7.2 (CsOH). For high internal Ca2+ buffering, the pipette saline contained (in mM) 120 CsCl, 2 CaCl2, 2 MgCl2, 0.05 Na2GTP, 0.05 K5Fura-2, 10 Hepes, 10 Cs4BAPTA, pH 7.2 (CsOH). K5Fura-2 and Cs4BAPTA were supplied by Molecular Probes (Leiden, The Netherlands). Applied Biosystems (Warrington, UK) supplied all real-time PCR reagents, whilst Qiagen Ltd (Crawley, UK) supplied all other molecular reagents and kits, unless otherwise stated. Cell culture reagents were supplied by Gibco (Paisley, UK). All other reagents were supplied by Sigma-Aldrich (Poole, UK).
RT-PCR
Total RNA and cDNA were prepared from individually extracted megakaryocytes as previously described [8]. Total RNA was extracted from platelets using a modified TRIzol protocol as described elsewhere [20]. Platelet cDNA was generated using random hexamer priming and TaqMan® Reverse transcription (Applied Biosystems, CA, USA) according to the manufacturer’s instructions. The extremely low grade of leukocyte contamination was confirmed through quantitative real-time PCR using leukocyte-common antigen (CD45) as a marker of leukocyte contamination, as described elsewhere [20]. Total RNA was purified from cultured cell lines using the RNeasy® mini kit and reverse-transcribed using the High Capacity cDNA RT kit with RNasin® inhibitor according to the manufacturer’s instructions (Promega, Southampton, UK). Relative quantification of gene targets was performed using the Applied Biosystems 7500 Real Time system. For each target and housekeeping gene, 2μl cDNA was added to the customised Taqman Gene Expression Assay and used according to manufacturer instructions. Separate assays based on either murine or human gene sequences were used for each target (Assay ID); β-ACTIN (Mm00607939_s1 & Hs99999903_m1), ORAI1 (also known as CRACM1 or TMEM142A) (Mm00774349_m1 & Hs00385627_m1), STIM1 (Mm00486423_m1 & Hs00162394_m1), TRPC1 (Mm00441975_m1 & Hs00608195_m1), TRPC6 (Mm00443441_m1 & Hs00175753_m1) and TRPM2 (Mm00663098_m1 & Hs00268573_m1). Analysis was performed using Applied Biosystems 7500 Fast System SDS Software and results displayed as average fold expression relative to the housekeeping gene β-ACTIN. Each target was assayed in duplicate or triplicate for each sample and the average fold expression derived from 3-6 samples.
Electrophysiology
Conventional whole-cell patch clamp recordings were carried out using an Axopatch 200B amplifier (Axon CNS Molecular Devices Corporation, Union City, CA, USA) in voltage clamp mode as described in detail elsewhere [21]. Current-voltage relationships of membrane conductances were assessed using voltage ramps of 100 msec duration from a holding potential of -70 mV to a peak of +70 mV. Membrane currents during voltage ramps were filtered at 1 kHz and acquired at a rate of 5 kHz, using a Digidata 1200 A/D converter and pClamp 6 (Axon CNS Molecular Device Corporation). Membrane currents were also digitized at a slower rate (60 Hz, filtered at 30 Hz) using a Cairn Research Ltd (Faversham, Kent) acquisition system to provide a continuous recording of holding current. All experiments were carried out at room temperature. Data were analysed using Clampfit v9.0 (Axon Instruments) or Cairn Research Ltd software and exported for presentation in Microcal Origin (Microcal Software Inc, Northampton, MA, USA).
Results and Discussion
A recent RT-PCR screen of all known transient receptor potential ion channels within primary murine megakaryocytes demonstrated the presence of messages for TRPC1, TRPC6, TRPM1, TRPM2 and TRPM7 [8]. Of these, TRPC1, TRPC6 and TRPM2 have been proposed to contribute to agonist-evoked Ca2+ signalling in the platelet via store-operated, store-independent or secondary superoxide-dependent mechanisms, respectively. However, Orai1, a transmembrane protein shown to be essential for store-operated Ca2+ influx through CRAC channels, has not been demonstrated in platelets or megakaryocytes. Using material from individually selected megakaryoctyes, a strong signal was obtained for Orai1 using qRT-PCR, and the relative gene expression for the different Ca2+-permeable ion channels displayed the order: Orai1>TRPC1>TRPC6>TRPM2 (Fig. 1A). In addition, the transcript for STIM1, the Ca2+ store sensor required for activation of CRAC channels showed a high relative expression in the megakaryocyte. Grosse et al. [17] have also recently shown, using immunohistochemical studies, that STIM1 is highly expressed in murine marrow megakaryocytes. Orai1 and STIM1 gene transcripts were also detected in human platelets at significantly higher levels than TRPC1, TRPC6 and TRPM2 (Fig. 1B). The ratio of Orai1 expression to that of TRPC1 and TRPC6 in the platelet was 36 and 32 fold, respectively. Finally, two human megakaryocytic cell lines, HEL and MEG-01 showed a clear gene expression for STIM1 and Orai1, with a smaller relative signal for TRPC1, whereas TRPC6 or TRPM2 were not detected (Fig. 1C). Taken together, these data demonstrate for the first time a strong expression of Orai1, an essential component of the store-operated CRAC channel [22-24], in the platelet and megakaryocyte. The gene for STIM1, the Ca2+ sensor required for activation of this channel upon store depletion, is also strongly expressed in the platelet and megakaryocyte
Figure 1. Quantitative PCR analysis of expression levels for candidate agonist-evoked Ca2+ entry pathways and STIM1 in human platelets, primary murine megakaryocytes and human megakaryocytic cell lines.
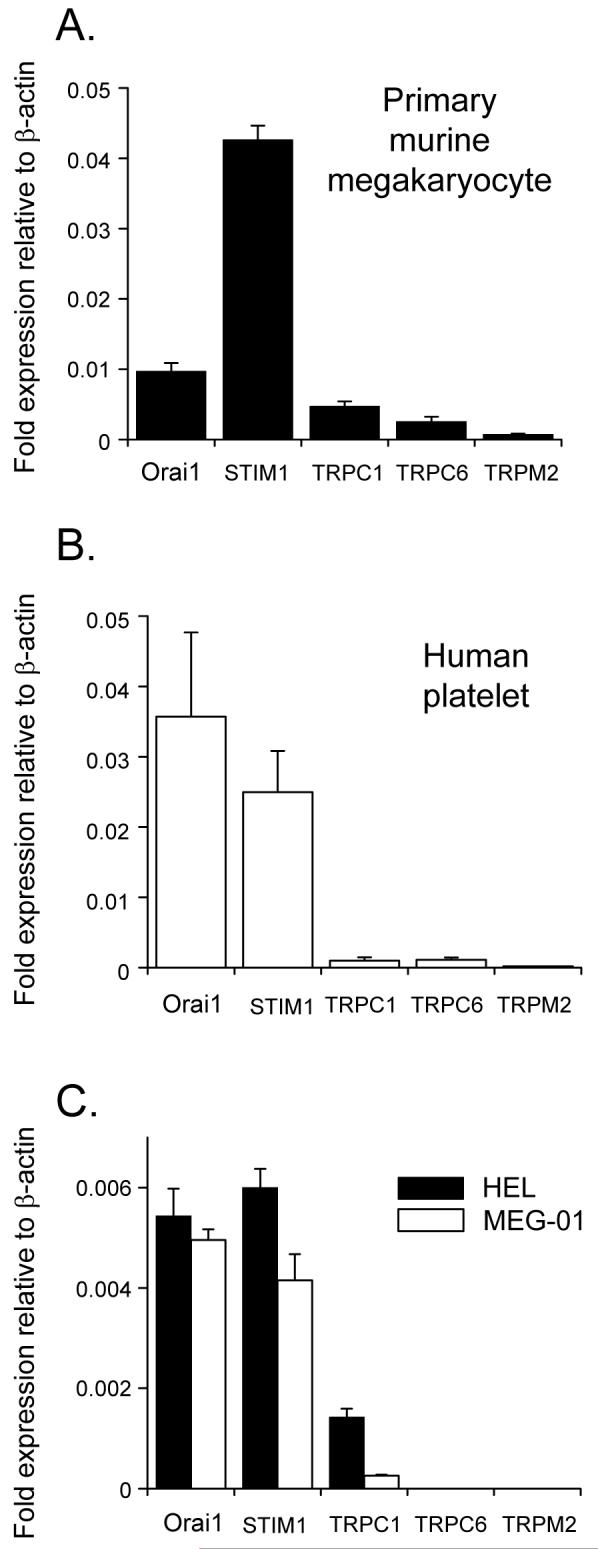
The expression level of each candidate gene is shown relative to β-actin for primary murine megakaryocytes (A), human platelets (B) and two human megakaryocytic cell lines, Meg-01 and HEL (C).
Although CRAC currents have been reported in HEL cells and rat megakaryocytes [25,26], this Ca2+ influx pathway has not been shown in murine megakaryocytes or platelets. Attempts to record store-dependent CRAC currents in thapsigargin-treated human platelets suggested that the underlying channel is below the limit of resolution of the patch clamp technique (Martyn Mahaut-Smith, unpublished observations). This may result from the low current density of this highly Ca2+-selective conductance and the small capacitance of the platelet (≈130 fF; [27]). We therefore further examined the agonist- and store-dependent ionic conductances in the primary murine megakaryocyte, which we and other groups have argued represents a valid model for studies of signalling in the anuclear platelet [28;29]. For example, the unique synergy between Gq and Gi-coupled receptors, leading to inside-out activation of αIIbβ3 receptors that is crucial for platelet function, is well established in the mature megakaryocyte [28;30]. The primary murine megakaryocyte also has the advantage that recent work has electrophysiologically characterised the non-selective cation influx currents coupled to P2Y receptor activation [8;28]. Selective activation of store-dependent over store-independent inward currents was achieved by depletion of intracellular Ca2+ stores with the ionophore ionomycin under high intracellular Ca2+ buffering conditions [31]. This induced, over a period of tens of seconds, a sustained inward current (Fig. 2Ai) with a pronounced inwardly rectifying current-voltage relationship (Fig. 2Aii), typical of CRAC currents recorded in HEL cells, rat megakaryocytes and other non-excitable cell types, including RBL cells and lymphocytes [25;26;31-33]. The very positive reversal potential for this conductance is indicative of a high selectivity for divalent cations. When the pipette saline contained low Ca2+ buffering levels (0.1 mM EGTA), the physiological agonist ADP activated an initial transient current which subsided into a smaller sustained event (Fig. 2Bi), as previously reported [8;28]. This ADP-evoked conductance exhibits a curvilinear relationship, with pronounced outward rectification and a reversal potential near 0mV (Fig. 2Bii) due to its non-selective permeability to cations [8;28]. Based on these characteristics and potentiation by flufenamic acid (FlFnA), we have recently concluded that the majority of the ADP-evoked current is carried through TRPC6, since FlFnA blocks other TRPC channels [8]. Transient currents through autocrine activation of P2X1 receptors are also variably observed during the plateau phase [28], although recordings in which such events were infrequent were used for the present study. ICRAC would also be expected to be activated by ADP, but will be masked by TRPC6 due to the small magnitude of the store-dependent CRAC current at pseudophysiological Ca2+ buffering levels [25;31;32]. Increasing the Ca2+ buffering power led to a more sustained initial ADP-evoked inward current (Fig. 2Ci), with a current-voltage relationship that represents a combination of Fig.2Aii and Fig.2Bii, and a reversal potential part-way between that of TRPC6 and ICRAC (Fig. 2Cii). We do not at present understand why the ADP-evoked current was not as maintained as observed with ionomycin, but one possibility is that this results from part-inactivation of CRAC currents due to partial store refilling, which can occur during agonist stimulation after the initial burst of IP and Ca2+ release. Taken together, these data suggest that physiological agonists will evoke Ca2+ influx through a combination of TRPC6 and CRAC channels. We cannot at present rule out a role for TRPC1, although TRPC1-deficient mice show no significant platelet-related phenotype and thus this channel is not essential for normal platelet function [19]. Further support for a lack of major role for TRPC1 comes from work on megakaryocytic cell lines. TRPC1, but not TRPC6, was detected in HEL and MEG-01 cells lines (Fig. 1C) and a previous study found evidence only for CRAC currents during stimulation by thrombin and store depletion [26]. The importance of TRPM2 in agonist-evoked Ca2+ entry remains to be investigated.
Figure 2. Store- and receptor-operated cation conductances in the primary megakaryocyte.
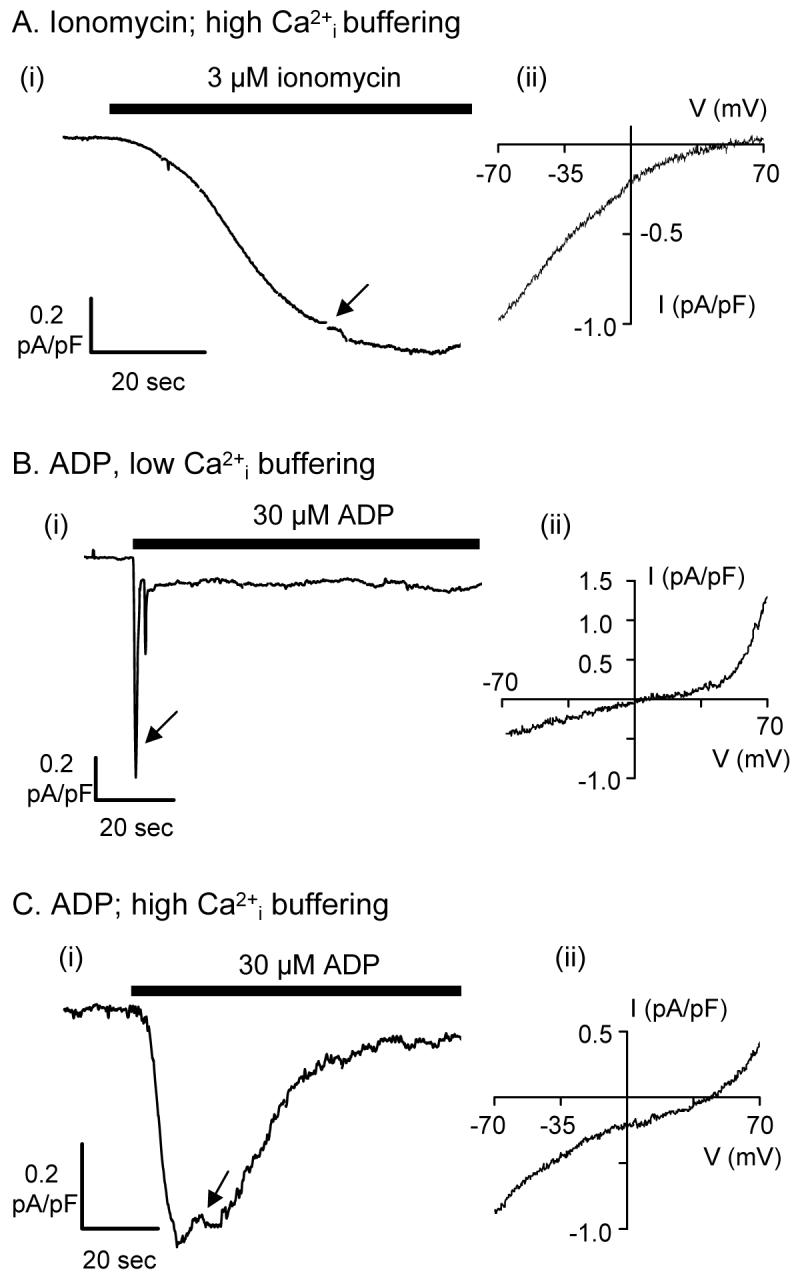
Megakaryocytes were voltage-clamped in the whole cell configuration under conditions designed to eliminate K+ currents (see methods). Left hand panels (i) show continuous recordings of membrane currents at -70mV. Right hand panels (ii) show the current-voltage (I-V) relationship for ionomycin- and ADP-evoked currents obtained using ramps from -70 to +70 mV (at the point indicated by the arrow in (i)). Prestimulus currents have been subtracted. A. With high intracellular Ca2+ buffering, 3 μM ionomycin evoked a slowly developing inward cationic current (i), that displayed the typical inwardly rectifying I-V relationship of ICRAC (ii). B. At low internal Ca2+ buffering, 30 μM ADP evoked an inward cationic current at -70 mV (i), with an outwardly rectifying I-V relationship (ii). C. With high intracellular Ca2+ buffering, 30 μM ADP evoked a more sustained inward current (i) that showed both outward and inward rectification (ii). Traces representative of 3 - 4 cells.
Although CRAC currents are small at normal levels of intracellular Ca2+ buffering, the high selectivity of the channel for Ca2+ under physiological conditions, even over Mg2+, allows significant Ca2+ influx [31-33]. Given the high surface area:volume ratio of the platelet, this pathway will efficiently elevate cytosolic Ca2+, a key signal during platelet activation [1]. Indeed, homozygote mice with an activating point mutation in the EF hand of STIM1 show severe haemorrhaging and exhibit embryonic lethality [17]. The platelets (and T lymphocytes) from heterozygote mice carrying this mutation have elevated basal intracellular Ca2+ concentrations and enhanced background Ca2+ influx, consistent with a role for STIM1 in the calcium entry pathway of these cells. Intracellular Ca2+ increases and functional responses resulting from stimulation of platelet PLCγ-coupled receptors (GPVI and CLEC-2) were compromised, whereas, unexpectedly, signalling via a number of PLCβ-coupled receptors showed no defects. The reasons for the effect on PLCγ rather than PLCβ-coupled receptor responses is unclear, however STIM1 may have other unrecognised roles in platelet function and further studies are required, particularly selective downregulation of STIM and Orai expression in the platelet/megakaryocyte lineage. Nevertheless, a case is emerging for a major role for STIM1 activation of Orai1 in Ca2+ signalling within the platelet and megakaryocyte. This prototypical store-dependent Ca2+ influx pathway may therefore serve as a clinical means to modulate thrombopoiesis and platelet function.
Acknowledgements
This work was funded by the British Heart Foundation (PG/06/028 & FS/03/117).
References
- [1].Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003;1:1602–1612. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- [2].Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989;69:58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- [3].Fung CY, Cendana C, Farndale RW, Mahaut-Smith MP. Primary and secondary agonists can use P2X1 receptors as a major pathway to increase intracellular Ca2+ in the human platelet. J Thromb Haemost. 2007;5:910–917. doi: 10.1111/j.1538-7836.2007.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sage SO, Reast R, Rink TJ. ADP evokes biphasic Ca2+ influx in fura-2-loaded human platelets. Evidence for Ca2+ entry regulated by the intracellular Ca2+ store. Biochem J. 1990;265:675–680. doi: 10.1042/bj2650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Putney JW., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- [6].Hassock SR, Zhu MX, Trost C, Flockerzi V, Authi KS. Expression and role of TRPC proteins in human platelets: evidence that TRPC6 forms the store-independent calcium entry channel. Blood. 2002;100:2801–2811. doi: 10.1182/blood-2002-03-0723. [DOI] [PubMed] [Google Scholar]
- [7].den Dekker E, Molin DG, Breikers G, van Oerle R, Akkerman JW, van Eys GJ, et al. Expression of transient receptor potential mRNA isoforms and Ca2+ influx in differentiating human stem cells and platelets. Biochim Biophys Acta. 2001;1539:243–255. doi: 10.1016/s0167-4889(01)00112-4. [DOI] [PubMed] [Google Scholar]
- [8].Carter RN, Tolhurst G, Walmsley G, Vizuete-Forster M, Miller N, Mahaut-Smith MP. Molecular and electrophysiological characterization of transient receptor potential ion channels in the primary murine megakaryocyte. J Physiol. 2006;576:151–162. doi: 10.1113/jphysiol.2006.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Krotz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- [10].Marcus AJ, Silk ST, Safier LB, Ullman HL. Superoxide production and reducing activity in human platelets. J Clin Invest. 1977;59:149–158. doi: 10.1172/JCI108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- [17].Grosse J, Braun A, Varga-Szabo D, Beyersdorf N, Schneider B, Zeitlmann L, et al. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest. 2007;117:3540–3550. doi: 10.1172/JCI32312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- [19].Varga-Szabo D, Braun A, Bender M, Dietrich A, Nieswandt B. Unaltered Ca2+ homeostasis and agonist-induced activation in platelets lacking trasnient receptor potential (TRP) C1. J Thromb Haemost. 2007;5:P-W-252. [Google Scholar]
- [20].Amisten S, Braun OO, Bengtsson A, Erlinge D. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb Res. 2008;122:47–57. doi: 10.1016/j.thromres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [21].Mahaut-Smith MP. Patch-clamp recordings of electrophysiological events in the platelet and megakaryocyte. Methods Mol Biol. 2004;273:277–300. doi: 10.1385/1-59259-783-1:277. [DOI] [PubMed] [Google Scholar]
- [22].Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- [23].Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Somasundaram B, Mahaut-Smith MP. Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes. J Physiol. 1994;480:225–231. doi: 10.1113/jphysiol.1994.sp020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Somasundaram B, Mason MJ, Mahaut-Smith MP. Thrombin-dependent calcium signalling in single human erythroleukaemia cells. J Physiol. 1997;501:485–495. doi: 10.1111/j.1469-7793.1997.485bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maruyama Y. A patch-clamp study of mammalian platelets and their voltage-gated potassium current. J Physiol. 1987;391:467–485. doi: 10.1113/jphysiol.1987.sp016750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tolhurst G, Vial C, Leon C, Gachet C, Evans RJ, Mahaut-Smith MP. Interplay between P2Y1, P2Y12, and P2X1 receptors in the activation of megakaryocyte cation influx currents by ADP: evidence that the primary megakaryocyte represents a fully functional model of platelet P2 receptor signaling. Blood. 2005;106:1644–1651. doi: 10.1182/blood-2005-02-0725. [DOI] [PubMed] [Google Scholar]
- [29].Shattil SJ, Leavitt AD. All in the family: primary megakaryocytes for studies of platelet αIIbβ3 signaling. Thromb Haemost. 2001;86:259–265. [PubMed] [Google Scholar]
- [30].Gaur M, Kamata T, Wang S, Moran B, Shattil SJ, Leavitt AD. Megakaryocytes derived from human embryonic stem cells: a genetically tractable system to study megakaryocytopoiesis and integrin function. J Thromb Haemost. 2006;4:436–442. doi: 10.1111/j.1538-7836.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- [31].Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- [33].Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]


