Summary
The CD8αβ heterodimer interacts with class I pMHC on antigen-presenting cells as a co-receptor for TCR-mediated activation of cytotoxic T cells. To characterize this immunologically important interaction, we used mAbs specific to either CD8α or CD8β to probe the mechanism of CD8αβ binding to pMHCI. The YTS156.7 mAb inhibits this interaction and blocks T cell activation. To elucidate the molecular basis for this inhibition, the crystal structure of the CD8αβ immunoglobulin-like ectodomains were determined in complex with mAb YTS156.7 Fab at 2.7 Å resolution. The YTS156.7 epitope on CD8β was identified and implies that residues in the CDR1 and CDR2-equivalent loops of CD8β are occluded upon binding to class I pMHC. To further characterize the pMHCI/CD8αβ interaction, binding of class I tetramers to CD8αβ on the surface of T cells was assessed in the presence of anti-CD8 mAbs. In contrast to YTS156.7, mAb YTS105.18, which is specific for CD8α, does not inhibit binding of CD8αβ to class I tetramers, indicating the YTS105.18 epitope is not occluded in the pMHCI/CD8αβ complex. Together, these data indicate a model for the pMHCI/CD8αβ interaction which is similar to that observed for CD8αα in the CD8αα/pMHCI complex, but in which CD8α occupies the lower orientation (membrane proximal to the antigen presenting cell), and CD8β occupies the upper position (membrane distal). The implication of this molecular assembly for the function of CD8αβ in T cell activation is discussed.
Keywords: CD8αβ, TCR co-receptor, antibody complex, T cell, crystal structure
Introduction
The CD8 glycoprotein is essential for the development, activation and biological functions of cytotoxic (CD8+) T cells. CD8 comprises two distinct subunits, CD8α and CD8β, and is expressed as both αβ heterodimers and αα homodimers. CD8αβ is the most abundant isoform on developing thymocytes and circulatory CD8+ T cells.1 CD8αβ interacts with peptide-bound class I major histocompatibility complex (pMHCI) molecules on the surface of antigen-presenting cells to form a tertiary complex with the T cell receptor (TCR) that is a prerequisite for triggering and activation of cytotoxic T lymphocytes (reviewed in 2). Homodimeric CD8αα predominates on the surface of intra-epithelial leucocytes (IEL), as well as on Natural Killer cells (NK cells), and has been further observed on γδ and CD4+ T cells.3 CD8αα also binds pMHCI and this interaction has been characterized from crystal structures of CD8αα with4,5 and without6,7 an MHC class I ligand. However, although soluble CD8αα and CD8αβ IgSF dimers bind soluble pMHCI with equivalent affinity (11–67 μM)8,9, CD8αα fails to compensate for CD8αβ in CD8-dependent TCR activation10 and is approximately 100-fold less effective as a TCR co-receptor.11
Despite the physiological importance of CD8αβ, information concerning how CD8αβ interacts with pMHCI is presently limited to biochemical and mutational analyses,12,13,14 and the structural basis for the enhanced co-receptor activity of CD8αβ remains unclear. Crystallographic analysis of a single chain CD8αβ immunoglobulin superfamily (IgSF) heterodimer has shown that the CD8β globular domain adopts an immunoglobulin-like fold that is similar in structure to that of CD8α and the overall topology of the CD8αβ dimer resembles that of CD8αα.15 The mouse CD8αα/H-2Kb co-crystal structure4 previously revealed a profound asymmetry in the interacting elements of the two CD8α subunits. A single CD8α protomer (designated α1) in the “upper” position, immediately below the α1/α2 peptide-binding platform, accounts for >60% of the total surface area buried upon complex formation and contacts residues in the α1, α2 and α3 domain of pMHCI, as well as β2-microglobulin (β2M). The second CD8α subunit (designated α2) in the “lower” membrane-proximal position (with respect to the antigen-presenting cell) interacts only with the pMHCI α3 domain. Structural complementarity in the CD8αα and CD8αβ ligand-binding domains has lead to speculation that both CD8 isoforms adopt a common mode of interaction with pMHCI but mutagenesis studies have given rise to fundamentally different models in which CD8αβ interacts with pMHCI (H-2Kb) in at least two distinct orientations, such that CD8β would occupy the position of either CD8α subunit in the CD8αα/pMHCI complex.12,14 However, the biological significance of such findings is currently unclear.
Monoclonal antibodies (mAbs) provide a means to probe the physiologically relevant interfaces of interacting proteins which avoids the pitfalls of interpreting results from incorrectly folded or non-expressed proteins that are inherent in mutagenesis studies. In this regard, mAbs have been widely used in the investigation of CD8 activity both in vivo and in vitro. Many CD8-specific mAbs block CD8/pMHCI interaction and inhibit CD8+ T cell activation in vivo through binding to either CD8α (YTS105.18, CT-CD8a, YTS169) or CD8β (YTS156.7, 53.5.8).16,17,18 However, mAb binding does not always abrogate CD8 interaction with pMHCI. Anti-CD8α mAb 53.6.7 and anti-CD8β mAb KT112 actually enhance binding of CD8 to class I tetramers.18,19,20 Hence, elucidation of the mAb epitopes on the surface of CD8 can provide a means to ascertain which regions of CD8 are occluded and which are exposed upon interaction with pMHCI.
To establish the nature of the interaction between class I MHC and CD8αβ, we investigated the effects of different antibodies against CD8αβ upon pMHCI complex formation. The YTS156.7 mAb is a rat IgG2b against mouse CD8β which depletes mouse CD8+ T cells in vivo21 and blocks interaction between CD8αβ and soluble pMHCI in vitro.10 Numerous studies have employed YTS156.7 to investigate CD8αβ-mediated T cell activation and, more recently, to probe the orientation of pMHCI/CD8αβ interactions.14 To determine the molecular basis for YTS156.7.7 activity, a soluble mouse CD8αβ dimer was crystallized in complex with YTS156.7.7 Fab. The structure enabled identification of the YTS156.7 epitope on the surface of CD8β and confirmed that the existing crystal structure of a single-chain mouse CD8αβ IgSF dimer15 was indeed properly configured. Location of the YTS156.7.7 epitope on CD8β implies that residues in the CDR1 and CDR2-eqivalent loops of CD8β are occluded in the pMHCI/CD8αβ complex, either by direct contact with pMHCI, or through steric hindrance.
To determine the disposition of the CD8α subunit in the pMHCI/CD8αβ complex, we investigated the effects of other anti-CD8 mAbs on pMHCI/CD8αβ interaction using tetramers of pMHCI. In this way, we could demonstrate that the mAb YTS105.18 epitope on the surface of CD8α is not occluded in the pMHCI/CD8αβ complex. This finding indicates that CD8α occupies a “lower” position (i.e. more proximal to the antigen-presenting cell (APC)) in the complex, and implies that CD8β occupies a binding site that is more proximal to the peptide-binding groove of class I MHC (i.e. more distal to the APC and, hence, closer to the T cell).
Results
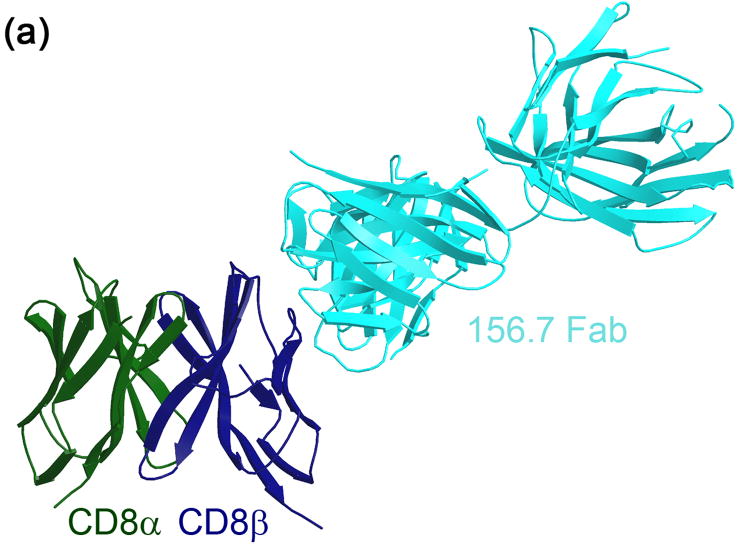
Overview of the CD8αβ/YTS156.7 Fab complex structure
A complex containing the soluble ectodomain of the mouse CD8αβ IgSF dimer and mAb YTS156.7 Fab was crystallized in orthorhombic space group P212121 at pH 6.2 and data collected to 2.7 Å resolution. Preliminary phases were determined by the molecular replacement method and the CD8αβYTS156.7 Fab structure was refined to 2.7 Å resolution. Data collection and refinement statistics are presented in Table 1. The asymmetric unit of the crystal contains two CD8αβ/YTS156.7 Fab complexes (complexes A and B), that are related by a translation along the Y axis. The two complexes are extremely similar and superimposition of all Cα atoms gives an rmsd value of 0.77 Å. Subsequent analyses of CD8αβ/YTS156.7 will, therefore, be confined to only one of the two complexes in the asymmetric unit (complex A, chain IDs LHAB), unless otherwise stated. The structure of the complex is shown in Figure 1a, and comprises one CD8αβ IgSF heterodimer bound to a single YTS156.7 Fab. The refined model contains 659 of the 680 residues that are present in the constructs and includes residues 4–121 of CD8α, 1–117 of CD8β, 1–230 of the YTS156.7 heavy chain and 1–214 of the YTS156.7 light chain. Three residues at the amino-terminus of CD8α as well as residues at the carboxyl-terminus of both CD8 subunits, including the thrombin protease recognition sequence, were not observed in the electron density maps and have not been included in the model.
Table 1.
Crystallographic data and refinement
| Data Processing | |
| Resolution range | 50.0 - 2.70 Å (2.80 - 2.70 Å) |
| Unique reflections | 44,334 (4,468) |
| Completeness | 97.5% (99.8%) |
| Redundancy | 3.3 (3.4) |
| Rsymb | 13.1 % (57.9%) |
| Average I/σ (I) | 8.6 (2.2) |
| Refinement | |
| Reflections | 42,077 (3,110) |
| Reflections (test) | 2,216 |
| Rcrystc | 23.5% |
| Rfreed | 28.4% |
| Residues/protein atoms | 1,320/10,298 |
| Water molecules | 36 |
| Coordinate errore | 0.33 Å |
| Rmsd bonds/angles | 0.015 Å/1.58° |
| Average B values | |
| Complex A (LHAB) | |
| YTS156.7.7 Fab | 68.4 Å2 |
| CD8αβ | 72.0 Å2 |
| Complex B (CDEF) | |
| YTS156.7.7 Fab | 80.2 Å2 |
| CD8αβ | 71.4 Å2 |
| Waters | 39.0 Å2 |
| Carbohydrates | 116.0 Å2 |
| Ramachandran statistics | |
| Most favored | 86.0 % |
| Additionally allowed | 12.9% |
| Generously allowed | 0.9% |
| Disallowed | 0.2%f |
Values in parenthesis refer to the highest resolution shell.
Rsym = 100·ΣhΣi|Ii(h)−<I(h)>|/ΣhI (h), where Ii(h) is the ith measurement of the h reflection and <I(h)> is the average value of the reflection intensity.
Rcryst = Σ|Fo|−|Fc|/Σ|Fo|, where FO and FC are the structure factor amplitudes for the experimental data and from the calculated model, respectively.
Rfree is Rcryst e)with 5% of the test set structure factors.
Values are based on maximum likelihood
Residues ThrL51 and ThrC51 lie within a well-defined γ-turn in the YTS156.7 Fab light chain, as in almost all antibodies.
Figure 1.
Crystal structure of mouse CD8αβ in complex with YTS156.7 Fab. (a) Ribbon diagram of the refined model of the CD8αβ/YTS156.7 Fab complex (complex A). CD8α and CD8β are colored in green and blue, respectively and YTS156.7 Fab is colored cyan. (b) Ribbon representation of the CD8αβ IgSF dimer showing the single carbohydrate moiety at Asnα42, as well as the position of asparagine side chains at the potential N-glycosylation sites at Asnα70 and Asnβ13. The CDR-equivalent loops of CD8αβ are numbered and the YTS156.7 epitope is represented as a molecular surface.
The refined model has Rcryst (23.5%), Rfree (28.4%) and Ramachandran plot values that are within the ranges commonly observed at this resolution22,23 (Table 1). ThrL51 incorrectly is assigned to a disallowed region of the Ramachandran plot according to Procheck,22 as it is located within a perfectly acceptable γ-turn that is observed in almost all antibodies.24 2Fo−Fc electron density is clearly defined at the 1σ level throughout the entire structure, although less-well defined for certain regions of the constant heavy chain (CH1) and constant light chain (CL) of YTS156, which have higher overall B values (83.9 Å2 and 99.1 Å2 for CH1and CL respectively, as compared to 69.7 Å2 for the overall complex), due to limited contacts in the crystal. In particular, Pro127-Ser137 in CH1 form part of a highly flexible loop that is disordered in most Fab crystal structures.25 Furthermore, the paucity of electron density for the CD and EF loops of CH1 indicates flexibility in this region of the structure, which lies within a solvent-filled cavity in the crystal.
Conformational flexibility in CD8αβ is restricted to a few distinct regions, as indicated by reduced quality of the 2Fo−Fc electron density map and higher B values in these regions. A schematic representation of the B values of Cα residues in CD8αβ is given in supplemental Figure 3. Overall, the electron density in the CD8β subunit is of higher quality than for CD8α, which does not contact YTS156.7, and is reflected by a higher B value (79.4 Å2 for CD8α, as compared to 64.6 Å2 for CD8β). The C′C and C″D loops of both CD8α and CD8β are highly flexible and exhibit weaker electron density, and correspondingly higher B values. Furthermore, Lysα1-Asnα3 that comprise the N-terminus of CD8α are disordered, as observed in other CD8 structures, and have been omitted from the model.
Structure of the CD8αβ heterodimer
The mouse CD8αβ IgSF dimer described here represents the crystal structure of a non-covalently associated CD8αβ heterodimer, albeit in complex with YTS156.7 Fab. The CD8α and CD8β constructs used in expression of our soluble CD8αβ both lack the connecting glycopeptides at the carboxyl terminus (31 and 27 residues for CD8α and CD8β, respectively) that couple the CD8 globular IgSF ectodomains to the T cell membrane. The CD8α and CD8β subunits are representative of the immunoglobulin V-set family, each comprising nine β-strands which form the characteristic anti-parallel, β-sheet. CD8α and CD8β heterodimerize through pairing of their respective C′ and G strands, such that the CD8αβ dimer resembles the antigen binding (Fv) region of antibodies (Figure 1b). Indeed, the B–C, C′-C″ and F–G loops of both CD8 subunits are spatially equivalent to the hyper-variable complementarity-determining region (CDR) 1, 2 and 3 loops of antibodies, respectively. In mouse CD8α, these loops comprise residues Glyα30-Glyα35 (CDR1-equivalent), Tyrα55-Lysα62 (CDR2-equivalent) and Valα104-Tyrα111 (CDR3-equivalent). The analogous loops on CD8β comprise Serβ24-Serβ30 (CDR1-equivalent), Trpβ51-Glyβ56 (CDR2-equivalent) and Thrβ98-Valβ105 (CDR3-equivalent). The CDR1-equivalent loop of CD8α (Glyα30-Glyα35) has a higher B value (93.1 Å2), as compared to CD8α as a whole (79.3 Å2), that indicates greater flexibility. In comparison, the CDR1-equivalent loop in CD8β forms part of the YTS156.7 epitope and is constrained to a single conformation. The CDR3-equivalent regions of both CD8α and β subunits are notably short compared with those normally found in antibodies, and the position and orientation of these loops are both fixed in the dimer interface.
Of the three potential N-linked glycosylation sites in CD8αβ, only one in CD8α, at Asnα70, shows well-defined electron density for a single N-acetyl glucosamine (GlcNAc) moiety, which occupies the pocket created between the C-C′ loop and E–F loop. The amide nitrogen of the GlcNAc hydrogen bonds with the carbonyl oxygen of Gluα95, forming a bridge which stabilizes the relative orientation of the C-C′ and E–F loops. Despite a lack of electron density for N-carbohydrates at the other two N-glycosylation motifs in CD8αβ (Asnα42 and Asnβ13), enzymic deglycosylation of each CD8 IgSF domain revealed the presence of carbohydrates in both subunits, indicating sugars are, indeed, attached to CD8β at Asn13 (data not shown). This N-glycosylation motif, in the A–B loop of CD8β, is conserved in both human and mouse and precludes interaction between pMHCI and this region of the molecule. The lack of interpretable electron density for carbohydrates at this site in the CD8αβ/YTS156.7 structure indicates a high degree of conformational flexibility and implies that N-carbohydrates do not have a direct influence on the structure of CD8β per se.
The structural aspects of the CD8αβheterodimer have previously been addressed in the crystal structure of a single-chain CD8αβ construct, which mimics the physiological form of the molecule, but employs a 29 amino-acid linker to connect the subunits. Comparison of the single-chain CD8αβ model with our CD8αβ heterodimer structure indicates that they are structurally homologous, and superimpose with rmsd values of 0.85 Å and 1.27 Å for 118 and 115 Cα atoms of the α and β subunits, respectively (see supplemental Figure 4). Given that the single-chain CD8αβ model (PDB ID 2ATP15) was not utilized for molecular replacement in structural determination of the CD8αβ/YTS156.7 complex structure, a high degree of complementarity between these structures confirms the validity of both models. In both structures, the extended N-terminus of CD8α projects into the solvent and is highly flexible; as a consequence, Lysα1, Proα2 and Glnα3 have been omitted from both models due to lack of interpretable electron density. These same residues are also disordered in the crystal structure of CD8αα in the absence of a class I binding partner,6 but are ordered in the interface between one CD8α subunit and pMHCI in the co-crystal structure.4,26 The most notable structural deviations between the two models occur in loop regions of the β subunit, specifically in the C-C′ and C″-D loops, which are flexible and exhibit high B values in both structures. Furthermore, conformational plasticity is indicated by alternative conformations in the solvent-exposed C-C′ loop of CD8β (Glnβ38-Aspβ42), as well as in the flexible C″-D loops of both CD8α (Gluα67-Leuα74) and CD8β (Gluβ61-Asnβ68) in both structures, consistent with relatively high B values in both models. The B–C (CDR1-equivalent) loop of CD8β is fixed by interaction with YTS156.7 and has a low B value, in contrast to the equivalent loop in the single-chain structure, which is shifted by 6 Å and exhibits high B values, indicative of significant flexibility in this loop. Importantly, high homology is observed in both the conformation and B values of residues in the F–G (CDR3-equiavalent) loops of both CD8α and CD8β in both models, suggesting these loops are more ordered and less flexible than the CDR1-equivalent B–C loops.
Despite the different roles of the two isoforms of CD8, the overall structure of the CD8αβ IgSF dimer closely resembles that of CD8αα. Comparison with CD8αα,6 by superimposition of 112 Cα atoms in the CD8α subunit of each model, illustrates the overall similarity of the two isoforms of the receptor (Figure 2a). Importantly, heterodimerization with the β subunit does not confer conformational changes on CD8α and the structural features of CD8α are highly conserved in both isoforms. Furthermore, the dimerization interfaces in both CD8αβ and CD8αα are highly conserved, with equivalent surface complementarity (Sc) values of 0.77 and 0.73, respectively.27 Comparison of the individual CD8α and CD8β IgSF domains indicates both subunits are topologically similar, despite low sequence identity (12%).28 Superimposition of 60 corresponding Cα atoms from the β-sheets within the Ig cores of both subunits produces an rmsd value of 1.1 Å and reveals striking similarity in the overall architecture of the α and β subunits (Figure 2b). The CD8β IgSF domain (117 residues) is smaller than that of CD8α (125 residues) and one of the most profound structural differences is in the length of the A strands, which extend 11 and 5 residues in CD8α and CD8β respectively, prior to the characteristic β-bulge. The similar topology of both subunits, together with conservation of the interacting elements within the CD8α/CD8β dimerization interface, accounts for the similar architectures of the CD8αα and CD8αβ IgSF dimers. Furthermore, spatial and conformational homology in the CDR-equivalent loops of both subunits likely accounts for the similarity in the pMHCI-binding characteristics of both CD8αα and CD8αβ and provides evidence for an equivalent mechanism of interaction.
Figure 2.
Comparison of mouse CD8αβ and CD8αα IgSF dimers. (a) Comparison of the CD8αβ and CD8αα IgSF dimers. Superimposition of CD8αβ onto CD8αα from the YTS105.18 complex structure (PDB ID 2ARJ6), by aligning the Cα atoms in CD8α, illustrates complementarity in the dimerization interface of both CD8 isoforms and overall structural similarity in the two receptors. CD8αα is colored maroon and CD8αβ is colored green/blue. (b) Stereo representation of the CD8α and CD8β IgSF domains, superimposed using the Cα atoms in the β-strands of each subunit. CD8α is colored green and CD8β is in blue. The N and C termini of each subunit are labeled for clarity.
The YTS156.7 Fab epitope
The YTS156.7 epitope is restricted to the CD8β subunit and is centered over the D–E loop, roughly at 45° to the plane of the CD8αβ dimerization interface (Figure 1a). The Fab interface has a shape complementarity (Sc) value of 0.76,27 which is in the upper range of values reported for antigen-antibody complexes,24 and buries a total surface area of 1,479 Å2, incorporating 717 Å2 on CD8β and 761 Å2 on YTS156.7.29 Interaction involves all six complementarity-determining regions (CDRs) of the Fab, with the majority (69%) of the buried surface being contributed from the heavy chain, as commonly observed in antibody-antigen interactions.30
The 2Fo−Fc electron density map is of excellent quality in the region of the YTS156.7 combining site. YTS156.7 contacts 27 residues on the surface of CD8β, including five from the B–C (CDR1-equivalent) loop and six from the C′-C″ (CDR2-equivalent) loop (Figure 3). The interface comprises three salt-bridge interactions (AspL92 to Argβ77, AspL92 to Argβ78 and AspH32 to Lysβ27), and 11 hydrogen bonds, as well as numerous van der Waals contacts, which are predominantly mediated by TyrL32, TrpH52 and TyrH99. The primary interaction surface centers on Argβ77 and Argβ78 in the D–E loop of CD8β, which hydrogen bond with TyrL32 and ThrL93, and salt-bridge to AspL92. CDR H1 contacts residues in the C-C′ loop and CDR H2 inserts within the cleft between the C″/D strands of CD8βand CDR H3.
Figure 3.
The YTS156.7epitope. Expanded view of the molecular surface buried by YTS156.7 on CD8β, showing the residues that contact YTS156.7. The CDR1 and CDR2-equivalent loops of CD8β are involved in this interaction.
Effects of mAbs on CD8αβ binding to tetramers of class I pMHCI
To probe the interacting elements between CD8αβ and class I pMHC, we evaluated the effects of YTS156.7 and two other anti-CD8 mAbs, YTS105.18 and 53.6.7, on binding of tetramers to OT-1 T cells transgenic for a T cell receptor specific for class I MHC H-2Kb and the SIINFEKL (OVA) peptide. It has been demonstrated that binding of tetramers of H-2Kb-OVA to TCR at the surface of OT-1 splenocytes is dependent upon the participation of CD8,18 and the OT-1 system is, therefore, ideal for monitoring the effects of mAbs upon CD8/pMHCI interaction. We assessed the effects of mAbs YTS156.7.7, YTS105.18 and 53.6.7 on T cell activation by measurement of induced Ca2+ mobilization following stimulation of OT-1 splenocytes with tetramers of H-2Kb-OVA. YTS105.18 and 53.6.7 are both rat IgG2a mAbs specific for mouse CD8α. In the absence of mAbs against CD8, exposure of OT-1 cells to tetramers of H-2Kb-OVA gave rise to high levels of calcium flux and subsequent T cell activation, whereas exposure to tetramers of H-2Kb loaded with the control peptide SIYRYYGL (SIYR) produced no measurable calcium flux (Figure 4a). Incubation of OT-1 T cells with YTS156.7 mAb prior to tetramers of H-2Kb-OVA completely blocked calcium flux, indicating inhibition of pMHCI/CD8 interaction. In contrast, incubation of OT-1 T cells with mAb YTS105.18 had no effect upon pMHCI/CD8 interaction, and exposure of these cells to H-2Kb-OVA tetramers gave rise to high levels of calcium flux, equivalent to that observed for the control cells, indicating that this mAb does not inhibit productive pMHCI/CD8 interactions. Prior incubation of OT-1 T cells with mAb 53.6.7 produced enhanced calcium flux in the presence of H-2Kb-OVA tetramers, suggesting that this mAb induced elevated T cell activation. To exclude the possibility that the observed effects of mAbs on T cell activation were influenced by mAb-mediated co-localization (cross-linking) of surface receptors, calcium flux measurements were repeated in the presence of mAb Fabs. Results obtained for each of the mAb Fabs were equivalent to those obtained for each of the intact IgGs, respectively (Figure 4b), indicating the observed effects of mAbs upon T cell activation are independent of Fc-mediated activities. These data were confirmed by direct measurement of tetramer binding to OT1 splenocytes in the presence of varied concentrations of each of the mAbs (supplemental Figure 5).
Figure 4.
YTS156.7.7, but not YTS105.18, blocks MHC class I and CD8 interaction and subsequent T cell activation. (a) Ca2+ mobilization in OT-I RAG− splenocytes treated with H-2Kb tetramers loaded with the OVA peptide (H-2Kb/OVA) after pre-staining with mAbs 53.6.7 (red line), YTS105.18 (purple line) or YTS156.7.7 (green line). The tetramers were added at 1 min (arrow) and the sample re-analyzed for a further 4 min. The H-2Kb/OVA tetramer trace is taken from a 5 min time course and overlaid here as a representation of a responding population. Response to the H-2Kb/OVA tetramers in the absence of CD8 antibodies is shown (blue line), as well as response to H-2Kb/SIYR tetramers (black line) as a negative control. All traces indicate the median of the responding population. (b) Equivalent plot to show relative Ca2+ mobilization in OT-I RAG− splenocytes treated with H-2Kb/OVA tetramers after pre-staining now with only the Fabs of mAbs 53.6.7 (red line), YTS105.18 (purple line) or YTS156.7.7 (green line). The baselines for all traces are normalized to the response to H-2Kb/OVA tetramers in the absence of any Fabs (blue line).
Taken together these findings imply that, whilst all three mAbs bind to regions of CD8 that are exposed at the surface of resting T cells, the YTS156.7 epitope is occluded during pMHCI/CD8αβ binding, whereas both the 53.6.7 and YTS105.18 epitopes remain exposed. The ability of YTS156.7 Fab to block pMHCI/CD8 interaction, and thereby inhibit T cell activation, is consistent with the renowned biological activity of this mAb in vivo. In contrast, the finding that YTS105.18 does not inhibit this interaction is surprising, given that this mAb has been associated with the blockade of T cell activation in vivo.16,31 However, the molecular mechanism for this inhibition has not been investigated and our in vitro data imply that the reported inhibitory effects of this mAb in vivo could occur through an indirect mechanism that is dependent upon the organization of molecules within the intercellular contact zone. Specifically, the absence of the antigen-presenting cell membrane and the spatial restraints of the intercellular contact zone in our tetramer-binding experiments represent a fundamental difference that may account for this inconsistency. In this regard, it is also important to note that the inhibitory activity of YTS156.7, conversely, is entirely independent of membrane-associated effects. The finding that mAb 53.6.7 induces an increased level of T cell activation is consistent with previous observations19; however, the molecular basis for this mechanism has yet to be established.
Mechanism of YTS156.7 activity and orientation of the pMHCI/CD8αβ complex
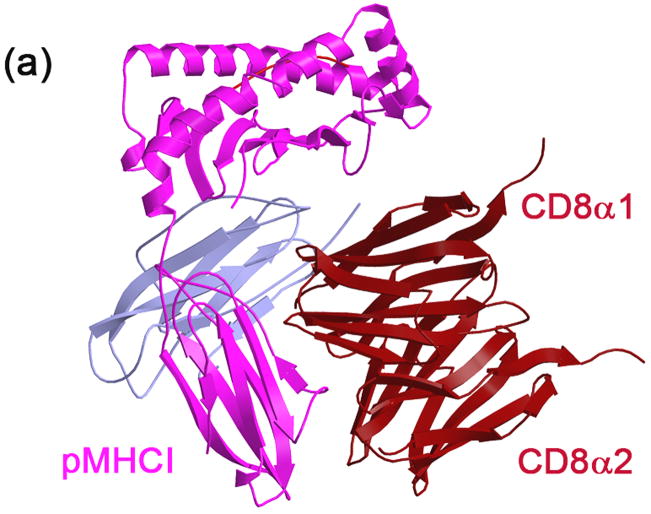
The structural basis for CD8αβ/YTS156.7 interaction, together with the biological activity of the YTS156.7 Fab, provides insight to the interaction of CD8αβ with pMHCI. Comparison of CD8αβ from the single-chain structure with that from our CD8αβ/YTS156.7 complex structure indicates both structures are highly similar and confirms that YTS156.7 does not induce conformational changes in CD8 that may inhibit pMHCI binding. Hence, inhibition of pMHCI/CD8αβ by YTS156.7 Fab implies that the YTS156.7 epitope, which includes residues within CDR-equivalent loops 1 and 2, as well as residues in the B, D and E strands of CD8β (Figure 3), overlaps with the binding site of pMHCI, or that YTS156.7 Fab precludes pMHCI/CD8αβ complex formation by steric clash with the pMHCI. Structural similarity in the CD8αα and CD8αβ IgSF domain dimers, as well as existing mutagenesis data,14 suggest that both CD8 isoforms interact with pMHCI in an approximately equivalent manner, such that CD8αβ binds to the acidic C–D loop on the side of pMHCI, below the antigen-presenting groove. In the CD8αα/H-2Kb co-crystal structure, interaction of the two CD8 subunits is asymmetric, with one subunit in an “upper” (α1) position and one subunit in a “lower” (α2) position (Figure 5a). To determine whether our data fit such a mode of binding, we created two models of the pMHCI/CD8αβ complex, based on the orientation of CD8αα in the CD8αα/H-2Kb structure, in which CD8β occupies either the α1 or α2 position when bound to pMHCI. Modeling of the interaction in this way indicates that, if CD8β occupies the α1 position, binding of YTS156.7 would inhibit pMHCI/CD8αβ interaction through a substantial steric clash with the pMHCI (Figure 5b). In the alternative model, in which CD8αβ binds pMHCI with CD8β in the α2 position, a clash between the B–C (CDR 2) loop of YTS156.7 VH and the A–B loop of the pMHCI α3 domain would also inhibit PMHCI/CD8αβ interaction (Figure 5c). Both of these models are consistent with the experimentally-determined activity of the YTS156.7 Fab and, hence, the location of the YTS156.7 epitope on CD8β supports a model in which both CD8αα and CD8αβ interact with pMHCI in an equivalent manner. Furthermore, our finding that mAb YTS105.18 Fab does not inhibit interaction between CD8αβ and pMHCI then provides additional information regarding the disposition and orientation of the CD8α subunit. The experimentally determined effects of YTS105.18 upon pMHCI/CD8αβ interaction indicate that, unlike YTS156.7, the YTS105.18 epitope is exposed in the pMHCI/CD8αβ complex. The interacting elements between mouse CD8α and the rat antibody YTS105.18 have been identified in the CD8αα/YTS105.18 Fab co-crystal structure.6 The two potential models for the pMHCI/CD8αβ complex, in which CD8α occupies an α1 or α2 position, respectively, indicate that inhibition of pMHCI/CD8αβ interaction by mAb YTS105.18 would occur only if CD8αβ binds pMHCI with CD8α in the α1 position (Figure 5d), whereas YTS105.18 Fab would not block pMHCI/CD8αβ interactions in which CD8α adopts an α2 position (Figure 5e). In summary, the finding that mAb YTS156.7 inhibits binding of soluble tetramers of class I pMHC to CD8αβ at the surface of T cells, whereas mAb YTS105.18 does not, indicates that both isoforms of CD8 interact with pMHCI in an equivalent manner, but that CD8β occupies a position equivalent to the α1 position observed in the CD8αα/H-2Kb co-crystal structure.
Figure 5.
Models of the pMHCI/CD8αβ complex, based on the CD8αα/H-2Kb co-crystal structure, illustrate the influence of mAbs YTS156.7 and YTS105.18 and indicate CD8α binds at, or near, the α2 position. (a) Ribbon diagram of the CD8αα/H-2Kb complex (PDB ID 1BQH4), illustrating the relative orientation of the CD8α α1 and α2 subunits. pMHCI is colored magenta, β2-microglobulin (β2M) is colored mauve and CD8αα is colored maroon. (b) Combination of the CD8αα/pMHCI and CD8αβ/YTS156.7 Fab structures by superimposition of Cα atoms in CD8α provides two alternative models for the YTS156.7/pMHCI/CD8αβ interaction, dependent upon the relative disposition of CD8β in the pMHCI/CD8αβ complex. If CD8β binds to pMHCI in the upper (α1) position, YTS156.7 would inhibit pMHCI/CD8αβ interaction by a molecular clash with the pMHCI α1 and α2 domains. (c) In the alternate model, CD8β would occupy the lower (α2) position and inhibition would occur through clash between the Fab and the AB loop of pMHCI α3. pMHCI is colored magenta, β2M is colored mauve, YTS156.7 Fab is colored cyan and CD8α and CD8β are colored green and blue, respectively. (d) An equivalent model, representing the experimental effects of mAb YTS105.18 Fab upon CD8αβ interaction with soluble pMHCI. Models were created using the CD8αα/pMHCI (PDB ID 1BQH4) and CD8α/YTS105.18 Fab (PDB ID 2ARJ6) structures. Superimposition of Cα atoms in the CD8α component of both models indicates that, if CD8α binds to pMHCI in the α1 position, YTS105.18 would inhibit pMHCI/CD8αβ interaction by a molecular clash with the pMHCI α1 and α2 domains. (e) If CD8α binds to pMHCI in the lower α2 position, YTS105.18 would have no effect upon interaction of CD8αβ with soluble pMHCI, consistent with the results of our tetramer binding assay.
Conclusions
The significance of the CD8β subunit for CD8 function is well documented, although the molecular basis for the enhanced co-receptor function of CD8αβ over that of CD8αα has yet to be fully elucidated. The extremely low affinity (~11–67 μM)8,9 of the class I pMHC/CD8αβ interaction, coupled with the instability of soluble recombinant CD8αβ proteins has rendered analysis of the pMHCI/CD8 αβ interaction a significant technical challenge. In the absence of a pMHCI/CD8αβ co-crystal structure, ongoing efforts have focused on alternative means to determine the nature of the pMHCI/CD8αβ interaction. To this end, mutagenesis studies, as well as mAbs against CD8 that inhibit or enhance interactions with pMHCI, have been applied to probe binding of CD8αβ with pMHCI.12,13,14,19,32 Analysis of the CD8β-specific inhibition of (CD8+) T cell activation and suppression of immune function by mAb YTS156.7 is tantamount to probing the biological significance of the β subunit. Hence, the CD8αβ/YTS156.7 co-crystal structure presented here provides valuable information pertaining to CD8+ T cell biology. Structural analysis of our physiological CD8αβ IgSF dimer confirms the previously determined structure of a single-chain CD8αβ construct and the analysis of the YTS156.7 epitope on CD8β identifies, or severely restricts the choice of, residues on CD8β that are involved in interaction of CD8αβ with class I pMHC. These findings provide a framework for further studies of CD8 involving mAb YTS156.7.7 interactions both in vivo and in vitro. Moreover, our data provide insights into the relative orientation of the CD8α and CD8β subunits in binding to pMHCI. Models of the pMHCI/CD8αβ complex, based upon the existing CD8αα/H-2Kb co-crystal structure, illustrate how YTS156.7 inhibits interaction between CD8αβ and pMHCI through a molecular clash and indicate that both CD8 isoforms do, indeed, adopt a similar mechanism of interaction with pMHCI. Furthermore, the finding that the YTS105.18 epitope is not occluded in the functional pMHCI/CD8αβ complex confirms that CD8α occupies a lower (α2) position, such that CD8β occupies an upper (α1) position that places the CD8β subunit proximal to the T cell membrane. This model for CD8αβ/pMHCI interaction is consistent with the findings of mutational and functional analyses.15 Given the structural similarities between the CD8αα and CD8αβ IgSF dimers, as well as the convincing support for a model in which both isoforms bind pMHCI in an equivalent manner, it is interesting to speculate as to the physiological differences between CD8αβ and CD8αα. There is clear evidence that CD8αα alone can provide some co-receptor activity, albeit less profound than that of CD8αβ, and given that CD8αα and CD8αβ IgSF dimers bind to soluble class I molecules with equivalent affinity, it is likely that observed differences in the functional activity of the two isoforms of CD8 are primarily mediated by the different binding characteristics of the homodimeric and heterodimeric receptors at the T cell surface. One such difference may result from diversity in the length of the extended stalk region, which is invariably shorter in CD8β than in CD8α and likely produces a bowed conformation in the CD8αβ stalk at the cell surface. In light of the fact that CD8α contacts the “lower” site on class I pMHC that is more proximal to the surface of antigen presenting cells, a bowed conformation in the stalk may position the CD8αβ IgSF dimer more optimally for interaction with class I pMHCI at the surface of antigen presenting cells and may account for the enhanced co-receptor activity over that of CD8αα. On the other hand, the stalk region of homotypic CD8αα is unlikely to produce a bowed conformation and may position the CD8αα IgSF dimer less optimally for binding to the side of class I pMHC. This concept is consistent with the observation that the stalk region of CD8β is critical to the enhanced co-receptor function of CD8αβ,33 and may explain the finding that changes to oligosaccharides in the stalk of CD8β can influence CD8αβ function.32,34,35 However, in the absence of a detailed analysis of the interacting elements between class I pMHC and CD8αβ, the molecular basis for the enhanced co-receptor function of CD8αβ remains an enigma and must await a pMHCI/CD8αβ complex crystal structure.
Materials and Methods
Soluble CD8αβ expression and purification
Expression constructs for soluble mouse CD8α and CD8β were generated as described previously for soluble mouse CD8αα.6 Briefly, soluble CD8αβ dimers (sCD8αβ) were expressed in insect cells as a heterodimer of their IgSF domains, comprising residues 1–123 of the mature CD8α and residues 1–117 of the mature CD8β subunits. Specific pairing between α and β subunits was achieved by addition of acidic or basic leucine-zipper motifs at the carboxyl terminus of each respective subunit which preclude expression of homodimeric CD8 species which would readily form otherwise. A hexa-histidine motif was also incorporated at the carboxyl terminus of each subunit to facilitate protein purification (see supplemental Figure 1). Both constructs were cloned into the pRMHa3 inducible expression vector, used previously in the expression of soluble CD8 proteins,6 and co-transfected at equimolar ratio into D. melanogaster SC2 cells. Stable cell lines were maintained in Schneider’s media (BioWhittaker/Lonza), containing 500 μg/ml gentamycin sulphate (G-418) (BioWhittaker/Lonza). Large-scale cell culture (16 L) was carried out in 2 L disposable roller bottles (Corning) at 28°C, using Insect Express serum-free insect cell medium (BioWhittaker/Lonza). Protein expression was induced with addition of 700 μM CuSO4 for three days and sCD8αβ was then purified from concentrated supernatant by metal affinity chromatography using Ni-NTA beaded-agarose resin (Qiagen). Subsequent purification was carried out by size exclusion gel filtration chromatography, using an AKTA FPLC system fitted with a HiLoad Superdex 200 16/60 column (GE Healthcare), in 25 mM Hepes, pH 7.8, 150 mM NaCl, and 0.04% sodium azide. The pooled protein fractions were concentrated to 12 mg/ml using a Centricon concentrator device with a 30 kDa MW-cut off (Millipore). Protein concentrations were measured using the Coomassie Plus Bradford assay (Pierce Biotechnology).
Preparation of YTS156.7.7 Fab
The YTS156.7.7 hybridoma was obtained as a kind gift from Dr H. Waldman, Sir William Dunn School of Pathology, University of Oxford, UK. Cells were cultured in RPMI media (Gibco-Invitrogen), containing 5% FCS, 100 mM L-glutamine, 25 mM Hepes pH 7.5 and 100 mM MEM non-essential amino acid solution (Gibco-Invitrogen). Large-scale antibody production was carried out using an Integra CL 1000 cell factory (Integra Biosciences) and IgG was purified from cell-free tissue culture supernatant by affinity chromatography using agarose-beaded protein G (Sigma-Aldrich). The YTS156.7 Fab fragment was isolated by digestion with 10 ng papaya papain (Sigma-Aldrich) protease/mg IgG for 4 hours at 37°C, and purified from the Fc fragment by affinity chromatography using agarose-beaded protein A (Sigma-Aldrich). The amino-acid sequence of the YTS156.7 Fab was determined by RT-PCR, using mRNA extracted from YTS156.7.7 clone (hybridoma) cells and subsequent sequencing of the resulting cDNA.
Purification of the CD8αβ/YTS156.7.7 Fab complex
YTS156.7 Fab bound sCD8αβ with nanomolar affinity (see Supplemental Figure 2) and the CD8αβ/YTS156.7 Fab complex was purified to homogeneity by size exclusion chromatography using an AKTA FPLC fitted with a HiLoad Superdex 200 10/30 column (GE Healthcare) in 25 mM Hepes, pH 7.8, 150 mM NaCl, and 0.04% sodium azide. Subsequent removal of the carboxyl-terminal, leucine-zipper and hexa-histidine motifs from sCD8αβ was carried out by digestion with bovine thrombin protease (Calbiochem) using 3 U/mg enzyme for 3 hours at 37°C. Finally, the CD8αβ/YTS156.7 Fab complex was purified again by size exclusion gel filtration, using an AKTA FPLC system fitted with a HiLoad Superdex 200 10/30 column (GE Healthcare), in 25 mM Hepes, pH 7.8, 150 mM NaCl, and 0.04% sodium azide.
Crystallization and data collection
Crystallization experiments were carried out using the sitting drop, vapor diffusion method at a protein concentration of 12 mg/ml. Each drop contained 0.45 μl of protein mixed with an equal quantity of precipitant solution. Crystals of CD8αβ/YTS156.7 Fab were obtained from a solution containing 10% polyethylene glycol (PEG 8,000), 0.1 M Na/K phosphate, 0.2 M NaCl, pH 6.2, at 23°C. Crystals were flash cooled to 95 K in a cryo-protectant containing precipitant supplemented with 25% glycerol, and a dataset was collected to 2.7 Å from a single crystal at the Stanford Synchrotron Radiation Laboratory (SSRL) beamline 11-1. Data were integrated and scaled using Denzo and Scalepack, as implemented in the HKL2000 suite.36 CD8αβ/YTS156.7 Fab crystallized in space group P212121, with unit cell dimensions a = 91.8 Å, b = 92.7 Å, c = 190.3 Å, α= β, =γ, = 90°. Data collection and processing statistics are detailed in Table 1.
Structural determination, refinement and validation of the CD8αβ/YTS156.7.7 Fab complex
The CD8αβ/YTS156.7 Fab structure was determined by molecular replacement (MR) with the program Phaser.37 Rat mAb YTS105.18 Fab from our CD8αα/YTS105.18 complex structure (PDB ID 2ARJ6) was utilized as a search model for the rat mAb YTS156.7 Fab and the CD8αα component from the same structure was utilized as a search model for CD8αβ. Preliminary phases were initially determined for the YTS156.7 Fab. These coordinates were fixed and a subsequent round of MR determined a solution for CD8αβ, which packed without clashes. The solution was confirmed by rigid-body refinement using the program Refmac-5,38 as implemented in the CCP4 program suite.
Model building, using the program O,39 and energy-minimization refinement, using Refmac-5, was carried out until the refinement converged. Rcryst (25.4%) and Rfree (28.5%) did not improve further due to pseudo-translational symmetry of the molecules in the asymmetric unit. Water molecules were identified from residual density greater than 2.0 in the 2Fo−Fc electron density map and were added if within hydrogen-bond distance of the protein.
Validation of geometry and overall quality of the final model was carried out using Molprobity,40 as well as the JCSG validation suite, incorporating the programs Procheck,22 and Whatcheck.41 All structural alignments and RMSD calculations were carried out using the McLachlan algorithm,42 as implemented in the program ProFit. Figures were generated using the program MOLSCRIPT43 and Pymol.44
Preparation of peptides and MHC tetramers
Peptides OVA (SIINFEKL) and SIYR (SIYRYYGL) were synthesized by Anaspec (San Jose, CA). Production and purification of the soluble form of H-2Kb was performed as described previously.45 Briefly, plasmids encoding the H-2Kb heavy chain with the BirA recognition sequence at the carboxyl-terminus and mouse β2M were transformed and over-expressed in D. melanogaster SC2 cells. The protein was purified by anion exchange chromatography on a Resource-Q column (GE Healthcare) and size exclusion gel filtration using a HiLoad Superdex 200 10/30 column (GE Healthcare). Following biotinylation using BirA (Avidity), the monomeric MHCI complexes (loaded with an endogenous peptide(s) acquired from the expression system46) were purified via a second round of size exclusion gel filtration using a HiLoad Superdex 200 10/30 column. The efficiency of biotinylation was assessed by sequential immunoprecipitation with avidin-conjugated beads (Pierce Biotechnology) and was estimated to be 95%. After concentration of the appropriate-sized fractions, biotinylated H-2Kb was mixed with streptavidin (SA)–PE (Biosource Inc) at a 4:1 molar ratio in the presence of a 10-fold excess of added peptide (OVA, SIYR) which was of sufficiently high affinity (~nM) to readily displace the endogenous peptide(s). Finally, tetramers of H-2Kb-peptide complexes were purified on a Superdex 200 PC 3.2/30 column (GE Healthcare).
Tetramer Staining and Calcium flux measurements
Isolated splenocytes from OT-I transgenic mice were treated with 0.165 M NH4Cl to remove red blood cells and washed in complete medium. For tetramer staining, OT-I splenocytes were first incubated in 5 μg/ml Fc-Block (BD-Pharmingen) at 4°C for 15 min then stained for 1 hr at 4°C with 50 nM H-2Kb/OVA PE-conjugated tetramers or with H-2Kb/SIYR PE-conjugated tetramers and washed in FACS buffer (PBS 2% FCS, 25 mM Hepes, 2 mM EDTA) before staining with FITC-conjugated anti-CD4 (BD-Pharmingen) and APC-conjugated anti-CD90.2 53.2.1 (eBioscience) mAbs. Lymphocytes were finally washed and re-suspended in FACS buffer and tetramer staining was measured as a function of mean fluorescence. For inhibition experiments, cells were incubated in dilutions of purified anti-CD8 antibodies (anti-CD8α YTS105.18, anti-CD8β YTS156.7.7, anti-CD8α 53-6.7, anti-CD8β 53-5.8 (BD-Pharmingen) and anti-CD8α KT15 (Serotec)) in FACS buffer for 15 min at 4°C and then washed prior to tetramer staining. To measure binding of anti-CD8 antibodies, 50% of the cells were incubated with a PE-conjugated secondary antibody (Donkey anti-Rat IgG, eBioscience) instead of tetramers and washed before subsequent tetramer staining.
For Ca2+ mobilization experiments, purified OT-I RAG− splenocytes were then incubated for 30 min at 37°C with 10 μg/ml of the Ca2+-sensitive dye Indo-1 (Molecular Probes) in RPMI medium (Gibco-Invitrogen) containing 1% FCS, 10 mM Hepes and 1% L-Glutamine. Cells were washed 3 times and re-suspended in Ca2+-containing medium (HBSS supplemented with 10 mM Hepes and 1% FBS). Splenocytes were first incubated in 5 μg/ml FcBlock (BD-Pharmingen) at 4°C for 15 min to saturate any unspecific binding sites before staining with PE-conjugated anti-CD4 RM4-5 (BD-Pharmingen) and APC-conjugated anti-CD90.2 53.2.1 (eBioscience) mAbs at 4°C for 15 min to isolate the CD4− T cells. Lymphocytes were finally washed and re-suspended in Ca2+-containing medium. Before stimulation, cells were warmed for 5 min in a 37°C water bath, and then analyzed on an LSR II flow cytometer (BD Biosciences). For stimulation, baseline Ca2+ was measured for 1 min, followed by the addition of purified tetramers at a final concentration of 50 nM. Ca2+ influx was measured for 5 min. For positive and negative controls, cells were stimulated either with PBS, ionomycin 1 μg/ml (Calbiochem) or 10 μg/ml anti-CD3 2C11.145 (BD-Pharmingen) followed by cross-linking with 50 μg/m anti-hamster Ig (Jackson ImmunoResearch Labs). For analysis of the effect of anti-CD8 antibodies upon tetramer binding, lymphocytes were pre-incubated at 4°C with the antibodies for 15 min and washed. Ca2+ flux results are presented as the ratio of Indo1-Violet (bound to Ca2+) against Indo1-Blue (unbound) over time. All data were acquired on an LSR II flow cytometer (BD Biosciences) and analyzed with the Flow-Jo software (Tree Star). Figures represent signal for CD8+ T cells, identified by gating on CD90.2+ CD4− T cells.
Protein Data Bank accession code
Coordinates and structure factors for the CD8αβ/YTS156.7 Fab co-crystal structure have been deposited in the RCSB Protein Data Bank with RCSB accession number RCSB045249 and PDB ID code 3B9K.
Supplementary Material
Schematic representation of the mouse CD8α and CD8β IgSF expression constructs used in the production of soluble CD8αβ (sCD8αβ). The complete aminoacid sequence of each construct is also given. Amino acids within the mature CD8α and CD8β protein are shown in green and blue, respectively. Potential N-glycosylation sequences within each construct are boxed.
sCD8αβ was determined as having correctly folded native structure by binding to a panel of monoclonal antibodies specific for the CD8α (YTS105.18 and YTS169) and CD8β subunit (YTS156.7), as determined by surface plasmon resonance (SPR) using a BIAcore 2,000 instrument (BIAcore). mAbs YTS105.18 and YTS169 came from our laboratory (L.T.). Approx. 10,000 response units (RU) of anti-rat IgG Fc (Pierce Biotechnology) was bound to a CM-5 sensor chip by standard amine conjugation. mAbs were passed over the surface of individual flow cells in PBS until an equivalent amount (approx. 250–350 RU) had been captured in each flow cell. Soluble CD8αβ IgSF was subsequently passed over each flow cell in the buffer at concentrations of 0, 3.25, 7.5, 15 and 30 nM. Both CD8 and mAbs were stripped from the surface by injection of 20 mM HCl following each injection.
Stereo view of the Cα trace of CD8αβ colored according to B value. Cα atoms with B values around the average for the main-chain of CD8αβ (70.6 Å2) are colored green, those below are blue and those above are yellow-red. Colors are interpolated according to B value, as indicated on the color bar.
Stereo view of the Cα trace of CD8αβ from the YTS156.7 complex structure overlaid with that from the single-chain structure (PDB ID 2ATP15). CD8αβ from the single-chain structure are in cyan and CD8α and CD8β from the YTS156.7 complex structure are in magenta and green, respectively. CD8αβ IgSF dimers superimpose with an rmsd of 0.85 Å and 1.27 Å for 118 and 115 Cα atoms of the α and β subunits, respectively. Significant deviations occur only in loop regions of the CD8β structures. Superimposition was carried out along Cα atoms in the β-strand regions of each subunit.
(a) OT-I splenocytes were incubated with a panel of antibodies specific for mouse CD8α (KT12, 53.6.7, YTS105.18) or CD8β (YTS156.7, 53.5.8), before being stained with 50 nM H-2Kb/OVA PE-tetramers (dark line, right panel), or with a PE-conjugated secondary antibody (dark line, left panel). The staining with tetramers of H-2Kb/OVA (right, dark grey) and tetramers of H-2Kb/SIYR (right, light grey) in absence of anti-CD8 antibody, as well as the background of the secondary antibody (left, light grey), are shown as controls. (b) Mean fluorescence of H-2Kb/OVA PE-tetramer staining on OT-I splenocytes in the presence of increasing concentrations of anti-CD8. The experiment was performed as in (a).
Acknowledgments
This work was supported by NIH grants AI042266 and CA58896 to I.A.W. and AI042267 to L.T. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. We thank Professor Raymond Dwek for helpful advice and encouragement, Sharon Ferguson, for assistance with large-scale tissue culture, Dr Xiaoping Dai as well as the staff at the SSRL beamline 11-1 for assistance with data collection and Drs Xueyong Zhu and Robyn Stanfield (TSRI) for assistance with structural determination. This is manuscript number 19190 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norment AM, Littman DR. A second subunit of CD8 is expressed in human T cells. EMBO J. 1988;7:3433–3439. doi: 10.1002/j.1460-2075.1988.tb03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 3.Gangadharan D, Cheroutre H. The CD8 isoform CD8αα is not a functional homologue of the TCR co-receptor CD8αβ. Curr Opin Immunol. 2004;16:264–270. doi: 10.1016/j.coi.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Kern PS, Teng MK, Smolyar A, Liu JH, Liu J, Hussey RE, Spoerl R, Chang HC, Reinherz EL, Wang JH. Structural basis of CD8 coreceptor function revealed by crystallographic analysis of a murine CD8αα ectodomain fragment in complex with H-2Kb. Immunity. 1998;9:519–530. doi: 10.1016/s1074-7613(00)80635-4. [DOI] [PubMed] [Google Scholar]
- 5.Gao GF, Tormo J, Gerth UC, Wyer JR, McMichael AJ, Stuart DI, Bell JI, Jones EY, Jakobsen BK. Crystal structure of the complex between human CD8α(α) and HLA-A2. Nature. 1997;387:630–634. doi: 10.1038/42523. [DOI] [PubMed] [Google Scholar]
- 6.Shore DA, Teyton L, Dwek RA, Rudd PM, Wilson IA. Crystal structure of the TCR co-receptor CD8αα in complex with monoclonal antibody YTS 105.18 Fab fragment at 2.88 Å resolution. J Mol Biol. 2006;358:347–354. doi: 10.1016/j.jmb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Leahy DJ, Axel R, Hendrickson WA. Crystal structure of a soluble form of the human T cell coreceptor CD8 at 2.6 Å resolution. Cell. 1992;68:1145–1162. doi: 10.1016/0092-8674(92)90085-q. [DOI] [PubMed] [Google Scholar]
- 8.Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, Teyton L. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 9.Kern P, Hussey RE, Spoerl R, Reinherz EL, Chang HC. Expression, purification, and functional analysis of murine ectodomain fragments of CD8αα and CD8αβ dimers. J Biol Chem. 1999;274:27237–27243. doi: 10.1074/jbc.274.38.27237. [DOI] [PubMed] [Google Scholar]
- 10.McNicol AM, Bendle G, Holler A, Matjeka T, Dalton E, Rettig L, Zamoyska R, Uckert W, Xue SA, Stauss HJ. CD8α/α homodimers fail to function as co-receptor for a CD8-dependent TCR. Eur J Immunol. 2007;37:1634–1641. doi: 10.1002/eji.200636900. [DOI] [PubMed] [Google Scholar]
- 11.Witte T, Spoerl R, Chang HC. The CD8β ectodomain contributes to the augmented coreceptor function of CD8αβ heterodimers relative to CD8αα homodimers. Cell Immunol. 1999;191:90–96. doi: 10.1006/cimm.1998.1412. [DOI] [PubMed] [Google Scholar]
- 12.Devine L, Thakral D, Nag S, Dobbins J, Hodsdon ME, Kavathas PB. Mapping the binding site on CD8β for MHC class I reveals mutants with enhanced binding. J Immunol. 2006;177:3930–3938. doi: 10.4049/jimmunol.177.6.3930. [DOI] [PubMed] [Google Scholar]
- 13.Devine L, Sun J, Barr MR, Kavathas PB. Orientation of the Ig domains of CD8αβ relative to MHC class I. J Immunol. 1999;162:846–851. [PubMed] [Google Scholar]
- 14.Chang HC, Tan K, Hsu YM. CD8αβ has two distinct binding modes of interaction with peptide-major histocompatibility complex class I. J Biol Chem. 2006;281:28090–28096. doi: 10.1074/jbc.M604931200. [DOI] [PubMed] [Google Scholar]
- 15.Chang HC, Tan K, Ouyang J, Parisini E, Liu JH, Le Y, Wang X, Reinherz EL, Wang JH. Structural and mutational analyses of a CD8αβ heterodimer and comparison with the CD8αα homodimer. Immunity. 2005;23:661–671. doi: 10.1016/j.immuni.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Qin SX, Wise M, Cobbold SP, Leong L, Kong YC, Parnes JR, Waldmann H. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur J Immunol. 1990;20:2737–2745. doi: 10.1002/eji.1830201231. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Cobbold S, Metcalfe S, Waldmann H. Tolerance in the mouse to major histocompatibility complex-mismatched heart allografts, and to rat heart xenografts, using monoclonal antibodies to CD4 and CD8. Eur J Immunol. 1992;22:805–810. doi: 10.1002/eji.1830220326. [DOI] [PubMed] [Google Scholar]
- 18.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devine L, Hodsdon ME, Daniels MA, Jameson SC, Kavathas PB. Location of the epitope for an anti-CD8α antibody 53.6.7 which enhances CD8α-MHC class I interaction indicates antibody stabilization of a higher affinity CD8 conformation. Immunol Lett. 2004;93:123–130. doi: 10.1016/j.imlet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature. 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 21.Wei Y, Chen K, Sharp GC, Yagita H, Braley-Mullen H. Expression and regulation of Fas and Fas ligand on thyrocytes and infiltrating cells during induction and resolution of granulomatous experimental autoimmune thyroiditis. J Immunol. 2001;167:6678–6686. doi: 10.4049/jimmunol.167.11.6678. [DOI] [PubMed] [Google Scholar]
- 22.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 23.Kleywegt GJ, Brunger AT. Checking your imagination: applications of the free R value. Structure. 1996;4:897–904. doi: 10.1016/s0969-2126(96)00097-4. [DOI] [PubMed] [Google Scholar]
- 24.Wilson IA, Stanfield RL. Antibody-antigen interactions: new structures and new conformational changes. Curr Opin Struct Biol. 1994;4:857–867. doi: 10.1016/0959-440x(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 25.Sheriff S, Jeffrey PD, Bajorath J. Comparison of CH1 domains in different classes of murine antibodies. J Mol Biol. 1996;263:385–389. doi: 10.1006/jmbi.1996.0582. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Xiong Y, Naidenko OV, Liu JH, Zhang R, Joachimiak A, Kronenberg M, Cheroutre H, Reinherz EL, Wang JH. The crystal structure of a TL/CD8αα complex at 2.1 Å resolution: implications for modulation of T cell activation and memory. Immunity. 2003;18:205–215. doi: 10.1016/s1074-7613(03)00027-x. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence MC, Colman PM. Shape complementarity at protein/protein interfaces. J Mol Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 28.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connolly ML. The molecular surface package. J Mol Graph. 1993;11:139–141. doi: 10.1016/0263-7855(93)87010-3. [DOI] [PubMed] [Google Scholar]
- 30.Stanfield RL, Wilson IA. Antigen-induced conformational changes in antibodies: a problem for structural prediction and design. Trends Biotechnol. 1994;12:275–279. doi: 10.1016/0167-7799(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 31.Parish NM, Bowie L, Zusman Harach S, Phillips JM, Cooke A. Thymus-dependent monoclonal antibody-induced protection from transferred diabetes. Eur J Immunol. 1998;28:4362–73. doi: 10.1002/(SICI)1521-4141(199812)28:12<4362::AID-IMMU4362>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Daniels MA, Devine L, Miller JD, Moser JM, Lukacher AE, Altman JD, Kavathas P, Hogquist KA, Jameson SC. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15:1051–1061. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 33.Wong JS, Wang X, Witte T, Nie L, Carvou N, Kern P, Chang HC. Stalk region of β-chain enhances the coreceptor function of CD8. J Immunol. 2003;171:867–874. doi: 10.4049/jimmunol.171.2.867. [DOI] [PubMed] [Google Scholar]
- 34.Moody AM, Chui D, Reche PA, Priatel JJ, Marth JD, Reinherz EL. Developmentally regulated glycosylation of the CD8αβ coreceptor stalk modulates ligand binding. Cell. 2001;107:501–512. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- 35.Moody AM, North SJ, Reinhold B, Van Dyken SJ, Rogers ME, Panico M, Dell A, Morris HR, Marth JD, Reinherz EL. Sialic acid capping of CD8β core 1-O-glycans controls thymocyte-major histocompatibility complex class I interaction. J Biol Chem. 2003;278:7240–7246. doi: 10.1074/jbc.M210468200. [DOI] [PubMed] [Google Scholar]
- 36.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 37.McCoy AJ. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr D Biol Crystallogr. 2007;63:32–41. doi: 10.1107/S0907444906045975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 39.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 40.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, 3rd, Snoeyink J, Richardson JS, Richardson DC. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooft RW, Vriend G, Sander C, Abola EE. Errors in protein structures. Nature. 1996;381:272. doi: 10.1038/381272a0. [DOI] [PubMed] [Google Scholar]
- 42.McLachlan AD. Rapid comparison of protein structures. Acta Crystallogr A. 1982;38:871–873. [Google Scholar]
- 43.Kraulis PJ. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- 44.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; Palo Alto, CA, USA: 2002. http://www.pymol.org. [Google Scholar]
- 45.Matsumura M, Fremont DH, Peterson PA, Wilson IA. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992;257:927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- 46.Stura EA, Matsumura M, Fremont DH, Saito Y, Peterson PA, Wilson IA. Crystallization of murine major histocompatibility complex class I H-2Kb with single peptides. J Mol Biol. 1992;228:975–982. doi: 10.1016/0022-2836(92)90881-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of the mouse CD8α and CD8β IgSF expression constructs used in the production of soluble CD8αβ (sCD8αβ). The complete aminoacid sequence of each construct is also given. Amino acids within the mature CD8α and CD8β protein are shown in green and blue, respectively. Potential N-glycosylation sequences within each construct are boxed.
sCD8αβ was determined as having correctly folded native structure by binding to a panel of monoclonal antibodies specific for the CD8α (YTS105.18 and YTS169) and CD8β subunit (YTS156.7), as determined by surface plasmon resonance (SPR) using a BIAcore 2,000 instrument (BIAcore). mAbs YTS105.18 and YTS169 came from our laboratory (L.T.). Approx. 10,000 response units (RU) of anti-rat IgG Fc (Pierce Biotechnology) was bound to a CM-5 sensor chip by standard amine conjugation. mAbs were passed over the surface of individual flow cells in PBS until an equivalent amount (approx. 250–350 RU) had been captured in each flow cell. Soluble CD8αβ IgSF was subsequently passed over each flow cell in the buffer at concentrations of 0, 3.25, 7.5, 15 and 30 nM. Both CD8 and mAbs were stripped from the surface by injection of 20 mM HCl following each injection.
Stereo view of the Cα trace of CD8αβ colored according to B value. Cα atoms with B values around the average for the main-chain of CD8αβ (70.6 Å2) are colored green, those below are blue and those above are yellow-red. Colors are interpolated according to B value, as indicated on the color bar.
Stereo view of the Cα trace of CD8αβ from the YTS156.7 complex structure overlaid with that from the single-chain structure (PDB ID 2ATP15). CD8αβ from the single-chain structure are in cyan and CD8α and CD8β from the YTS156.7 complex structure are in magenta and green, respectively. CD8αβ IgSF dimers superimpose with an rmsd of 0.85 Å and 1.27 Å for 118 and 115 Cα atoms of the α and β subunits, respectively. Significant deviations occur only in loop regions of the CD8β structures. Superimposition was carried out along Cα atoms in the β-strand regions of each subunit.
(a) OT-I splenocytes were incubated with a panel of antibodies specific for mouse CD8α (KT12, 53.6.7, YTS105.18) or CD8β (YTS156.7, 53.5.8), before being stained with 50 nM H-2Kb/OVA PE-tetramers (dark line, right panel), or with a PE-conjugated secondary antibody (dark line, left panel). The staining with tetramers of H-2Kb/OVA (right, dark grey) and tetramers of H-2Kb/SIYR (right, light grey) in absence of anti-CD8 antibody, as well as the background of the secondary antibody (left, light grey), are shown as controls. (b) Mean fluorescence of H-2Kb/OVA PE-tetramer staining on OT-I splenocytes in the presence of increasing concentrations of anti-CD8. The experiment was performed as in (a).