Abstract
We have previously shown that the ability of buprenorphine to activate the opioid receptor-like (ORL1) receptor compromises its antinociceptive effect. Furthermore, morphine has been shown to alter the level of orphanin FQ/nociceptin (OFQ/N), the endogenous ligand of the ORL1 receptor, raising the possibility that the endogenous OFQ/N/ORL1 receptor system may be involved in the actions of these opioids. Thus, using mice lacking the ORL1 receptor and their wild-type littermates, the present study assessed the role of the ORL1 receptor in psychomotor stimulant and rewarding actions of buprenorphine and morphine. Morphine (5, 10 mg/kg) dose-dependently increased motor activity and induced conditioned place preference. However, the magnitude of each response was comparable for the mutant mice and their wild-type littermates. In contrast, buprenorphine (1 mg/kg) induced greater motor stimulation in ORL1 receptor knockout mice as compared to their wild-type littermates. Further, single conditioning with buprenorphine (3 mg/kg) induced place preference in mutant mice but not in their wild-type littermates. The results of binding assay showed that buprenorphine concentration-dependently (0–1000 nM) displaced specific binding of [3H]-orphanin FQ/nociceptin (OFQ/N) in brain membrane of wild-type mice. Together, the present results suggest that the ability of buprenorphine to interact with the ORL1 receptor modulates its acute motor stimulatory and rewarding effects.
Keywords: Buprenorphine, Morphine, ORL1 receptor (NOP), Knockout Mouse, Conditioned Place Preference (CPP), Motor Activity
Introduction
Buprenorphine, an opioid with mixed agonist/antagonist activity at the opioid receptors, is used clinically as an analgesic (Finco et al., 1995; Picard et al., 1997) and for the treatment of opiate dependency (Jasinski et al., 1978; Fudala et al., 1990; Johnson et al., 1995; Kuhlman, Jr. et al., 1998; Greenwald et al., 1999; Heit and Gourlay, 2003; Johnson et al., 2003). Buprenorphine is described as a partial agonist at the mu opioid receptor (Martin et al., 1976). While the sub-maximal dose-response curve of buprenorphine can be explained by its ability to act as a partial agonist at the mu opioid receptor, its bell-shaped dose-response curves cannot be described by this property of the drug. Thus, other mechanisms may be involved which need to be elucidated.
Previous studies have shown that buprenorphine interacts with the opioid receptor-like (ORL1) receptor (Martin et al., 1976; Wnendt et al., 1999; Hawkinson et al., 2000; Bloms-Funke et al., 2000; Huang et al., 2001; Lutfy et al., 2003). Interestingly, orphanin FQ/nociceptin (OFQ/N), the endogenous agonist ligand of the ORL1 receptor (Reinscheid et al., 1995; Meunier et al., 1995), is considered as an anti-opioid peptide in the brain (for review, see (Mogil and Pasternak, 2001), raising the possibility that the mu opioid receptor-mediated actions of buprenorphine could be altered by its ability to interact with the ORL1 receptor. Consistent with this hypothesis, we have previously shown that the antinociceptive effect of buprenorphine mediated via the mu opioid receptor (Kamei et al., 1995; Kamei et al., 1997; Lutfy et al., 2003) was enhanced in mice lacking the ORL1 receptor (Lutfy et al., 2003). We have also demonstrated that the descending part of the bell-shaped dose-response curve of buprenorphine disappeared in wild-type mice treated with J-113397, an ORL1 receptor antagonist (Lutfy et al., 2003). Together, these results suggest that the activity of buprenorphine at the ORL1 receptor plays a functional role in the unique pharmacology of buprenorphine. However, it is not known whether this modulatory role of the ORL1 receptor is limited to the antinociceptive effect of buprenorphine or it could be generalized to other actions of the drug. Thus, using mice lacking the ORL1 receptor and their wild-type littermates, the present study was designed to determine the role of this receptor system in buprenorphine-induced motor stimulation and conditioned place preference (CPP), an animal model of reward (Bardo and Bevins, 2000).
Previous studies have shown that intracerebroventricular OFQ/N administration blocks morphine-induced CPP (Murphy et al., 1999; Ciccocioppo et al., 2000) and decreases the ability of morphine to increase extracellular dopamine in the nucleus accumbens in rats (Di Giannuario et al., 1999). Chronic morphine treatment has been shown to increase the level of OFQ/N-immunoreactivity in some brain regions in the rat (Yuan et al., 1999), raising the possibility that the endogenous OFQ/N/ORL1 receptor system may also be involved in the rewarding and addictive properties of morphine. Thus, we also assessed the role of the ORL1 receptor in the motor stimulatory and rewarding actions of morphine.
Experimental Procedures
Animals and husbandry
Male ORL1 knockout (Nishi et al., 1997) and their wild-type littermates (3–4 months) were the offspring of ORL1 heterozygous breeding pairs backcrossed on C57BL/6J mice for six generations. Mice were housed 2–4 per cage with free access to food and water and maintained under a 12-h light/12-h dark cycle. All experiments were conducted according to the National Institute of Health (NIH) guideline for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences (Pomona, CA, USA).
Drugs
Morphine sulfate and buprenorphine hydrochloride were purchased from Sigma/Aldrich (St Louis, MO, USA) and dissolved in normal saline (0.9% NaCl in sterile water). The doses are as the salt form of the drugs. The choice of the doses of each drug was based on our published results showing that the antinociceptive effect of buprenorphine but not morphine was altered in mice lacking the ORL-1 receptor (Lutfy et al., 2003).
Motor activity test
Distance traveled (cm) was recorded using a Videomex-V system (Columbus Instruments, Inc.; Columbus, Ohio, USA) and used as a measure of motor activity. To determine the role of the ORL1 receptor in motor stimulation induced by buprenorphine, mice lacking ORL1 receptor and their wild-type littermates were habituated to motor activity chambers (14 cm length × 14 cm width × 22 cm height) for one hour, injected with saline or buprenorphine (1 mg/kg, s.c.) and motor activity was recorded for one hour (4 × 15-min epochs). In a separate set of experiments, the motor stimulatory action of morphine (0, 5 or 10 mg/kg, s.c.) was also assessed.
CPP test
The description of the CPP apparatus is provided elsewhere (Marquez et al., 2006). In brief, the CPP apparatus was a three-chambered compartment: a central smaller neutral grey chamber (16.5 cm length × 16.5 cm width × 26.5 cm height) and two conditioning chambers (26.5 cm length × 16.5 cm width × 26.5 cm height) distinguishable by visual (decorated with 1-inch black and white horizontal or vertical stripes) and olfactory (almond or orange scent) cues. Mice lacking the ORL1 receptor and their wild-type littermates were tested for baseline place preference toward the CPP chambers on day 1. On this day, each mouse was individually placed in the neutral central grey chamber and allowed to freely explore all three chambers of the CPP apparatus for 15 minutes. The amount of time that the mice spent in each chamber was recorded. On day 2, mice were injected with saline or buprenorphine (3 mg/kg, s.c.) and confined to the vehicle-paired or drug-paired chamber for one hour, respectively. We used one hour confinement to the conditioning chambers since our motor activity data revealed that the action of both buprenorphine and morphine lasted at least for one hour. On day 3, mice were treated with the alternate treatment and confined to the opposite conditioning chamber for one hour. On each conditioning day, immediately following placement of the mice in the conditioning chambers, 10 µL of pure almond or orange scent (McCormick, Hunt Valley, DM, USA) were dispensed on a filter paper (2 cm × 2 cm) hanging on the upper corner (19 cm above the floor) of the conditioning chambers. Every attempt was made to balance the assignment of the mice to the treatments and conditioning chambers including the olfactory and visual cues. On day 4, mice were tested for postconditioning place preference in which each mouse was placed in the neutral central grey chamber and allowed to freely explore all the CPP chambers for 15 minutes. The amount of time that the mice spent in the conditioning chambers was recorded and used for data analysis. In a separate set of experiments, the rewarding action of morphine (5 or 10 mg/kg, s.c.) was also determined.
Binding assay
Mice were sacrificed, their brain were removed and homogenized in Tris buffer (50 mM, pH = 7.4). The homogenates were spun at 18,000 g for 15 min and the pellets were resuspended in 40 volumes of buffer and spun again. The pellets were resuspended in 40 volumes of buffer and incubated at 25°C for 30 min and spun for 15 min for the third time. The pellets were then resuspended in 40 volumes of buffer and assayed in triplicates for 3H-OFQ/N (5 nM) binding in the presence and absence of buprenorphine (0, 1, 10, 100 or 1000 nM). Non-specific binding was defined in the presence of non-labeled OFQ/N (5 µM).
Statistical Analysis
Data are expressed as mean ± S.E.M. Dose-response data were analyzed using one- or two-factor analysis of variance (ANOVA). Time-course data were analyzed using repeated measures ANOVA. The post-hoc Student-Newman-Keuls or Tukey-Compromise test was used to reveal significant differences between various groups. A p<0.05 was considered statistically significant.
Results
Motor activity test
Figure 1 illustrates the motor stimulatory action of buprenorphine in mice lacking the ORL1 receptor and their wild-type littermates. Although the saline-treated mice displayed a higher level of basal motor activity as compared to buprenorphine-treated mice during habituation, there was no significant difference in basal motor activity between wild-type and mutant mice during habituation or in response to saline injection (compare saline-treated ORL1 knockout mice to their wild-type littermates). Buprenorphine increased motor activity in both genotypes (Fig. 1; compare buprenorphine-treated mice of each genotype to their respective saline-treated controls). A two-factor (genotype and treatment) ANOVA of the total distance traveled during the entire one hour test period revealed a significant effect of treatment (saline versus buprenorphine; F1,35 = 36.63, p<0.001), but no significant effect of genotype (F1,35 = 1.81, p>0.05) or interaction between treatment and genotype (F1,35 = 2.26, p>0.05). Further analysis of the data showed that buprenorphine increased motor activity to a greater extent in mutant mice as compared to their wild-type littermates (p<0.05). On the other hand, the motor stimulatory action of morphine was not altered in ORL1 knockout mice (Fig. 2). There was no difference in basal motor activity between the morphine-treated and saline-treated groups during the habituation period. Also, there was no significant difference in basal motor activity between the mutant mice and their wild-type littermates following saline injection Fig. 2; (compare saline-treated ORL1 knockout mice to saline-treated wild-type mice). A two-factor ANOVA (genotype and morphine dose) of the collapsed data (total distance traveled during the entire one hour test period following saline or morphine) revealed a significant effect of morphine dose (F2,39 = 38.71, p<0.001) but no significant effect of genotype (F1,39 = 0.12; p<0.05) or interaction between time and genotype (F1,39 = 0.05; p>0.05). Further analysis of the data showed that morphine dose-dependently increased motor activity in both mutant mice and their wild-type littermates [(p<0.05; compare morphine (10 mg/kg) and morphine (5 mg/kg) versus saline for each genotype as well as morphine (10 mg/kg) versus morphine (5 mg/kg)]. However, the magnitude of this response was not altered in mice lacking the ORL1 receptor (p>0.05; compare mutant mice to their wild-type littermates at each morphine dose).
Fig. 1. The motor stimulatory action of buprenorphine was enhanced in mice lacking the ORL1 receptor.
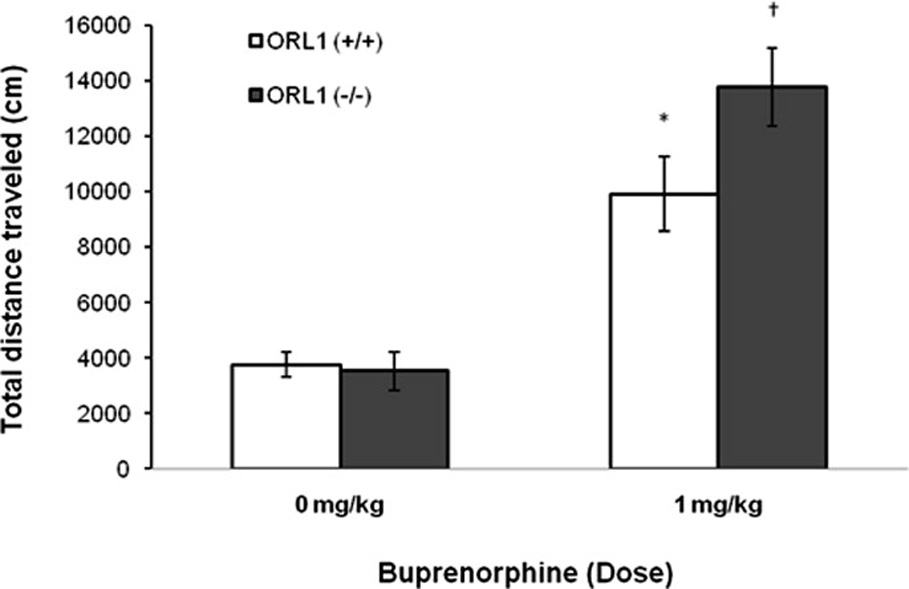
Mice lacking the ORL1 receptor [ORL1 (−/−)] and their wild-type littermates [ORL1 (+/+)] were habituated to motor activity chambers for one hour, then injected with saline (n = 7 mice/genotype) or buprenorphine (1 mg/kg, s.c.; n = 12–13 mice/genotype) and motor activity was recorded for an additional one hour (4 ×15-min bins). Data are presented as mean (±SEM) of total distance traveled (cm) by the mutant mice and their wild-type littermates during the entire one hour test period. *indicates a significant increase as compared to its respective saline-treated control group (p<0.05); †indicates a significant increase as compared to all other groups (p<0.05).
Fig. 2. The motor stimulatory action of morphine was not altered in mice lacking the ORL1 receptor.
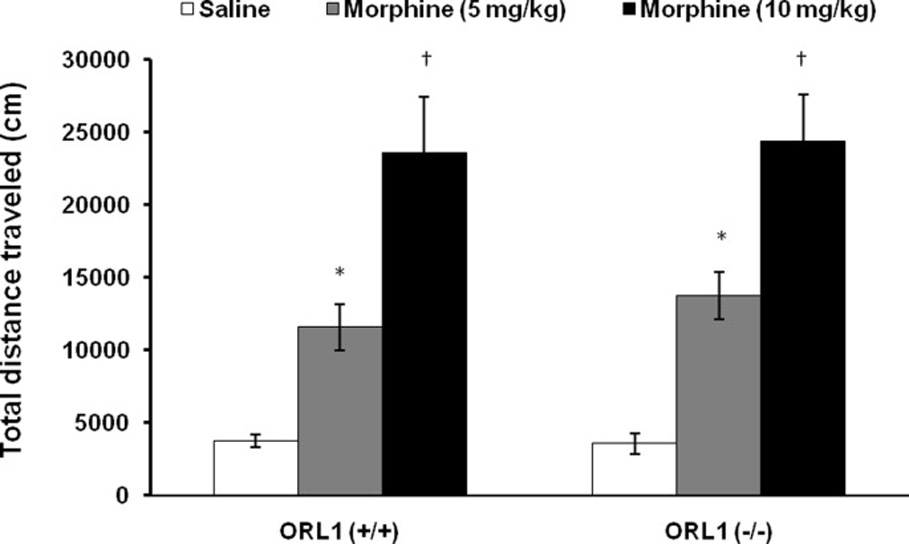
Mice lacking the ORL1 receptor [ORL1 (−/−)] and their wild-type littermates [ORL1 (+/+)] were habituated to motor activity chambers for one hour, then injected with morphine (0, 5 or 10 mg/kg, s.c.) and motor activity was recorded for an additional one hour (4 × 15-min bins; Fig. 2A). Data are presented as mean (±SEM) of the entire one hour test period of 7–8 mice per genotype. *indicates a significant increase as compared to their respective saline-treated groups (p<0.05). †indicates a significant increase as compared to saline- and morphine (5 mg/kg)-treated groups (p<0.05).
CPP test
Figure 3 illustrates the amount of time that the mutant mice and their wild-type littermates spent in conditioning chambers prior to (Day 1) and following (Day 4) a single alternate-day saline/buprenorphine conditioning session. A two-factor ANOVA with repeated measures on the amount of time that the mice spent in the drug-paired chamber on day 4 versus day 1 showed a significant interaction between genotype and time (F1,16 = 6.53; p<0.02). Further analysis of the data revealed that ORL1 receptor knockout mice spent significantly greater time in the drug-paired chamber on day 4 as compared to day 1 (p<0.05), showing that mutant mice expressed CPP. In contrast, buprenorphine failed to induce CPP in wild-type mice, which was evident as no change in the amount of time that the wild-type mice spent in the drug-paired chamber on day 4 as compared to day 1 (Fig. 3; p>0.05). Additionally, mutant mice as compared to their wild-type littermates spent significantly greater amount of time in the drug-paired chamber on day 4 (p<0.05) but not on day 1 (p<0.05). Figure 4 depicts the amount of time that mutant mice and their wild-type littermates spent in conditioning chambers prior to (Day 1) and following (Day 4) single alternate-day saline/morphine conditioning. There was no initial preference toward the drug-paired as compared to vehicle-paired chamber in mutant mice or in their wild-type littermates (p>0.05). Conditioning in the presence of a lower dose of morphine (5 mg/kg) failed to induce CPP (Fig. 4A). On the other hand, a higher dose of morphine (10 mg/kg) induced a significant CPP (Fig. 4B). A two-factor ANOVA with repeated measures on the amount of time that the mice spent in the drug-paired chamber prior to and following conditioning with this dose of morphine revealed a significant effect of time (F1,16 = 19.79; p<0.001) but no significant effect of genotype (F1,16 = 0.01; p>0.05) or interaction between genotype and time (F1,16 = 0.11; p>0.05), showing that both genotypes expressed CPP. However, the magnitude of this response was not different between mutant mice and their wild-type littermates (p>0.05).
Fig. 3. Buprenorphine induced CPP in ORL1 receptor knockout mice but not in their wild-type littermates.
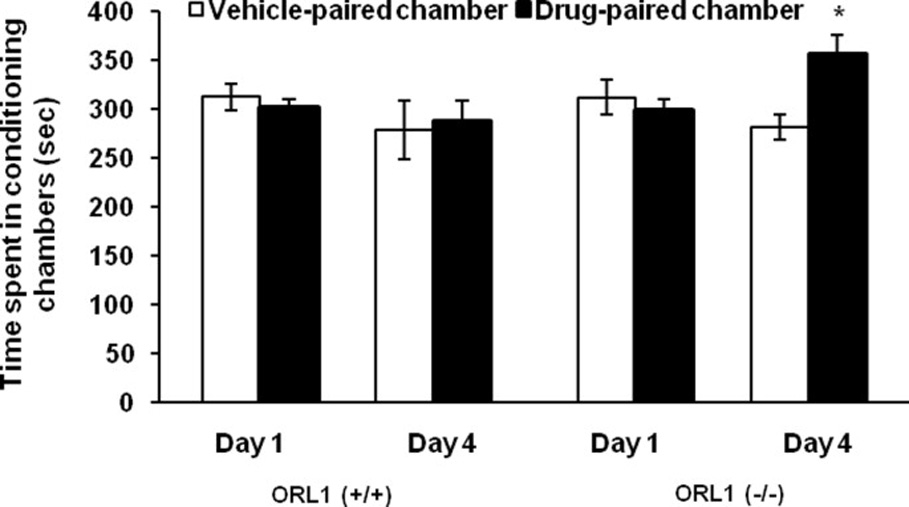
Mice lacking the ORL1 receptor [ORL1 (−/−)] and their wild type littermates [ORL1 (+/+)] were tested for baseline place preference on day 1. Mice then received alternate-day saline/buprenorphine (3 mg/kg) or buprenorphine/ saline conditioning sessions on days 2 and 3. Mice were then tested for postconditioning preference on day 4. Data are expressed as mean (±SEM) of 9 mice per genotype. *indicates a significant increase in the amount of time that the mice spent in the drug-paired chamber on day 1 (p<0.05).
Fig. 4. Morphine-induced CPP was not altered in mice lacking the ORL1 receptor.
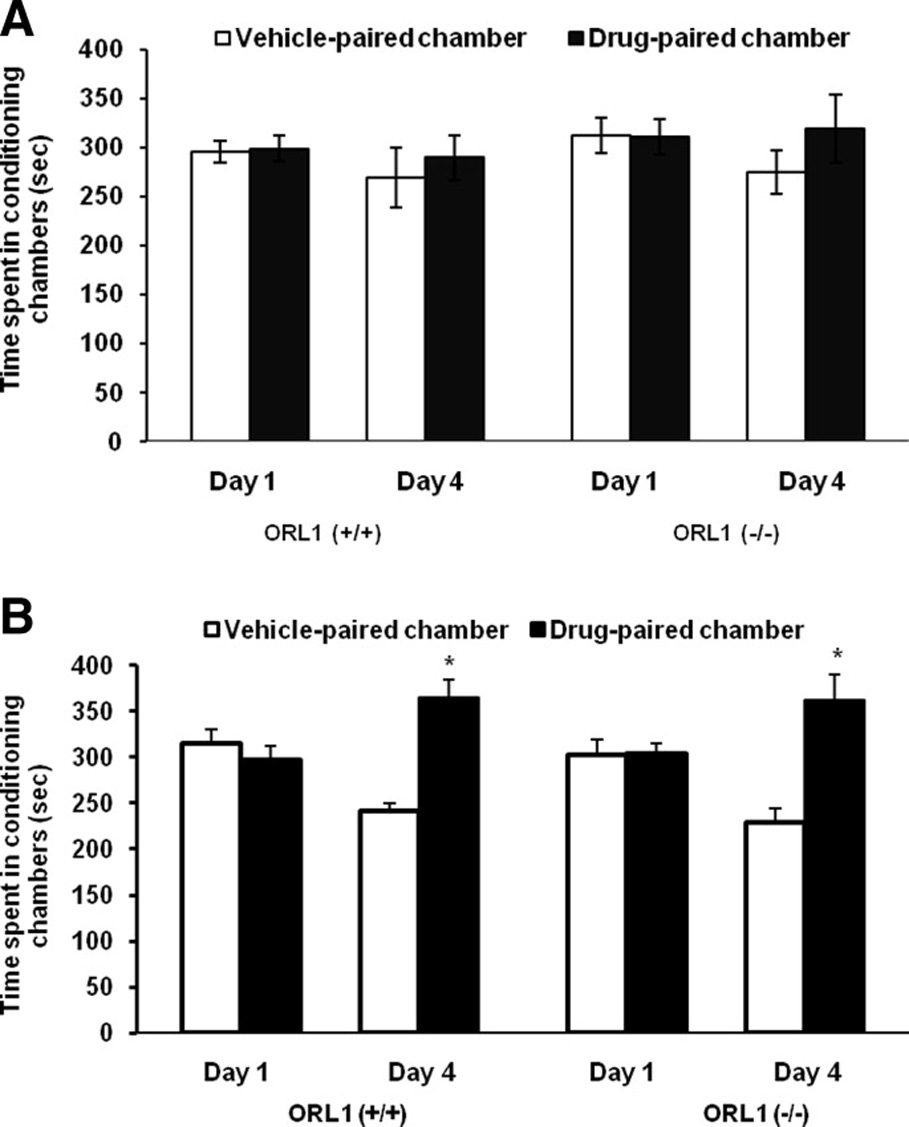
Mice lacking the ORL1 receptor [ORL1 (−/−)] and their wild-type littermates [ORL1 (+/+)] were tested for baseline preference on day 1. Mice then received alternate-day saline/morphine [5 mg/kg (4A) 10 mg/kg (4B)] or morphine/saline conditioning sessions on days 2 and 3 and tested for post-conditioning preference on day 4. Data are expressed as mean (±SEM) of 6–9 mice per genotype. *indicates a significant increase in the amount of time that the mice spent in the drug-paired chamber on day 1 (p<0.05).
Binding assay
Buprenorphine concentration-dependently displaced specific binding of [3H]-OFQ/N in mouse brain homogenates (Fig. 5). One-way ANOVA revealed a significant effect of buprenorphine concentration (F4,10 = 52.44; p<0.001). The post-hoc test showed that buprenorphine at concentrations ≥ 10 nM significantly displaced the specific binding of [3H]-OFQ/N. Furthermore, the inhibitory effect of buprenorphine was significantly greater at the highest concentration (1000 nM) as compared to all other concentrations of the drug tested (p<0.05).
Fig. 5. Buprenorphine concentration-dependently displaced specific binding of [3H]-OFQ/N in mouse brain homogenates.
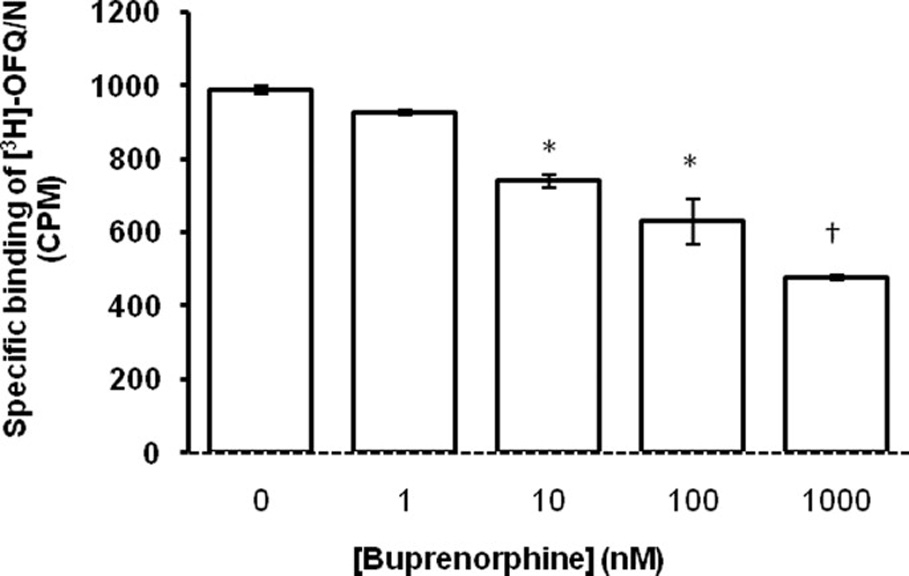
Wild-type mice (n = 3) were euthanized under isoflurane anesthesia, their brains removed and homogenized in Tris buffer (50 mM; pH = 7.4). Brain membrane homogenates were prepared (for details, see Method section) and assayed in triplicates for 3H-OFQ/N (5 nM) binding in the presence and absence of buprenorphine (0, 1, 10, 100 or 1000 nM). Non-specific binding was defined in the presence of non-labeled OFQ/N (5 µM). *indicates a significant increase as compared to control (p<0.05). †indicates a significant increase as compared to all other groups (p<0.05).
Discussion
The main finding of the present study is that the psychostimulant and rewarding actions of buprenorphine were enhanced in mice lacking the ORL1 receptor as compared to their wild-type littermates. However, these actions of morphine were not altered in mutant mice. Buprenorphine was found to concentration-dependently displace specific binding of [3H]-OFQ/N in brain homogenate of wild-type mice. Together, the present results suggest that the ability of buprenorphine to interact with the ORL1 receptor compromises its acute motor stimulatory and rewarding actions.
Buprenorphine, an opioid with mixed agonist/antagonist activity, has a high affinity for the mu opioid receptor (Sadee et al., 1982; Richards and Sadee, 1985; Negus et al., 1989; Huang et al., 2001; Negus et al., 2002; Lutfy et al., 2003). Previous studies have shown that buprenorphine also interacts with the ORL1 receptor (Wnendt et al., 1999; Hawkinson et al., 2000; Bloms-Funke et al., 2000; Huang et al., 2001; Lutfy et al., 2003; Hou et al., 2004; Yamamoto et al., 2006; Ciccocioppo et al., 2007). We have recently shown that the motor stimulatory and rewarding actions of buprenorphine are mediated primarily via the mu opioid receptor (Marquez et al., 2007). We have also shown that the mu opioid receptor-mediated antinociceptive effect of buprenorphine was enhanced in ORL1 receptor knockout mice (Lutfy et al., 2003), raising the possibility that the mu opioid receptor-mediated actions of buprenorphine could be modulated by its ability to interact with the ORL1 receptor. The results of the present study provide further support for this notion by demonstrating that the motor stimulatory action of buprenorphine (1 mg/kg) was enhanced in mice lacking the ORL1 receptor as compared to their wild-type littermates. The increase in motor stimulatory action of buprenorphine in ORL1 receptor knockout mice was rather selective because basal motor activity was comparable between the mutant mice and their wild-type littermates (compare saline-treated ORL1 knockout mice to their wild-type littermates). Importantly, morphine (5 or 10 mg/kg) produced comparable motor stimulation in mutant mice and their wild-type littermates. An earlier report also demonstrated that the motor stimulatory action of heroin was not altered in ORL1 receptor knockout mice (Murphy et al., 2002), suggesting that the endogenous OFQ/N/ORL1 receptor system may not be involved in the acute actions of opioid. However, our data cannot rule out the possibility that the actions of morphine and other opioids could be altered following their chronic administration since the level of OFQ/N was shown to increase after repeated but not single morphine administration (Yuan et al., 1999). Thus, further studies are needed to assess this possibility.
Previous studies have shown that activation of the mu opioid receptor increases extracellular dopamine in the nucleus accumbens (Di Chiara and Imperato, 1988). On the other hand, stimulation of the ORL1 receptor reduces extracellular levels of dopamine in this brain region (Murphy et al., 1996; Di Giannuario et al., 1999; Murphy and Maidment, 1999). Likewise, OFQ/N reduces the ability of morphine to increase accumbal dopamine (Di Giannuario et al., 1999) or to induce CPP (Murphy et al., 1999; Ciccocioppo et al., 2000), showing that the ORL1 and mu opioid receptors represent two opposing mechanisms on hedonic homeostasis. Thus, we determined whether the rewarding action of buprenorphine (3 mg/kg) would be altered in ORL1 receptor knockout mice as compared to their wild-type littermates. As tolerance and/ or sensitization could develop following repeated opioid administration, we assessed CPP following a single alternate-day saline/buprenorphine conditioning session. ORL1 knockout mice expressed a robust CPP following this treatment. However, wild-type mice failed to display CPP, indicating that the rewarding action of buprenorphine is compromised by its ability to interact with the ORL1 receptor. On the other hand, morphine (10 mg/kg) induced a comparable CPP in mice lacking the ORL1 receptor and their wild-type littermates, suggesting that the "rewarding" effect of buprenorphine is under the control of the endogenous OFQ/N/ORL-1 receptor system. However, it should be kept in mind that we are studying a constitutive knockout; thus, any genotype-dependent differences in the magnitude of expressed CPP could be due to differences in acquisition of CPP (e.g. "reward", associative learning) or differences in expression of CPP (e.g. expression of autoshaped responses, incentive motivation, memory recall, etc.), which could theoretically be specific to particular drugs (see also below).
We have previously shown that buprenorphine and OFQ/N each activates mitogen-activated protein (MAP) kinase in CHO cells expressing ORL1 receptors (Lutfy et al., 2003). In the present study, we also demonstrated that buprenorphine concentration-dependently displaced specific binding of [3H]-OFQ/N in mouse brain membrane homogenates (Fig. 5), providing support that buprenorphine interacts with the mouse brain ORL1 receptor. Thus, our current results indicate that the ability of buprenorphine to concomitantly activate the ORL1 receptor compromises its motor stimulatory and rewarding actions. It is of interest to note that the affinity of buprenorphine for the mu opioid receptor is about 100 folds higher than its affinity for the ORL1 receptor (Huang et al., 2001; Lutfy et al., 2003). Thus, it is not known how this weak action of buprenorphine at the ORL1 receptor compromises its mu opioid receptor-mediated actions.
Previous studies have implicated the OFQ/N/ORL1 receptor system in learning and memory (Yu et al., 1997; Sandin et al., 1997). Interestingly, enhanced learning and memory has been reported in mice lacking the ORL1 receptor (Mamiya et al., 1999). However, the present data cannot be explained by alterations in memory performance or other compensatory changes in the knockout mice because the motor stimulatory and rewarding actions of morphine were not altered in mutant mice. Thus, further studies are needed to elucidate whether the direct interaction of buprenorphine with the ORL1 receptor leads to this enhancement or other mechanisms may be involved. Although the results of our binding assay suggest a direct interaction of buprenorphine with the ORL1 receptor, it is tempting to propose that buprenorphine, but not morphine, may also cause the release of OFQ/N following its single administration. Alternatively, buprenorphine may interact with a mu-ORL1 receptor heterodimer (Pan et al., 2002) in wild-type mice but only with the mu opioid receptor in ORL1 knockout mice. On the other hand, morphine, which has negligible affinity for the ORL1 receptor (Lutfy et al., 2003), acts on the mu opioid receptor in both mutant mice and their wild-type littermates and therefore its actions are comparable between the wild-type and mutant mice.
In summary, the present data demonstrate that the motor stimulatory and rewarding actions of buprenorphine, mediated by the mu opioid receptor (Marquez et al., 2007), were enhanced in mice lacking the ORL1 receptor. In contrast, these actions of morphine were not altered in mutant mice. Taken together, the present results indicate that the ability of buprenorphine to interact with the ORL1 receptor compromises its mu opioid receptor-mediated actions.
Acknowledgments
The authors wish to thank Dr. Arezoo Campbell for her suggestions and Professor Hiroshi Takeshima for providing the ORL1 receptor knockout breeding pairs. The present study was supported in part by an intramural grant from Western University of Health Sciences and in part by a NIDA grant R01 DA016682.
Abbreviations
- ORL1
the opioid receptor-like
- OFQ/N
orphanin FQ/nociceptin
- CPP
conditioned place preference
- ORL1 (+/+)
wild-type mice
- ORL1 (−/−)
ORL1 receptor knockout mice
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21:1141–1146. doi: 10.1016/s0196-9781(00)00252-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry. 2007;61:4–12. doi: 10.1016/j.biopsych.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S, Catalani A, Loizzo A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- Finco G, Polati E, Gottin L, Bartoloni A, Milan B, Zanoni L, Valle L. Intravenous patient-controlled analgesia (PCA) in the treatment of postoperative pain: rationale and clinical application. Chir Ital. 1995;47:20–25. [PubMed] [Google Scholar]
- Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opioid addiction. II. Physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clin Pharmacol Ther. 1990;47:525–534. doi: 10.1038/clpt.1990.67. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Johanson CE, Schuster CR. Opioid reinforcement in heroin-dependent volunteers during outpatient buprenorphine maintenance. Drug Alcohol Depend. 1999;56:191–203. doi: 10.1016/s0376-8716(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Hawkinson JE, costa-Burruel M, Espitia SA. Opioid activity profiles indicate similarities between the nociceptin/orphanin FQ and opioid receptors. Eur J Pharmacol. 2000;389:107–114. doi: 10.1016/s0014-2999(99)00904-8. [DOI] [PubMed] [Google Scholar]
- Heit HA, Gourlay DL. Office-based treatment of opiate addiction. N Engl J Med. 2003;349:2567–2568. doi: 10.1056/NEJM200312253492619. [DOI] [PubMed] [Google Scholar]
- Hou Y, Tan Y, Belcheva MM, Clark AL, Zahm DS, Coscia CJ. Differential effects of gestational buprenorphine, naloxone, and methadone on mesolimbic mu opioid and ORL1 receptor G protein coupling. Brain Res Dev Brain Res. 2004;151:149–157. doi: 10.1016/j.devbrainres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995;40:17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Kamei J, Saitoh A, Suzuki T, Misawa M, Nagase H, Kasuya Y. Buprenorphine exerts its antinociceptive activity via mu 1-opioid receptors. Life Sci. 1995;56:L285–L290. doi: 10.1016/0024-3205(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Kamei J, Sodeyama M, Tsuda M, Suzuki T, Nagase H. Antinociceptive effect of buprenorphine in mu1-opioid receptor deficient CXBK mice. Life Sci. 1997;60:L-7. doi: 10.1016/s0024-3205(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Kuhlman JJ, Jr., Levine B, Johnson RE, Fudala PJ, Cone EJ. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93:549–559. doi: 10.1046/j.1360-0443.1998.93454910.x. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ. Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci. 2003;23:10331–10337. doi: 10.1523/JNEUROSCI.23-32-10331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Nishi M, Takeshima H, Nabeshima T. Nociceptin system plays a role in the memory retention: involvement of naloxone benzoylhydrazone binding sites. Neuroreport. 1999;10:1171–1175. doi: 10.1097/00001756-199904260-00003. [DOI] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Gajawada N, Friedman TC, Lutfy K. Differential involvement of enkephalins in analgesic tolerance, locomotor sensitization, and conditioned place preference induced by morphine. Behav Neurosci. 2006;120:10–15. doi: 10.1037/0735-7044.120.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Kieffer BL, Lutfy K. The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology. 2007;52:1336–1341. doi: 10.1016/j.neuropharm.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Murphy NP, Lam HA, Chen Z, Pintar JE, Maidment NT. Heroin-induced locomotion and mesolimbic dopamine release is unchanged in mice lacking the ORL.1 receptor gene. Brain Res. 2002;953:276–280. doi: 10.1016/s0006-8993(02)03398-x. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ly HT, Maidment NT. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- Negus SS, Bidlack JM, Mello NK, Furness MS, Rice KC, Brandt MR. Delta opioid antagonist effects of buprenorphine in rhesus monkeys. Behav Pharmacol. 2002;13:557–570. doi: 10.1097/00008877-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Negus SS, Picker MJ, Dykstra LA. Kappa antagonist effects of buprenorphine in the rat drug-discrimination procedure. NIDA Res Monogr. 1989;95:518–519. [PubMed] [Google Scholar]
- Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukada T, Nabeshima T, Yamashita T, Noda T, Sugimoto T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YX, Bolan E, Pasternak GW. Dimerization of morphine and orphanin FQ/nociceptin receptors: generation of a novel opioid receptor subtype. Biochem Biophys Res Commun. 2002;297:659–663. doi: 10.1016/s0006-291x(02)02258-1. [DOI] [PubMed] [Google Scholar]
- Picard PR, Tramer MR, McQuay HJ, Moore RA. Analgesic efficacy of peripheral opioids (all except intra-articular): a qualitative systematic review of randomised controlled trials. Pain. 1997;72:309–318. doi: 10.1016/s0304-3959(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Richards ML, Sadee W. In vivo opiate receptor binding of oripavines to mu, delta and kappa sites in rat brain as determined by an ex vivo labeling method. Eur J Pharmacol. 1985;114:343–353. doi: 10.1016/0014-2999(85)90379-6. [DOI] [PubMed] [Google Scholar]
- Sadee W, Rosenbaum JS, Herz A. Buprenorphine: differential interaction with opiate receptor subtypes in vivo. J Pharmacol Exp Ther. 1982;223:157–162. [PubMed] [Google Scholar]
- Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Wnendt S, Kruger T, Janocha E, Hildebrandt D, Englberger W. Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol Pharmacol. 1999;56:334–338. doi: 10.1124/mol.56.2.334. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shono K, Tanabe S. Buprenorphine activates mu and opioid receptor like-1 receptors simultaneously, but the analgesic effect is mainly mediated by mu receptor activation in the rat formalin test. J Pharmacol Exp Ther. 2006;318:206–213. doi: 10.1124/jpet.105.100859. [DOI] [PubMed] [Google Scholar]
- Yu TP, Fein J, Phan T, Evans CJ, Xie CW. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus. 1997;7:88–94. doi: 10.1002/(SICI)1098-1063(1997)7:1<88::AID-HIPO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yuan L, Han Z, Chang JK, Han JS. Accelerated release and production of orphanin FQ in brain of chronic morphine tolerant rats. Brain Res. 1999;826:330–334. doi: 10.1016/s0006-8993(99)01337-2. [DOI] [PubMed] [Google Scholar]


