Abstract
INTRODUCTION
Peripheral arterial disease is a coronary risk equivalent; a low ankle-brachial index (ABI) is indicative of systemic vascular disease, and should place a patient in the high-risk category. Few physicians measure ABI because it is technically challenging and time consuming. Oscillometric blood pressure monitors are readily available and easy to use. The use of a simple method of documenting ABI was assessed and compared with the conventional method.
METHODS
The oscillometric ABI (OABI) was measured for normal volunteers, patients attending a cardiovascular risk clinic (Cardiovascular Risk Factor Reduction Unit [CRFRU] at the University of Saskatchewan, Saskatoon) and patients referred to a vascular laboratory (vasc lab). The latter group had Doppler ABI (DABI) measurements and served to validate OABI. An Omron HEM 711C oscillometric system (Omron Canada Inc) with appropriate cuff size for arm and leg circumference was used.
RESULTS
The mean ± SEM OABI was 1.13±0.08 in normal volunteers (n=26), 1.10±0.10 in CRFRU patients (n=11, P not significant) and 1.03±0.14 in vasc lab patients (n=57, P<0.05 compared with normal volunteers). No difference was found between sexes, and there was no correlation with age. In the vasc lab group, the correlation with DABI was 0.71 (P<0.05). The sensitivity of OABI to detect DABI of less than 0.9 was 0.71, and the specificity was 0.89. OABI was found to be less sensitive at detecting low values in patients with nonpalpable pulses on physical examination.
CONCLUSION
The OABI is feasible and operator-independent, but does not detect low ABI efficiently. If OABI is abnormal, low DABI is likely. The OABI is less likely to detect disease in patients with nonpalpable peripheral pulses. Such patients are better referred directly to a vascular laboratory for DABI testing.
Keywords: Ankle-brachial index, Atherosclerosis, Diagnosis, Peripheral vascular disease, Risk factors
Abstract
INTRODUCTION
La maladie artérielle périphérique correspond à un équivalent de risque coronarien; un indice cheville/bras (ICB) faible est un signe de maladie vasculaire systémique et de ce fait, le patient se trouve dans une catégorie à risque élevé. Peu de médecins mesurent l’ICB, parce que la technique est complexe et fastidieuse. Les moniteurs de pression artérielle oscillométriques sont faciles à se procurer et à utiliser. Une technique simple de suivi de l’ICB a été évaluée et comparée à la méthode classique.
MÉTHODES
L’ICB oscillométrique (ICBO) a été mesuré chez des volontaires normaux, des patients inscrits dans une clinique de risque cardiovasculaire (la Cardiovascular Risk Factor Reduction Unit [CRFRU] de l’Université de la Saskatchewan à Saskatoon) et chez des patients adressés en laboratoire vasculaire. Ce dernier groupe a subi des mesures Doppler (OCBD) qui ont servi à valider l’ICBO. Un système HEM 711C d’Omron pourvu de brassards de la taille appropriée pour la circonférence du bras et de la jambe a été utilisé.
RÉSULTATS
L’ICBO était de 1,13±0,08 chez les volontaires normaux (écart-type de la moyenne, n=26), de 1,10±0,10 chez les patients de la CRFRU (n=11, P non significatif) et de 1,03±0,14 chez les patients du laboratoire vasculaire (n=57, P<0,05 comparativement aux témoins normaux). Aucune différence n’a été observée entre les sexes et on n’a noté aucune corrélation avec l’âge. Dans le cas du groupe du laboratoire vasculaire, la corrélation avec l’ICBD a été de 0,71 (P<0,05). La sensibilité de l’ICBO à détecter une ICBD inférieur à 0,9 a été de 0,71 et la spécificité a été de 0,89. L’ICBO s’est révélé moins sensible pour ce qui est de détecter les valeurs faibles chez les patients présentant des pouls non palpables à l’examen médical.
CONCLUSION
L’ICBO est accessible et indépendant de l’examinateur, mais ne permet pas de déceler efficacement un ICB faible. Si l’ICBO est anormal, il est probable que l’ICBD est faible. L’ICBO est moins susceptible de permettre le dépistage de la maladie chez les patients qui ont des pouls périphériques non palpables. Il est préférable que ces patients soient adressés directement en laboratoire vasculaire pour faire mesurer leur ICBD.
Peripheral arterial disease (PAD) is a widely prevalent manifestation of systemic atherosclerosis. Patients who have PAD are three times more likely to suffer death, myocardial infarction or stroke than patients who do not (1). It is therefore considered to be a coronary risk equivalent, and warrants treatment via nonpharmacological means, including diet modification, exercise and smoking cessation, as well as pharmacological means, including an antiplatelet agent, a statin and an angiotensin-converting enzyme inhibitor (2).
According to the Canadian Cardiovascular Society consensus statement on PAD (2), PAD affects 16% of the population in North America and Europe. This corresponds to approximately 27 million people. Of these 27 million, 16.5 million (or three in five) are asymptomatic. As a result, PAD is underdiagnosed and undertreated.
In a recent study documenting recognition of PAD in the primary care setting (3), PAD was diagnosed by a low ankle-brachial index (ABI) or amputation in 1825 of 6979 patients. Fifty-five per cent of these patients were previously undiagnosed. Furthermore, among patients with previously diagnosed PAD, 83% of patients were aware of their diagnosis, while only 49% of their physicians were aware. Thus, many patients with PAD are going unrecognized by primary care physicians, and opportunities for secondary prevention of cardiovascular events are being missed.
The diagnosis of PAD can be confirmed using Doppler ultrasound methodology, which is used to calculate the ABI. By convention, the ABI is the ratio of the highest systolic pressure at the ankle to the highest systolic pressure measured in the arms (3). A ratio of 0.9 or lower by Doppler ultrasound confirms 50% or greater stenosis in one or more major vessels with 95% sensitivity and 100% specificity (4,5). However, this relatively simple calculation is rarely performed in the office because of a lack of equipment, the time required to do the procedure and the technical difficulties involved in making the measurements. For this reason, we tested the possibility of using an automated oscillometric blood pressure monitor for the calculation of the ABI.
Oscillometric blood pressure monitors are commonly available, reliable and simple to use (6). Thus, we proposed that oscillometric ABI (OABI) measurement is feasible and correlates with the ‘gold standard’ Doppler ABI (DABI) measurement. We further hypothesized that the OABI can be used to detect differences in ABI among groups (normal control patients, Cardiovascular Risk Factor Reduction Unit [CRFRU] patients and patients referred for vascular studies).
METHODS
Ninety-four subjects were enrolled. Exclusion criteria included inability to consent to the study, calf circumference larger than 45 cm, atrial fibrillation, significant aortic regurgitation or a pulse pressure lower than 30 mmHg.
Three groups were recruited: normal volunteers with no cardiac risk factors other than age and sex (normal control group, n=26), patients with significant cardiac risk profiles recruited from the CRFRU (7) (CRFRU group, n=11) and patients suspected of having PAD who had been referred to a vascular laboratory (vasc lab group, n=57). The Omron HEM 711C oscillometric monitor (Omron Canada Inc) was used for all OABI measurements, while Doppler measurements were acquired with the Parks Flo-Lab 2100-SX system (Parks Medical Electronics Inc, USA). A trained vascular laboratory technician performed all Doppler readings, while the authors performed the OABI readings.
Each subject completed a short questionnaire, in which demographic information and cardiovascular risk factors were recorded. Hypertension, hyperlipidemia, diabetes and current cigarette smoking habits were reported. Four pedal pulses were palpated by one of the investigators.
Blood pressure was measured twice in each arm using both the Omron monitor and a mercury manometer, with appropriately sized cuffs. If these readings differed by more than 10 mmHg, a third confirmatory reading was obtained. If the difference persisted, the higher of the two arms was used. Otherwise, the average of all systolic readings was taken as arm blood pressure.
The oscillometric cuff was placed in the mid-calf area. Cuff size was determined by calf circumference at its mid-point: 35 cm or larger required the use of a large cuff. Three oscillometric pressure readings were taken in each leg and the average was used as the ankle blood pressure. An average OABI for each leg was determined. In the subjects of the present study, no difference in OABI was found between the two sides, so the average of all OABI readings was used. In some patients (see below) oscillometric blood pressure could not be recorded in one leg; in these patients, the other leg was used as ‘average’.
The DABI was obtained by the vascular laboratory technician using a mercury sphygmomanometer to measure arm blood pressure, and a continuous-wave Doppler system was used to acquire signals at the posterior tibial and dorsalis pedis sites. Once the signals were obtained, a blood pressure cuff was placed around the ankle, inflated, then deflated. According to convention, the higher of the two pressures was taken as the ankle blood pressure. Again, the average of the two sides was taken as the DABI.
In comparing the vasc lab OABI and DABI readings, it was assumed that a difference of 0.05 units was clinically significant and that the SD of all readings was 0.1 unit; thus, a sample size of approximately 55 was necessary.
Differences among groups were compared using one-way ANOVA (SPSS; SPSS Inc, USA), correlations with linear regression, and sensitivity and specificity measurements using standard techniques. Positive and negative likelihood ratios were also calculated. For categorical variables (presence of pedal pulses), χ2 analysis was used. In all cases, P<0.05 was considered to be significant.
RESULTS
Subject characteristics are shown in Table 1. In general, there were more men than women in each group; the CRFRU and vasc lab groups had more cardiovascular risk factors than the normal control group.
TABLE 1.
Subject characteristics
| Normal control group | CRFRU group | Vascular laboratory group | |
|---|---|---|---|
| Patients, n (male/female) | 26 (18/8) | 11 (7/4) | 57 (48/9) |
| Age, years (range) | 40 (24–84) | 66 (49–81) | 65 (16–91) |
| CV risk factors, n (range) | 0 (0–1) | 3 (1–4) | 4 (0–4) |
| OABI ± SD | 1.13±0.08 | 1.10±0.10 | 1.03±0.14*¶ |
| Patients with OABI <0.9 | 0 | 4 | 24 |
Cardiovascular (CV) risk factors include hypertension, hyperlipidemia, diabetes and smoking.
P<0.05 compared with control group;
P<0.05 compared with CRFRU group. CRFRU Cardiovascular Risk Factor Reduction Unit; OABI Oscillometric ankle-brachial index
Good OABI recordings were possible in all normal and CRFRU patients. For 26 subjects in the vasc lab group, an oscillometric blood pressure reading could not be obtained in one leg. Twenty-four such subjects had a DABI of 0.9 or lower in that leg. Moreover, the 26 patients with unobtainable OABI in one leg had an average of 1.0 (of four) pulse palpable in both feet compared with 2.5 palpable pulses in the group with good OABI readings (Table 2). There were no other differences between these groups.
TABLE 2.
Patients in the vascular laboratory group with and without oscillometric ankle-brachial index (OABI) readings in both legs
| With OABI readings (n=31) | Without OABI readings (n=26) | |
|---|---|---|
| Age, years (mean ± SD) | 64±4 | 66±5 |
| Male/female, n | 27/3 | 18/6 |
| Hypertension, n (%) | 25 (80) | 20 (77) |
| Hyperlipidemia, n (%) | 20 (65) | 14 (54) |
| Diabetes, n (%) | 7 (23) | 8 (31) |
| Current smoker, n (%) | 10 (32) | 9 (35) |
| Arm – systolic BP (mean ± SD) | 132±8 | 135±8 |
| Arm – diastolic BP (mean ± SD) | 72±7 | 70±9 |
| Palpable pedal pulses (mean ± SD) | 2.5±0.8 | 1.0±0.4* |
| OABI (mean ± SD) | 1.03±0.10 | 1.05±0.10† |
| DABI (mean ± SD) | 0.97±0.09 | 0.95±0.10 |
P<0.05 by χ2 analysis;
OABI calculated on a single leg. BP Blood pressure; DABI Doppler ABI
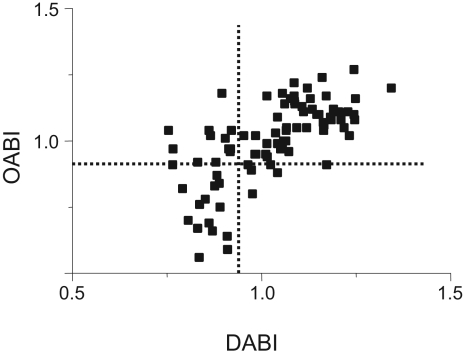
The correlation between OABI and DABI, shown in Figure 1, was 0.71 (P<0.05). The sensitivity of OABI to detect DABI lower than 0.9 was 0.71 and the specificity was 0.89. Similarly, the positive likelihood ratio was 6.45 and the negative likelihood ratio was 0.33.
Figure 1.
Correlation between vascular laboratory (Doppler) ankle-brachial index (DABI) and oscillometric ABI (OABI) in 57 patients referred to the vascular laboratory. The dotted lines represent an ABI of 0.9
The mean OABI was 1.13±0.08 in normal volunteers, 1.10±0.10 in CRFRU patients (P not significant) and 1.03±0.14 in vasc lab patients (P<0.05 compared with normal controls and CRFRU patients). Thus, the OABI method was capable of detecting differences among the three patient groups. No difference was found between sexes, and there was no correlation with age in the subjects as a whole or in any group.
DISCUSSION
In the present study, we found that measuring the ABI using an oscillometric system is feasible. In 26 normal subjects and 11 CRFRU patients, we obtained interpretable readings. Furthermore, four of 11 CRFRU patients had an ABI of 0.9 or lower. These subjects had no claudication or physical examination evidence of PAD.
The difficulty in using clinical parameters to diagnose PAD is well recognized. In a meta-analysis of 17 papers, Khan et al (8) found no symptoms or signs to have a positive likelihood ratio of greater than 6.0. Symptomatic claudication, for example, has a likelihood ratio of 3.30. This underlines the need for a simple, reliable way to measure the ABI as an aid in establishing the diagnosis.
An obvious problem for OABI is large calf circumference. We found no subject in the vasc lab or CRFRU groups with a calf circumference larger than 45 cm. We tested a single normal volunteer with such a large lower leg, but the ankle blood pressure could not be recorded.
The OABI is capable of finding differences in ABI among groups of patients. As expected, OABI was significantly lower in high-risk, likely symptomatic patients referred to our vascular laboratory than in normal or CRFRU patients.
The OABI cannot be substituted for a DABI. In patients with a strong clinical suspicion of PAD referred to our vascular laboratory, we were able to measure OABI in both legs in only approximately 60%. Furthermore, we found relatively higher ABIs using oscillometry in the calf compared with Doppler signals at the ankle or the foot. Indeed, the sensitivity to confirm ABI lower than 0.9 in this group was only approximately 70%; on the other hand, the specificity was 89%. We suggest that if OABI can be measured, and if it is lower than 0.9, the likelihood of a low ABI in the vascular laboratory is very high.
Measurement of ABI in the office setting is rarely performed. A 2004 survey in our institution (unpublished data), found that fewer than 5% of consultants and residents routinely measured it in the clinic or the hospital. Those who did used a handheld Doppler device and a calf blood pressure cuff, which requires considerable dexterity and practice. Automated systems, such as the one used in our vascular laboratory, are expensive and also technically challenging.
In general, OABI readings were somewhat higher than those of the DABI. While this may represent a technical difference, there may also be a biological one. Blood pressure in the calf may be higher than blood pressure in the ankle or foot. Moreover, oscillometry depends on recording small heart beat-related volume changes in the limb. It was found empirically that the point of maximal oscillation is equal to the mean arterial pressure. Systolic and diastolic pressure are computed using a proprietary algorithm (9). Whether such algorithms are applicable to the lower limb is uncertain. Thus, more data are needed on the OABI to establish the ‘normal’ range; it may well be higher than 0.9.
A low ABI can be due to partial obstruction of any part of the arterial tree from aorta to low leg arteries. Moreover, occasionally an ABI greater than 1.2 is recorded. These unusually high ABIs are usually attributed to ‘noncompressible arteries’. Neither OABI nor DABI can obtain accurate readings in this circumstance.
While our study was being performed, Beckman et al (10) published their findings with the OABI. They compared OABI with DABI in approximately 200 patients referred to a vascular laboratory. Fifty-five patients had PAD. The correlation between the two methods was 0.78, and the mean difference 0.04 to 0.06. They found a sensitivity of 73% to 88% and a specificity of 85% to 95% to detect a DABI lower than 0.9. This study looked only at patients who were referred to the vascular laboratory, and did not use a normal control group or a subset of clinic patients.
A potential limitation to our study is the small number of patients enrolled in each group, especially in the CRFRU group. A screening test is most useful when applied to a group of patients with a moderate pretest probability of disease, as opposed to those with a low or high pretest probability. The CRFRU patients represented an asymptomatic group with multiple cardiovascular risk factors; these are the patients who should be screened for PAD. Thus, it will be useful to study more CRFRU patients and compare OABI and DABI in this setting.
In summary, the present study showed that OABI is feasible, has a reasonable correlation with the ‘gold standard’ DABI and detects DABI lower than 0.9 with moderate sensitivity and good specificity. A patient with OABI lower than 0.9 is likely to have significant PAD and should be aggressively treated. The OABI is less useful in patients with absent peripheral pulses on physical examination. Such patients may be clinically inferred to have PAD and are best referred for formal vascular testing.
ACKNOWLEDGEMENTS
The authors thank Dr Brian Ulmer, director of the Vascular Laboratory at St Paul’s Hospital (Saskatoon, Saskatchewan), for his cooperation in ensuring access to the vascular laboratory patients, and Ms Michelle Diemer RN BScN RVT, vascular laboratory technician, for performing the DABI measurements.
REFERENCES
- 1.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 2.Abramson BL, Huckell V, Anand S, et al. Canadian Cardiovascular Society. Canadian Cardiovascular Society Consensus Conference: Peripheral arterial disease – executive summary. Can J Cardiol. 2005;21:997–1006. [PubMed] [Google Scholar]
- 3.McPhail IR, Spittell PC, Weston SA, Bailey KR. Intermittent claudication: An objective office-based assessment. J Am Coll Cardiol. 2001;37:1381–5. doi: 10.1016/s0735-1097(01)01120-2. [DOI] [PubMed] [Google Scholar]
- 4.Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg. 1969;56:676–9. doi: 10.1002/bjs.1800560910. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 6.Yarows SA, Julius S, Pickering TG. Home blood pressure monitoring. Arch Intern Med. 2000;160:1251–7. doi: 10.1001/archinte.160.9.1251. [DOI] [PubMed] [Google Scholar]
- 7.Wilson TW, Quest DW, Wilson M, et al. A cardiovascular risk factor reduction clinic. Can J Cardiol. 1999;15:887–91. [PubMed] [Google Scholar]
- 8.Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536–46. doi: 10.1001/jama.295.5.536. [DOI] [PubMed] [Google Scholar]
- 9.Yong P, Geddes LA. A surrogate arm for evaluating the accuracy of instruments for indirect measurement of blood pressure. Biomed Instrum Technol. 1990;24:130–5. [PubMed] [Google Scholar]
- 10.Beckman JA, Higgins CO, Gerhard-Herman M. Automated oscillometric determination of the ankle-brachial index provides accuracy necessary for office practice. Hypertension. 2006;47:35–8. doi: 10.1161/01.HYP.0000196686.85286.9c. [DOI] [PubMed] [Google Scholar]