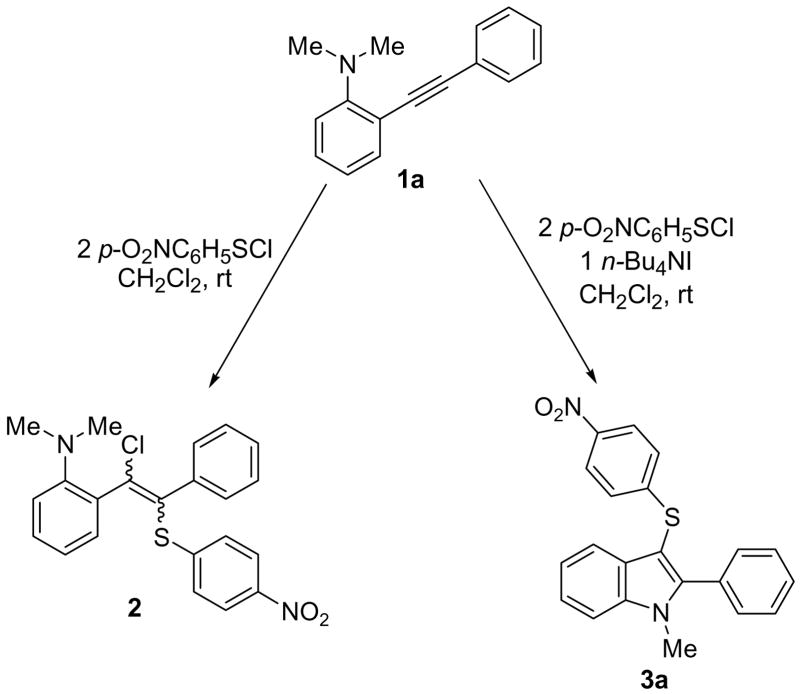
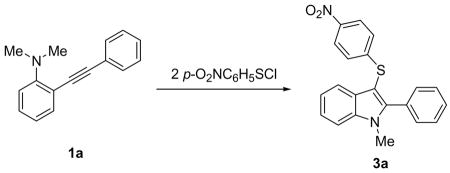
Abstract
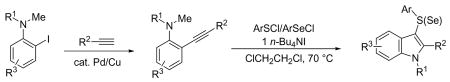
3-Sulfenyl- and 3-selenylindoles are prepared in excellent yields by the palladium/copper-catalyzed crossing coupling of N,N-dialkyl-ortho-iodoanilines and terminal alkynes, followed by electrophilic cyclization with arylsulfenyl chlorides and arylselenyl chlorides in the presence of a stoichiometric amount of n-Bu4NI.
The indole ring is a ubiquitous heterocycle in a wide variety of biologically important compounds, as well as pharmaceutical agents.1 Among the numerous indole derivatives, 3-thioindoles have recently attracted considerable interest from the pharmaceutical industry due to their therapeutic value in diseases, such as HIV,2 cancer,3 obesity,4 heart disease,5 and allergies.6 A number of synthetic routes to 3-sulfenylindoles have been demonstrated in the literature, including the direct sulfenylation of indoles by disulfides7 and quinone mono-O,S-acetals;8 halide-catalyzed sulfenylation by N-thioalkyl(aryl)phthalimides;9 sulfenylation using thiols activated in situ by N-chlorosuccinimide,10 phenyliodine(III) bis(trifluoroacetate),11 Selectfluor,12 or transition-metal catalysts;13 oxidant-promoted thiocyanation with ammonium thiocyanate;14 and treatment of 3,3′-dithiobisindoles with metalated aromatics or heterocycles.15 In general, all these protocols have focused on direct sulfenylation at the 3-position of the indole nucleus using different sulfenylating agents.
Recently, our group has shown that the palladium/copper-catalyzed coupling of functionally-substituted aryl halides and terminal alkynes provides aromatic acetylenes, which readily undergo electrophilic cyclization in the presence of halogen, sulfur and selenium electrophiles to produce an extraordinary range of medicinally-interesting, functionally-substituted heterocycles and carbocycles, including indoles,16 benzofurans,17 benzothiophenes,18 coumestans,19 chromones,20 isocoumarins,21 isochromenes,22 isoquinolines,23 quinolines,24 and isoxazoles.25 Although we have successfully prepared 3-sulfenyl-benzofurans17 and -benzothiophenes18 by electrophilic cyclization using arylsulfenyl chlorides as the electrophile, all previous attempts to prepare 3-sulfenylindoles using similar methods have thus far been unsuccessful. The pharmaceutical interest in 3-sulfenylindoles has inspired us to explore this approach further. In this letter, we report our preliminary results on the synthesis of 3-sulfenylindoles using electrophilic sulfur cyclization chemistry. To the best of our knowledge, this is the first synthetic protocol that installs the sulfenyl group in the 3-position of an indole ring while simultaneously constructing the indole nucleus itself.
Our previous results indicated that under common electrophilic cyclization conditions, the reaction between N,N-dimethyl-(2-phenylethynyl)aniline (1a) and 4-nitrobenzenesulfenyl chloride leads predominantly to the simple triple bond addition product 2. After several unsuccessful trials, we were pleased to find that in the presence of one equivalent of n-Bu4NI the triple bond addition reaction was completely shut down and the reaction slowly produced the desired cyclization product 3a solely (Scheme 1).
Scheme 1.
The Effect of n-Bu4NI in the Electrophilic Cyclization
Our preliminary results indicated that the cyclization reaction can be substantially accelerated at an elevated temperature. Thus, when the reaction is run at 70 °C in dichloroethane (DCE), instead of room temperature in dichloromethane (DCM) (Table 1, entries 1 and 2), a 90% yield of the desired 3-(arylsulfenyl)indole 3a was obtained in 5 h. An equimolar amount of n-Bu4NI is found necessary for exclusive formation of the cyclization product. When 0.5 equiv of n-Bu4NI is used, a mixture of both indole 3a and triple bond addition products is obtained in approximately a 1:1 ratio (Table 1, entry 3).
Table 1.
n-Bu4NI-Induced Electrophilic Cyclization of N,N-Dimethyl-(2-phenylethynyl) aniline with 4-Nitrobenzenesulfenyl Chloride
 | |||||
|---|---|---|---|---|---|
| entry | n-Bu4NI (equiv) | solvent | temp (°C) | time (h) | % yielda |
| 1 | 1 | CH2Cl2 | rt | 60 | 86 (3a) |
| 2 | 1 | (CH2Cl)2 | 70 | 5 | 90 (3a) |
| 3 | 0.5 | (CH2Cl)2 | 70 | 5 | 48 (3a) + 45 (2) |
Isolated yields after column chromatography.
The starting N,N-dialkyl-2-(1-alkynyl)anilines 1 are readily prepared by the Sonogashira coupling26 of N,N-dialkyl-o-iodoanilines 4 and terminal alkynes (eq 1). The results of this palladium/copper-catalyzed coupling process are summarized in the Supporting Information.
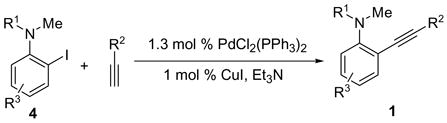 |
(1) |
The cyclization has proved to be a very general route to a variety of 3-substituted indoles (Table 2). Besides 4-nitrobenzenesulfenyl chloride, several other arylsulfenyl chlorides have also been successfully employed as electrophiles in this cyclization. When electron-deficient pentafluorobenzenesulfenyl chloride was employed, an 87% isolated yield of the corresponding indole was obtained (Table 2, entry 2). The more electron-rich arylsulfenyl chlorides phenylsulfenyl chloride and p-toluenesulfenyl chloride afforded similar high yields (Table 2, entries 3 and 4). When the more sterically demanding 2-nitrobenzenesulfenyl chloride was used, the yield of the cyclization product 3e decreased to 52% (Table 2, entry 5), although the starting material 1a was completely consumed. However, products of simple addition of the 2-nitrobenzenesulfenyl chloride to the triple bond of 1a were observed. Besides arylsulfenyl chlorides, an alkylsulfenyl chloride, trichloromethylsulfenyl chloride, has also been employed in this cyclization; however, this reagent only afforded a complex reaction mixture under our current reaction conditions.
Table 2.
Preparation of 3-Sulfenyl- and 3-Selenylindoles by n-Bu4NI-Induced Electrophilic Cyclizationa
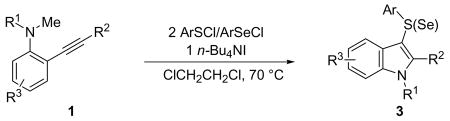 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | 1 | R1 | R2 | R3 | ArSCl/ArSeCl | time (h) | 3 | % yieldb |
| 1 | 1a | Me | C6H5 | H | p-O2NC6 H5SCl | 5 | 3a | 90 |
| 2 | 1a | Me | C6H5 | H | F5C6 SCl | 5 | 3b | 87 |
| 3 | 1a | Me | C6H5 | H | C6H5 SCl | 6 | 3c | 87 |
| 4 | 1a | Me | C6H5 | H | p-MeC6 H5SCl | 6 | 3d | 92 |
| 5 | 1a | Me | C6H5 | H | o-O2NC6 H5SCl | 5 | 3e | 52 |
| 6 | 1a | Me | C6H5 | H | C6H5SeCl | 5 | 3f | 84 |
| 7 | 1b | Me | C6H5 | 6-Me | p-O2NC6 H5SCl | 9 | 3g | 78 |
| 8 | 1c | Me | C6H5 | 5-Br | p-O2NC6 H5SCl | 6 | 3h | 85 |
| 9 | 1d | Me | C6H5 | 5-CO2Me | C6H5 SCl | 8 | 3i | 75 |
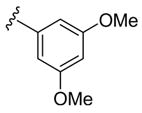
| 10 | 1e | Me |
|
H | p-O2NC6 H5SCl | 9 | 3j | 74 |
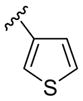
| 11 | 1f | Me |
|
H | p-O2NC6 H5SCl | 3 | 3k | 85 |
| 12 | 1g | Me |
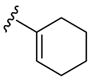
|
H | p-O2NC6 H5SCl | 3 | 3l | 91 |
| 13 | 1h | Me |
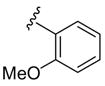
|
H | C6H5 SCl | 4 | 3m | 79 |
| 14 | 1i | C6H5 |
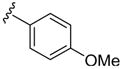
|
H | p-MeC6 H5SCl | 3 | 3n | 99 |
Representative procedure: N,N-dialkyl-2-(1-alkynyl)aniline1 (0.50 mmol), n-Bu4NI (0.50 mmol), arylsulfenyl/arylselenenyl chloride (1.00 mmol), and 5 mL of DCE were mixed in a sealed 4-dram vial. The reaction was stirred at 70 °C for the indicated time.
Isolated yields after column chromatography.
Despite our previous lack of success with the synthesis of 3-selenylindoles via analogous electrophilic cyclization chemistry,16a the cyclization of aniline 1a by PhSeCl plus n-Bu4NI was investigated. We were quite pleased to find that our current reaction conditions were equally suitable for the synthesis of 3-selenylindoles (Table 2, entry 6).
The electronic effect of the substituents on the aniline moiety in this electrophilic cyclization process has also been investigated. It turns out that this process is not particularly sensitive to electronic effects, which is in a good agreement with our previous experience with these electrophilic cyclization reactions, although the presence of a strong electron-withdrawing group can significantly reduce the nucleophilicity of the dialkylamino group.16 Thus, this cyclization proceeds nicely in the presence of either electron-withdrawing or electron-releasing groups (Table 2, entries 7–9). In all cases examined, high yields have been obtained with reaction times similar to those of the parent system 1a.
Besides 2-(phenylethynyl) anilines, other 2-(arylethynyl)anilines have also been successfully employed in this process (Table 2, entries 10 and 11). In the presence of an electron-rich thiophene ring, the reaction rate is considerably accelerated (Table 2, entry 11). The same high reaction rate was observed, when a vinylic moiety, such as a 1-cyclohexenyl group, was present on the triple bond (Table 2, entry 12). Interestingly, no product of addition of the 4-nitrobenzenesulfenyl chloride to the double bond of the 1-cyclohexenyl moiety was observed, although such an addition reaction has previously been reported.27
We have previously shown that ortho-methoxyaryl alkynes undergo electrophilic cyclization in the presence of an arylsulfenyl chloride, without the addition of n-Bu4NI, to form 3-sulfenylbenzofurans.17 We therefore investigated the reactivity of substrate 1h under our current cyclization conditions. Note that this alkyne contains an ortho-methoxyphenyl group at one end of the ethynyl functionality and an ortho-(N,N-dimethylamino)phenyl group at the other end. Thus, two cyclization paths are possible leading to the formation of an indole, a benzofuran or possibly a mixture of both. In practice, the 3-(phenylsulfenyl)indole 3m was generated exclusively with no benzofuran product being observed (Table 2, entry 13).
Finally, substrate 1i containing both a methyl and a phenyl group on the aniline nitrogen was studied in this cyclization. As expected, the N-phenylindole 3n was produced exclusively in essentially a quantitative yield (Table 2, entry 14).
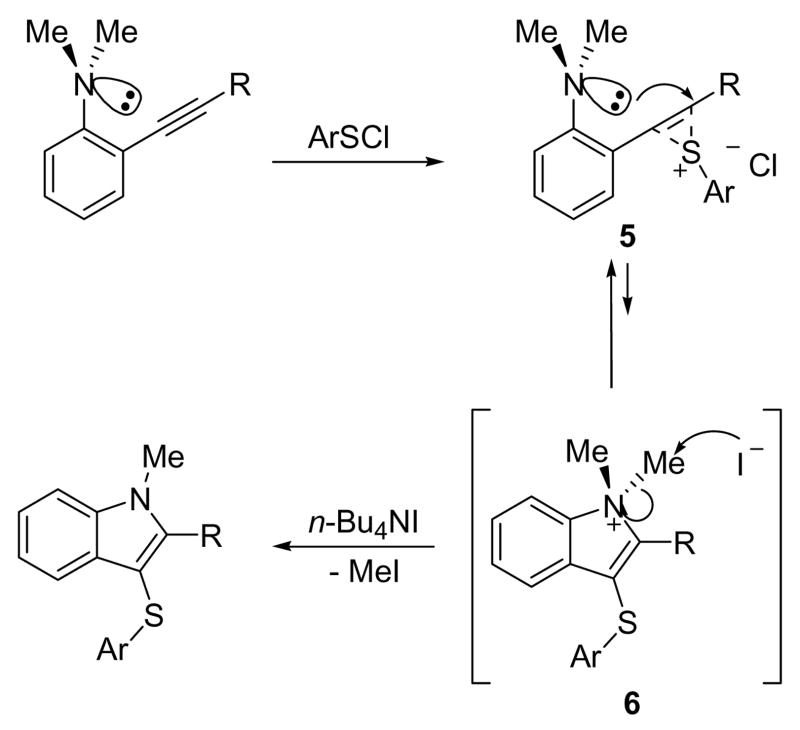
The role the n-Bu4NI plays in this process is uncertain at this point. However, we believe that this cyclization involves an anti addition of the sulfur electrophile and the nitrogen moiety of the aniline to the alkyne triple bond to form a transient 3-sulfenylindolium salt 6 via a sulfonium intermediate 5 (Scheme 2). In the presence of n-Bu4NI, 6 undergoes methyl group removal via SN2 displacement by the external iodide to complete indole ring construction, which is possibly the driving force to shift the equilibrium from the sulfonium species 5 to the indolium intermediate 6. However, our attempts to detect MeI and the indolium intermediate28 by 1H NMR spectroscopy in a reaction between 1a and 4-nitrobenzenesulfenyl chloride have been unsuccessful.29 We also cannot rule out activation of the arylsulfenyl chloride by n-Bu4NI through halogen exchange to form the corresponding arylsulfenyl iodide as the real electrophile.30
Scheme 2.
Plausible Mechanism for the Electrophilic Cyclization
The success of this cyclization reaction presumably relies on two factors: the presence of the two organic groups on the aniline nitrogen atom and the one equivalent of n-Bu4NI. In general, delocalization of the lone-pair of electrons on the aniline nitrogen into the aromatic ring π electron system through orbital overlap dramatically decreases its basicity and nucleophilicity. However, the steric bulkiness of the two organic groups on the nitrogen and the ortho-substituted internal triple bond forces rotation of the aromatic C-N bond and reduces this orbital overlap, resulting in considerable enhancement of the nitrogen nucleophilicity. Inductive electron donation by the two organic groups on nitrogen also raises the nitrogen nucleophilicity. With respect to n-Bu4NI, it provides the highly nucleophilic iodide ions needed to remove the methyl group from the indolium intermediate 6 and thus facilitates construction of the indole nucleus.
In conclusion, we have described a novel synthetic approach to 3-sulfenylindoles and 3-selenylindoles by the n-Bu4NI-induced electrophilic cyclization of N,N-dialkyl-2-(1-alkynyl)anilines and arylsulfenyl chlorides or arylselenyl chlorides. A wide variety of N,N-dialkyl-2-(1-alkynyl)anilines undergo this cyclization process in good to excellent yields. This procedure allows simultaneous construction of the indole ring system and the installation of a sulfenyl or selenyl functionality at the 3-position of the indole nucleus, which is a useful complement to the current synthetic approaches to 3-sulfenyl- and 3-selenylindoles. The preparation of biologically active 3-sulfonylindoles and 3-sulfenyl-5-substituted indoles, as well as a mechanistic study of the role of n-Bu4NI in this cyclization process, are currently underway.
Supplementary Material
Experimental procedures, characterization data of the new compounds and copies of 1H, 13C, and 19F NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We would like to acknowledge the National Institute of General Medical Sciences (GM070620 and GM079593) and the National Institutes of Health Kansas University Center of Excellence for Chemical Methodologies and Library Development (P50 GM069663) for financial support of this project. We also thank Johnson Matthey, Inc. and Kawaken Fine Chemicals Co., Ltd. for donations of palladium catalysts.
References
- 1.For selected recent reviews, see: Weng JR, Tsai CH, Kulp SK, Chen CS. Cancer Lett. 2008;262:153. doi: 10.1016/j.canlet.2008.01.033.Rieck GC, Fiander AN. Mol Nutr Food Res. 2008;52:105. doi: 10.1002/mnfr.200700138.Brancale A, Silvestri R. Med Res Rev. 2007;27:209. doi: 10.1002/med.20080.
- 2.Ragno R, Coluccia A, La Regina G, De Martino G, Piscitelli F, Lavecchia A, Novellino E, Bergamini A, Ciaprini C, Sinistro A, Maga G, Crespan E, Artico M, Silvestri R. J Med Chem. 2006;49:3172. doi: 10.1021/jm0512490. [DOI] [PubMed] [Google Scholar]
- 3.La Regina G, Edler MC, Brancale A, Kandil S, Coluccia A, Piscitelli F, Hamel E, De Martino G, Matesanz R, Díaz JF, Scovassi AI, Prosperi E, Lavecchia A, Novellino E, Artico M, Silvestri R. J Med Chem. 2007;50:2865. doi: 10.1021/jm061479u. [DOI] [PubMed] [Google Scholar]
- 4.Ramakrishna, V. S. N.; Shirsath, V. S.; Kambhampati, R. S.; Vishwakarma, S.; Kandikere, N. V.; Kota, S.; Jasti, V. PCT Int. Appl. WO 2007020653, 2007.
- 5.Funk CD. Nat Rev Drug Discovery. 2005;4:664. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 6.Armer, R. E.; Wynne, G. M. PCT Int. Appl. WO 2008012511, 2008.
- 7.Atkinson JG, Hamel P, Girard Y. Synthesis. 1988:480. [Google Scholar]
- 8.Matsugi M, Murata K, Gotanda K, Nambu H, Anilkumar G, Matsumoto K, Kita Y. J Org Chem. 2001;66:2434. doi: 10.1021/jo001710q. [DOI] [PubMed] [Google Scholar]
- 9.Tudge M, Tamiya M, Savarin C, Humphrey GR. Org Lett. 2006;8:565. doi: 10.1021/ol052615c. [DOI] [PubMed] [Google Scholar]
- 10.Schlosser KM, Krasutsky AP, Hamilton HW, Reed JE, Sexton K. Org Lett. 2004;6:819. doi: 10.1021/ol049956v. [DOI] [PubMed] [Google Scholar]
- 11.Campbell JA, Broka CA, Gong L, Walker KAM, Wang JH. Tetrahedron Lett. 2004;45:4073. [Google Scholar]
- 12.Yadav JS, Reddy BVS, Reddy YJ. Tetrahedron Lett. 2007;48:7034. [Google Scholar]
- 13.Maeda Y, Koyabu M, Nishimura T, Uemura S. J Org Chem. 2004;69:7688. doi: 10.1021/jo048758e. [DOI] [PubMed] [Google Scholar]
- 14.(a) Pezzella A, Palma A, Iadonisi A, Napolitano A, d’Ischia M. Tetrahedron Lett. 2007;48:3883. [Google Scholar]; (b) Wu G, Liu Q, Shen Y, Wu W, Wu L. Tetrahedron Lett. 2005;46:5831. [Google Scholar]; (c) Yadav JS, Reddy BVS, Krishna AD, Reddy CS, Narsaiah AV. Synthesis. 2005:961. [Google Scholar]; (d) Chakrabarty M, Sarkar S. Tetrahedron Lett. 2003;44:8131. [Google Scholar]
- 15.Shirani H, Stensland B, Bergman J, Janosik T. Synlett. 2006:2459. [Google Scholar]
- 16.(a) Yue D, Yao T, Larock RC. J Org Chem. 2006;71:62. doi: 10.1021/jo051549p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yue D, Larock RC. Org Lett. 2004;6:1037. doi: 10.1021/ol0498996. [DOI] [PubMed] [Google Scholar]
- 17.Yue D, Yao T, Larock RC. J Org Chem. 2005;70:10292. doi: 10.1021/jo051299c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue D, Larock RC. J Org Chem. 2002;67:1905. doi: 10.1021/jo011016q. [DOI] [PubMed] [Google Scholar]
- 19.Yao T, Yue D, Larock RC. J Org Chem. 2005;70:9985. doi: 10.1021/jo0517038. [DOI] [PubMed] [Google Scholar]
- 20.Zhou C, Dubrovskiy AV, Larock RC. J Org Chem. 2006;71:1626. doi: 10.1021/jo0523722. [DOI] [PubMed] [Google Scholar]
- 21.Yao T, Larock RC. J Org Chem. 2003;68:5936. doi: 10.1021/jo034308v. [DOI] [PubMed] [Google Scholar]
- 22.Yue D, Della Cá N, Larock RC. J Org Chem. 2006;71:3381. doi: 10.1021/jo0524573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Q, Hunter JA, Larock RC. J Org Chem. 2002;67:3437. doi: 10.1021/jo020020e. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Campo MA, Yao T, Larock RC. Org Lett. 2005;7:763. doi: 10.1021/ol0476218. [DOI] [PubMed] [Google Scholar]
- 25.Waldo JP, Larock RC. J Org Chem. 2007;72:9643. doi: 10.1021/jo701942e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(a) Sonogashira K. In: Metal-Catalyzed Cross-Coupling Reactions. Diederich F, Stang PJ, editors. Wiley-VCH; Weinheim, Germany: 1998. p. 203. Chapter 5. [Google Scholar]; (b) Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;50:4467. [Google Scholar]
- 27.(a) Schmid GH, Strukelj M, Dalipi S, Ryan MD. J Org Chem. 1987;52:2403. [Google Scholar]; (b) Akguen E, Hartke K, Kaempchen T. Arc der Pharm. 1981;314:72. [Google Scholar]
- 28.For an example of the preparation and characterization of a 3-iodoindolium triiodide species, see: Ten Hoedt RWM, Van Koten G, Noltes JG. Synth Commun. 1977;7:61.
- 29.We have been able to observe MeBr and the analogous selenium intermediate in our synthesis of benzoselenophenes. See: Kesharwani T, Worlikar SA, Larock RCJ. Org Chem. 2006;71:2307. doi: 10.1021/jo0524268.
- 30.For a selected example of the synthesis and reactivity of an arylsulfenyl iodide, see: Goto K, Yamamoto G, Tan B, Okazaki R. Tetrahedron Lett. 2001;42:4875.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, characterization data of the new compounds and copies of 1H, 13C, and 19F NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.