Abstract
To sense its population density and to trigger entry into the stress-resistant dauer larval stage, Caenorhabditis elegans uses the dauer pheromone, which consists of ascaroside derivatives with short, fatty acid-like side chains. Although the dauer pheromone has been studied for 25 years, its biosynthesis is completely uncharacterized. The daf-22 mutant is the only known mutant defective in dauer pheromone production. Here, we show that daf-22 encodes a homolog of human sterol carrier protein SCPx, which catalyzes the final step in peroxisomal fatty acid β-oxidation. We also show that dhs-28, which encodes a homolog of the human d-bifunctional protein that acts just upstream of SCPx, is also required for pheromone production. Long-term daf-22 and dhs-28 cultures develop dauer-inducing activity by accumulating less active, long-chain fatty acid ascaroside derivatives. Thus, daf-22 and dhs-28 are required for the biosynthesis of the short-chain fatty acid-derived side chains of the dauer pheromone and link dauer pheromone production to metabolic state.
Keywords: ascaroside, daf-22, dhs-28
Under favorable growth conditions, including low population density and high food availability, the nematode Caenorhabditis elegans progresses from the egg through 4 larval stages (L1 to L4) to the reproductive adult stage. However, if an L1 larva encounters high population density and low food availability, it will develop into an alternative L3 larval stage, the dauer (1–3). The dauer larva accumulates excess fat, its pharynx becomes sealed from the environment, and its cuticle becomes thicker, enabling it to survive harsh environmental conditions for prolonged periods. Dauer formation is controlled by multiple signaling pathways, including the transforming growth factor TGFβ (4–7) and the insulin/insulin-like growth factor IGF-1 (8–12) signaling pathways that converge on the DAF-12 nuclear hormone receptor (13–18), which is expressed in many of the tissues that undergo remodeling during dauer formation (19).
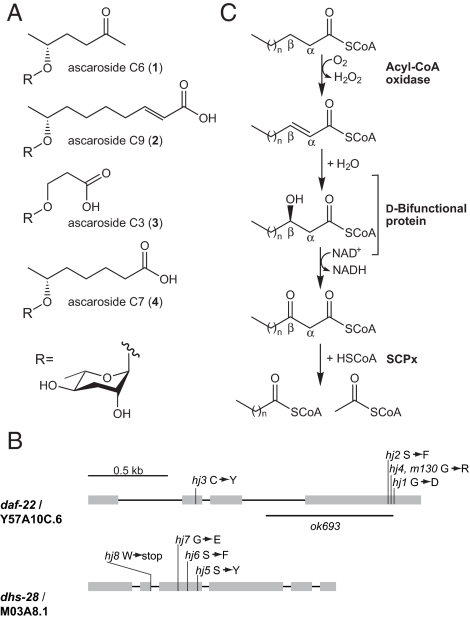
C. elegans senses its population density by a small-molecule pheromone, the dauer pheromone, which it continually secretes into its environment. More than 25 years ago, Golden and Riddle provided the first evidence for the dauer pheromone by showing that the “conditioned medium” from densely grown cultures of C. elegans could be used both to induce dauer formation and to inhibit dauer recovery (2, 3, 20). A crude dauer pheromone extract could be generated by drying down the conditioned medium and extracting the dried residue with organic solvent. By using activity-guided fractionation and NMR-based structure elucidation, the dauer pheromone has recently been shown to consist of several derivatives of the dideoxysugar ascarylose (21–23). The ascarosides differ only in the identity of the fatty acid-like side chain, and thus, we refer to them based on the carbon length of the side chains as ascaroside C6 (1) (22), ascaroside C9 (2) (22), ascaroside C3 (3) (23), and the much less potent ascaroside C7 (4) (21) (Fig. 1A).
Fig. 1.
Implicating DAF-22 and DHS-28 in dauer pheromone production. (A) The structures of the dauer pheromone components, ascaroside C6 (1), ascaroside C9 (2), ascaroside C3 (3), and the less active ascaroside C7 (4). (B) Gene structures of daf-22 and dhs-28. hj4 harbors the same lesion as the original daf-22 allele m130. ok693 is a deletion allele. The corresponding amino acid changes for each allele are shown. (C) The noninducible peroxisomal pathway for fatty acid β-oxidation. DHS-28 is homologous to human d-bifunctional protein, and DAF-22 is homologous to human SCPx thiolase.
The daf-22 mutant was originally identified as a mutant that fails to produce active conditioned medium that inhibits dauer recovery (24). No pheromone activity was detected in either the daf-22 conditioned medium or in organic extracts of the daf-22 worms themselves. Interestingly, the conditioned medium from daf-22 cultures did gradually develop the ability to inhibit dauer recovery if the cultures were grown for extended periods (24). In addition, if aqueous extracts from lysed adult daf-22 worms were incubated for extended periods at room temperature, they developed pheromone activity over time (D. Riddle, personal communication). This evidence led to the speculation that either (i) the daf-22 mutant could not secrete/excrete the dauer pheromone or (ii) the daf-22 mutant was able to synthesize some inactive precursor of the dauer pheromone that over time was processed to the dauer pheromone.
Here, we implicate 2 genes involved in fatty acid β-oxidation in dauer pheromone biosynthesis—daf-22 that encodes a homolog of the thiolase domain of human SCPx and dhs-28 that encodes a homolog of the dehydrogenase domain of human d-bifunctional protein. Extended culture of these strains results in the accumulation of dauer-inducing long-chain ascarosides, both in the worms themselves and in the culture medium. Thus, these strains are defective not in pheromone secretion/excretion, but rather pheromone biosynthesis, and produce an alternative set of weakly active, dauer-inducing molecules that accumulate over time. Additionally, both daf-22 and dhs-28 are expressed in the intestine, which is likely the site of pheromone biosynthesis.
Results
Cloning of daf-22 and dhs-28.
A fluorescently labeled fatty acid analog, C1-BODIPY-C12, has been used to monitor fat storage in live C. elegans animals (25, 26). In a forward genetic screen for fat-storage mutants that showed abnormal C1-BODIPY-C12 staining, we isolated multiple alleles that fell into 4 complementation groups. Here, we report the cloning of mutants from two of them. The first complementation group includes hj1, hj2, hj3, and hj4, and was mapped to chromosome II close to the genetic interval where daf-22 resided. Transgenic rescue and sequencing experiments led to the identification of a single ORF, Y57A10C.6, which harbored mutations in hj1, hj2, hj3, hj4, and m130, the canonical allele of daf-22 (Fig. 1B). Sequence analysis (supporting information (SI) Fig. S1A) suggests that daf-22 encodes a thiolase that is homologous to the thiolase domain of mammalian SCPx, a peroxisomal 3-ketoacyl-CoA thiolase that participates in straight-chain and branched-chain fatty acid β-oxidation and processing of bile acids (27) (Fig. 1C). Based on genetic mapping and transgenic rescue experiments (data not shown), we determined that the second complementation group, including hj5, hj6, hj7, and hj8, defined dhs-28 (Fig. 1B). dhs-28 encodes a dehydrogenase that shares greatest homology with the dehydrogenase domain of mammalian peroxisomal d-bifunctional protein, which acts upstream of SCPx (see Fig. S1B). We therefore speculated that DHS-28 may also act upstream of DAF-22 in C. elegans and asked whether they are part of a biosynthetic pathway for the production of dauer pheromone.
Characterization of Short-Term Cultures of daf-22 and dhs-28 Worms.
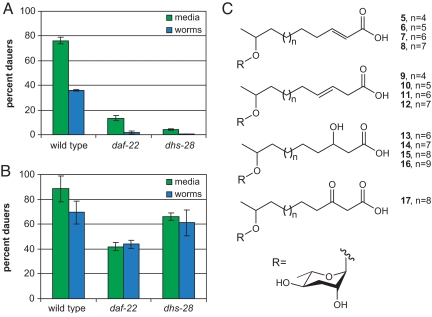
To study whether the dauer pheromone is produced by daf-22 and dhs-28 worms, wild-type, daf-22, and dhs-28 worms were grown in liquid culture until just after the Escherichia coli food source had been exhausted (10 d). The worms were filtered from the conditioned medium and washed, and both the worms and the conditioned medium were collected, freeze dried, and extracted with ethanol. Unlike the extracts obtained from wild-type cultures, the worm and conditioned medium extracts obtained from the daf-22 and dhs-28 cultures did not show any dauer-inducing activity in a dauer formation assay, in which the extracts were incorporated into an agar plate on which eggs were allowed to hatch and develop and dauer formation was monitored (Fig. 2A). The worm and conditioned medium extracts from the wild-type, daf-22, and dhs-28 cultures were compared by liquid chromatography mass spectrometry (LCMS). Peaks with the same mass and retention time as ascarosides C6, C9, and C7 were present in the worm and conditioned medium extracts from the wild-type cultures, but were absent in the worm and conditioned medium extracts from the daf-22 and dhs-28 cultures (Fig. S2A and data not shown). In addition, analysis by double quantum filtered (dqf)-COSY NMR confirmed the presence of the ascarosides, including C3 (which could not be detected by LCMS), in the wild-type worm and conditioned medium extracts and their absence in the daf-22 and dhs-28 worm and conditioned medium extracts (Fig. S2B and data not shown).
Fig. 2.
Comparison of the dauer-inducing activity in short-term and long-term cultures of wild-type, daf-22 and dhs-28 strains. (A) The dauer-inducing activity of worm and conditioned medium extracts from short-term (10 d) wild-type, daf-22 and dhs-28 cultures. The data represent the average of 2 experiments (±1 SD). (B) The dauer-inducing activity of worm and conditioned medium extracts from long-term (20 d) wild-type, daf-22 and dhs-28 cultures. The data represent the average of 2 experiments (±1 SD). (C) The chemical structures of the long-chain ascarosides from conditioned medium extracts from long-term dhs-28 cultures. The structural assignment of long-chain ascaroside 17 is tentative.
Characterization of Long-Term Cultures of daf-22 and dhs-28 Worms.
Prolonged culturing of daf-22 worms has previously been shown to result in active conditioned medium that can be used to block dauer recovery (24). To characterize the active molecules in long-term cultures, wild-type, daf-22, and dhs-28 worms were grown in liquid culture for an additional 10 d after the food had been exhausted (for a total duration of 20 d). The worms were filtered from the conditioned medium and washed, and both the worms and the conditioned medium were collected, freeze dried, and extracted with ethanol. The worm and conditioned medium extracts obtained from the daf-22 and dhs-28 cultures both showed dauer-inducing activity in a dauer formation assay (Fig. 2B). In particular, the activity of the conditioned medium from the dhs-28 culture approached that of the conditioned medium from the wild-type culture. LCMS and dqf-COSY analysis of the worm and conditioned medium extracts from the long-term daf-22 and dhs-28 cultures showed that ascarosides C6, C9, C3, and C7 were not present (Fig. S3 and data not shown).
To characterize the molecules responsible for this activity, we fractionated in parallel the conditioned medium and worm extracts from the long-term wild-type, daf-22, and dhs-28 cultures by high-pressure liquid chromatography (HPLC) and analyzed the activity of the fractions in the dauer formation assay. As indicated by their elution time, the active fractions from worm and media extracts from the daf-22 and dhs-28 cultures are of similar polarity and are less polar than the active fractions from the extracts from the wild-type cultures. Analysis of the dhs-28 conditioned medium active fractions by dqf-COSY and high-resolution mass spectrometry (HRMS) indicated that the active molecules are complex mixtures of ascaroside derivatives with longer-chain fatty acids that appear to be stalled at different steps in the β-oxidation pathway. The side chains of the longer-chain ascarosides present in the dhs-28 conditioned medium active fractions include α,β-unsaturated fatty acids (5-8), β-hydroxyl fatty acids (13–16), a β-keto fatty acid (17), as well as unusual β,γ-unsaturated fatty acids (9-12) (Fig. 2C; see Fig. S4 and Tables S1–S4 for NMR data and Materials and Methods for HRMS data). There was some degree of culture-to-culture variation in the molecules that were present. For comparison, ascaroside C9 is similar in structure to 5 through 8, but with n = 1. The daf-22 conditioned medium active fractions also appeared to contain long-chain ascarosides, based on the presence of characteristic peaks in the dqf-COSY spectrum, although these ascarosides were not present in sufficient quantities for further characterization (data not shown). None of the long-chain ascarosides (5–17) were detected in wild-type conditioned medium, as judged by LCMS. To verify the structural assignments of the long-chain ascarosides, a representative long-chain ascaroside (6) was synthesized (see SI Methods), and its polarity (as judged by its LCMS retention time) and dqf-COSY NMR spectrum were shown to be identical to that of natural 6 (Table S1). The EC50 of 6, when assayed in parallel with ascaroside C9 in the dauer formation assay at 25 °C, was 2.7 μM, compared with 820 nM for ascaroside C9, indicating that an ascaroside with a longer α,β-unsaturated fatty acid side chain is somewhat less potent than ascaroside C9, but still retains significant activity.
Dauer Pheromone Biosynthesis in the Intestine by DAF-22.
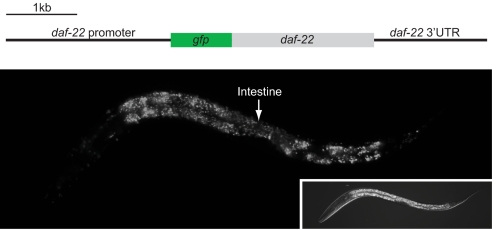
DAF-22 bears the canonical peroxisomal targeting signal, SKI, at its C terminus. To study the expression pattern and intracellular localization of DAF-22, we generated transgenic animals that expressed a DAF-22 fusion protein that was tagged with green fluorescent protein (GFP) at its N terminus, under the control of the native daf-22 promoter. The GFP-DAF-22 protein is strongly expressed in the intestine, the hypodermis, and the body wall muscle at all developmental stages (Fig. 3) and this expression pattern is shared by dhs-28 (data not shown). The punctate signal is consistent with peroxisomal targeting of the GFP-DAF-22 fusion protein. Furthermore, GFP-DAF-22 was retained in the cytoplasm when transgenic animals were subjected to prx-5 RNAi, which disrupts import of peroxisomal proteins (28) (data not shown).
Fig. 3.
Localization of the DAF-22 using a DAF-22 fusion protein tagged with GFP at its N terminus, the expression of which is under the control of the native daf-22 promoter. An L1 animal is shown.
To determine the tissue in which daf-22 is required for dauer pheromone biosynthesis, we expressed GFP-DAF-22 under the control of its native promoter or an intestine-specific promoter (vha-6). The 2 transgenic lines were grown in liquid culture, and the conditioned medium was collected, freeze-dried, and extracted with ethanol. Titration of the extracts in the dauer formation assay indicated that transgenic expression of DAF-22 in the intestine (compared with transgenic expression of DAF-22 under the control of the native promoter) was sufficient to restore production of a significant amount of dauer-inducing activity (16 ± 3% vs. 53 ± 9% dauers). Ascaroside C6, C9, and C3 were present in the extracts, as judged by LCMS and dqf-COSY analysis (Fig. S5). Therefore, the intestine is most likely the major site where dauer pheromone is synthesized.
Discussion
The biosynthesis of the active dauer pheromone components, ascarosides C6, C9, and C3, requires 2 genes involved in fatty acid β-oxidation, daf-22, a homolog of human SCPx thiolase, and dhs-28, a homolog of the human d-bifunctional protein. In humans, β-oxidation occurs in both the mitochondria and the peroxisomes. Mitochondrial β-oxidation degrades short-chain (<C8), medium-chain (C8-C12), and long-chain (C14-C20) fatty acids to acetyl-CoA for the generation of energy. Peroxisomal β-oxidation, however, degrades long- and very-long (>C20)-chain fatty acids to medium-chain fatty acids for subsequent export to the mitochondria and occurs through both inducible and noninducible pathways (27). SCPx and d-bifunctional protein are involved in the noninducible pathway in the peroxisome and catalyze the β-oxidation of straight-chain and branched-chain fatty acyl-CoAs and bile acid intermediates. The requirement for DAF-22 and DHS-28 in the production of the dauer pheromone suggests that in C. elegans pheromone production and thus developmental decisions are closely coupled to basic metabolic pathways in the peroxisomes.
The daf-22 and dhs-28 cultures latently accumulate ascarosides with long-chain fatty acid side chains, stalled at different stages in the β-oxidation process. These long-chain ascarosides may be intermediates in the dauer pheromone biosynthetic pathway. The long-chain ascarosides appear to accumulate in both the worms and in the culture media, but it is unclear whether the long-chain ascarosides are actively secreted/excreted from the worms or whether they are released on the death and eventual lysis of the worms. Earlier work suggested that dauer-inducing activity could be extracted from actively growing daf-22 worms by lysing them in aqueous buffer (24) and incubating the extract at room temperature for prolonged periods of time (D. Riddle, personal communication). We have been able to repeat these results, but we only obtain activity after incubating the extract at room temperature for ≈2 weeks, and even then we often do not obtain any activity for unknown reasons (data not shown). Thus, it is likely that the compounds present in the initial daf-22 worm extract are not active, but must instead be modified either enzymatically or nonenzymatically over time to become active. It is possible that long-chain ascarosides are responsible for the dauer-inducing activity in the aged daf-22 worm lysate and that the same processes that enable long-chain ascarosides to accumulate in daf-22 worms on prolonged culturing generate the long-chain ascarosides in the aqueous lysate. However, we have been unable to confirm this possibility given the low abundance of the active molecules.
There are at least 2 plausible models for the role of peroxisomal fatty acid β-oxidation in the biosynthesis of the dauer pheromone. In the first model, daf-22 and dhs-28 shorten long-chain fatty acids to short-chain fatty acids that are subsequently ω or ω-1 hydroxylated and attached to the ascarylose sugar to generate the dauer pheromone, ascarosides C6, C9, and C3. The accumulation of the long-chain ascarosides in the mutant strains may thus be a consequence of the accumulation of long-chain fatty acids, which are then nonspecifically hydroxylated and attached to the ascarylose sugar over time because of the promiscuity of the enzymes involved. In the second model, daf-22 and dhs-28 shorten long-chain ascarosides through β-oxidation to generate the dauer pheromone, ascarosides C6, C9, and C3. The presence of the long-chain ascarosides in the mutant strains may thus represent the accumulation of upstream intermediates in the dauer pheromone biosynthetic pathway. Interestingly, there is some evidence for the presence of very long-chain ascarosides in nematodes; they have been isolated from the internal lipid layer of the eggs of a range of nematode species (29, 30), where they provide a highly resistant barrier that protects the eggs from chemical treatments (31, 32). Typically, the side chains of these ascarosides are 32–34 carbons in length, and are either C-2 monols or C-2, ω-1 diols. Furthermore, SCPx thiolase and d-bifunctional protein have been shown to process bulky substrates such as bile acids, and thus, their C. elegans homologs DAF-22 and DHS-28 could potentially process a bulky long-chain ascaroside.
Both daf-22 and dhs-28 are expressed primarily in the intestine, suggesting that the ascarosides are produced in the intestine. Furthermore, expression of daf-22 in the intestine is sufficient to restore the production of ascarosides C6, C9, and C3. It is possible that the worms excrete rather than secrete the ascarosides. It is unclear why transgenic expression of DAF-22 is not sufficient to restore all dauer-inducing activity to the conditioned medium, given that it is able to restore production of ascarosides C6, C9, and C3. However, it is possible that DAF-22 expression in tissues other than the intestine is required for the production of some as-yet-unidentified dauer-inducing ascaroside.
Dauer pheromone biosynthesis in C. elegans is coupled to peroxisomal fatty acid β-oxidation. Thus, the dauer pheromone may reflect the metabolic activity of a population, not just the population density, and therefore may provide information to the animals regarding nutrient availability—another determinant for dauer arrest. Our findings suggest how the flux of fat metabolism may be translated into environmental signals (the dauer pheromone ascarosides) that govern reproductive development of a population of animals. Further study of dauer pheromone biosynthetic pathways will provide additional insights into the control mechanisms for dauer pheromone production.
Materials and Methods
Strains and General Culture Methods.
All strains were maintained at room temperature on NGM agar plates, which were made with granulated agar (BD Biosciences) and seeded with OP50 bacteria.
General Chemical Procedures.
NMR spectra of all natural compounds were recorded on a Varian VNMRS 600 NMR (600 MHz for 1H, 151 MHz for 13C). HRMS data for natural compounds were obtained on a Q-TOF-2 spectrometer (Micromass), equipped with an Alliance 2690/2695 HPLC system (Waters).
Genetic Screen.
Wild-type N2 animals were mutagenized with ethyl methanesulfonate (EMS) by using standard procedures. F2 progeny from mutagenized animals were grown on plates with C1-BODIPY-C12 (Invitrogen, D-3823), and adult animals that showed altered staining were selected. We screened 36,000 haploid genomes and retrieved mutants in 4 complementation groups. Genetic mapping was performed based on a SNP-based mapping strategy by crossing the mutants with the Hawaiian C. elegans isolate CB4856 (33). We mapped hj1 to LGII (uCE2–2131) and noted that animals bearing hj1 shared the same abnormal C1-BODIPY-C12 staining phenotype as the deletion mutant Y57A10C.6(ok693), which we studied in an independent survey of thiolase mutants. We isolated genomic DNA from animals bearing hj1, hj2, hj3, and hj4 and identified missense mutations in Y57A10C.6 in all cases by sequencing. Because Y57A10C.6 resides in the same genetic interval as daf-22, which also displays abnormal C1-BODIPY-C12 staining phenotype, we sequenced Y57A10C.6 in animals bearing the canonical daf-22 allele, m130, and found that it was identical to hj4. The daf-22p::gfp::daf-22 transgene rescued both the Daf-d and abnormal fat storage phenotypes of daf-22(ok693). We mapped hj5 to LGX between SNPs pkP6154 and pkP6155. Of the candidate genes we sequenced in this interval, we found mutations in dhs-28 in hj5, hj6, hj7 and hj8. All analysis on dauer pheromone was performed by using daf-22(ok693) and dhs-28(hj8).
Preparation of Conditioned Medium and Worm Extracts.
Crude dauer pheromone extract was prepared essentially according to the method of Golden and Riddle (2, 20); 4.5 L of worms were cultured in 4-L flasks (1.5 L per flask) for 10–20 d at 22.5 °C on a rotary shaker. Of saturated OP50, 4.5 L was resuspended in a small volume of S medium and half was added as a food source at day 1 and half at day 6. Conditioned medium was filtered through Whatman paper, and worms were collected from the paper by using distilled water. The worms were washed once and freeze-dried. The filtered conditioned medium was centrifuged to remove any remaining worms and freeze dried. The freeze-dried conditioned medium was pulverized by using a mortar and pestle, and the freeze-dried worms were pulverized with sodium chloride also by using a mortar and pestle. The freeze-dried conditioned medium and worms were extracted with 95% aqueous ethanol (0.45 L × 3) to afford conditioned medium and worm extracts. These extracts were then dried down, dissolved in 3 mL of methanol, filtered through cotton, and either analyzed by LCMS or dried down again and resuspended in 1 mL of CD3OD for dqf-COSY analysis.
Dauer Formation Assay.
The dauer formation assay was performed as described (22) by using plates made with Noble agar (BD Biosciences).
Analysis of the Long-Chain Ascarosides.
The wild-type, daf-22(ok693), and dhs-28(hj8) worm and conditioned medium extracts were fractionated directly by reversed phase HPLC on a C18 column (Supelco) by using an aqueous acetonitrile gradient (10–100%). Active fractions were identified by using the dauer formation assay and were characterized by dqf-COSY (see Fig. S4 and Tables S1–S4) and HRMS. HR-ESIMS (m/z): [M+Na]+ calculated for C18H32O6Na (5,9) 367.2097, found 367.2113; HR-ESIMS (m/z): [M+Na]+ calculated for C19H34O6Na (6,10) 381.2253, found 381.2255; HR-ESIMS (m/z): [M+Na]+ calculated for C20H36O6Na (7,11) 395.2410, found 395.2419; HR-ESIMS (m/z): [M+Na]+ calculated for C21H38O6Na (8,12) 409.2566, found 409.2578; HR-ESIMS (m/z): [M+Na]+ calculated for C20H38O7Na (13) 413.2515, found 413.2528; HR-ESIMS (m/z): [M+Na]+ calculated for C21H40O7Na (14) 427.2672, found 427.2667; HR-ESIMS (m/z): [M+Na]+ calculated for C22H42O7Na (15) 441.2828, found 441.2833; HR-ESIMS (m/z): [M+Na]+ calculated for C23H44O7Na (16) 455.2985, found 455.3013; HR-ESIMS (m/z): [M+Na]+ calculated for C22H40O7Na (17) 439.2672, found 439.2679.
Synthesis of Long-Chain Ascaroside, 6.
Experimental details regarding the synthesis of 6, as well as full characterization of synthetic intermediates and 6, can be found in SI Methods. Because natural 6 was isolated as a salt, synthetic 6 was converted to the salt (by addition of a 2-fold molar excess of sodium hydroxide) before comparative analysis with natural 6 by dqf-COSY (see Table S1).
Data Analysis.
EC50 values were determined by using Prism software. Each titration curve was fit with a sigmoidal curve with variable slope, in which the lower limit was set at 0 and the upper limit was not defined. The EC50 was defined as the concentration at which each ascaroside reached half its maximal activity (as calculated by Prism).
Transgenic Expression of daf-22.
All constructs are based on the pPD49.26 vector from Andy Fire that was modified by Jihong Bai. For daf-22p::gfp::daf-22, the 2.3-kb daf-22 promoter was amplified by PCR by using 5′-atatgcatgcatggctttaccaccaattgtaac-3′ and 5′-atatcccgggttttctggaacaatatttttttttcg-3′ and subcloned via SphI/SmaI sites. The daf-22 coding sequence and 3′ UTR was amplified by PCR by using 5′-aaatgcggccgctacgccaaccaagccaaaggtat-3′ and 5′-tatagctagcagttagttttttactagaagctg-3′ and subcloned via NotI/NheI sites. For vha-6p::gfp::daf-22, the vha-6 promoter was amplified by PCR by using 5′-gccagcatgctcaacgttgccagtga-3′ and 5′-aaatggatcctttttatgggttttggtaggttttagtc-3′ and subcloned via SphI/BamHI sites. The daf-22 cDNA was amplified by PCR by using 5′-aaatgcggccgctacgccaaccaagccaaaggtat-3′ and 5′-aaatgctagctcaaatcttggactgtgcag-3′ and subcloned via NotI/NheI sites. All transgenic lines were established by injecting the daf-22 constructs at 2 ng/μL, ttx-3::dsRed at 30 ng/μL, and pBluescript at 68 ng/μL into daf-22(ok693) mutant animals.
Supplementary Material
Acknowledgments.
We thank Donald Riddle for unpublished information; Jason Crawford and Dong-Chan Oh for discussion; and Andy Fire (Stanford University School of Medicine), Jihong Bai (Harvard Medical School), and Jeremy Dittman (Weill Cornell Medical College) for vectors. Some worm strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. This work was supported by Grant CA24487 (to J.C.) and by the Stowers Institute for Medical Research (H.Y.M.). R.A.B. was supported by National Research Service Award Postdoctoral Fellowship GM077943.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810338106/DCSupplemental.
References
- 1.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 2.Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 3.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: Developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 4.Georgi LL, Albert PS, Riddle DL. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990;61:635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- 5.Estevez M, et al. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- 6.Ren P, et al. Control of C. elegans larval development by neuronal expression of a TGFβ homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 7.Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003;17:844–858. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce SB, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 11.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 12.Ogg S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 13.Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development (Cambridge, U.K.) 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- 14.Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development (Cambridge, U.K.) 2004;131:1765–1776. doi: 10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- 15.Mak HY, Ruvkun G. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development (Cambridge, U.K.) 2004;131:1777–1786. doi: 10.1242/dev.01069. [DOI] [PubMed] [Google Scholar]
- 16.Rottiers V, et al. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Motola DL, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Gerisch B, et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- 20.Golden JW, Riddle DL. A Caenorhabditis elegans dauer-inducing pheromone and an antagonistic component of the food supply. J Chem Ecol. 1984;10:1265–1280. doi: 10.1007/BF00988553. [DOI] [PubMed] [Google Scholar]
- 21.Jeong PY, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 22.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 23.Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in C. elegans that acts synergistically with other components. Proc Natl Acad Sci USA. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden JW, Riddle DL. A gene affecting production of the Caenorhabditis elegans dauer-inducing pheromone. Mol Gen Genet. 1985;198:534–536. doi: 10.1007/BF00332953. [DOI] [PubMed] [Google Scholar]
- 25.Ashrafi K, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 26.Mak HY, Nelson LS, Basson M, Johnson CD, Ruvkun G. Polygenic control of Caenorhabditis elegans fat storage. Nat Genet. 2006;38:363–368. doi: 10.1038/ng1739. [DOI] [PubMed] [Google Scholar]
- 27.Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: An adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 28.Thieringer H, Moellers B, Dodt G, Kunau WH, Driscoll M. Modeling human peroxisome biogenesis disorders in the nematode Caenorhabditis elegans. J Cell Sci. 2003;116:1797–1804. doi: 10.1242/jcs.00380. [DOI] [PubMed] [Google Scholar]
- 29.Bartley JP, Bennett EA, Darben PA. Structure of the ascarosides from Ascaris suum. J Nat Prod. 1996;59:921–926. doi: 10.1021/np960236+. [DOI] [PubMed] [Google Scholar]
- 30.Jezyk PF, Fairbairn D. Ascarosides and ascaroside esters in Ascaris lumbricoides (Nematoda) Comp Biochem Physiol. 1967;23:691–705. doi: 10.1016/0010-406x(67)90334-9. [DOI] [PubMed] [Google Scholar]
- 31.Fairbairn D. The biochemistry of Ascaris. Exp Parasitol. 1957;6:491–554. doi: 10.1016/0014-4894(57)90037-1. [DOI] [PubMed] [Google Scholar]
- 32.Foor WE. Ultrastructural aspects of oocyte development and shell formation in Ascaris lumbricoides. J Parasitol. 1967;53:1245–1261. [PubMed] [Google Scholar]
- 33.Wicks SR, Yeh RT, Gish WR, Waterston RH, Plasterk RH. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet. 2001;28:160–164. doi: 10.1038/88878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.