Abstract
Hepatocyte death results in a sterile inflammatory response that amplifies the initial insult and increases overall tissue injury. One important example of this type of injury is acetaminophen-induced liver injury, in which the initial toxic injury is followed by innate immune activation. Using mice deficient in Tlr9 and the inflammasome components Nalp3 (NACHT, LRR, and pyrin domain–containing protein 3), ASC (apoptosis-associated speck-like protein containing a CARD), and caspase-1, we have identified a nonredundant role for Tlr9 and the Nalp3 inflammasome in acetaminophen-induced liver injury. We have shown that acetaminophen treatment results in hepatocyte death and that free DNA released from apoptotic hepatocytes activates Tlr9. This triggers a signaling cascade that increases transcription of the genes encoding pro–IL-1β and pro–IL-18 in sinusoidal endothelial cells. By activating caspase-1, the enzyme responsible for generating mature IL-1β and IL-18 from pro–IL-1β and pro–IL-18, respectively, the Nalp3 inflammasome plays a crucial role in the second step of proinflammatory cytokine activation following acetaminophen-induced liver injury. Tlr9 antagonists and aspirin reduced mortality from acetaminophen hepatotoxicity. The protective effect of aspirin on acetaminophen-induced liver injury was due to downregulation of proinflammatory cytokines, rather than inhibition of platelet degranulation or COX-1 inhibition. In summary, we have identified a 2-signal requirement (Tlr9 and the Nalp3 inflammasome) for acetaminophen-induced hepatotoxicity and some potential therapeutic approaches.
Introduction
Acetaminophen (N-acetyl-para-aminophenol [APAP]) hepatotoxicity is the most common cause of death due to acute liver failure in the developed world and is increasingly recognized as a significant public health problem (1, 2). The initial event in APAP-induced hepatotoxicity is a toxic-metabolic injury leading to hepatocyte death by necrosis and apoptosis. This results in secondary activation of the innate immune response involving upregulation of inflammatory cytokines with activation of NK cells, NKT cells, and neutrophils (3, 4). The molecular pathways for innate immune activation after hepatocyte death are of great interest, as they are likely common to sterile inflammation.
IL-1β is a very potent proinflammatory cytokine, and IL-1β levels are known to be increased during APAP hepatotoxicity (5, 6). In addition, signaling through the IL-1 receptor (IL-1R) was recently shown to be important in APAP-induced hepatotoxicity (7). The mechanisms by which IL-1β is upregulated during a sterile inflammatory response are not known. There are, however, extensive data on IL-1β upregulation by a variety a pathogens. Activation of TLRs by pathogen-associated molecular patterns (PAMPs) results in upregulation of pro–IL-1β via a MyD88/NF-κB pathway. Analogous to other potent inflammatory steps, production of IL-1β requires a second signal resulting in caspase-1–mediated cleavage of pro–IL-1β to release the active molecule (8–10).
We wished to identify the 2 signals that were responsible for IL-1β production in APAP hepatotoxicity. Tlr9 was of interest to us as a candidate molecule responsible for the first signal in sterile inflammation, because in addition to being activated by bacterial DNA rich in unmethylated CpG motifs, it can also be activated by DNA from mammalian cells (11, 12). When mammalian cells undergo apoptosis, genomic DNA is modified by caspase-activated DNase–mediated (CAD-mediated) cleavage and also aberrant methylation and oxidative damage (13–15). These apoptosis-mediated changes increase the ability of mammalian DNA to activate Tlr9 (16).
The activity of caspase-1 is regulated by a cytosolic protein complex called the inflammasome consisting of an NLR (nucleotide-binding domain, leucine-rich repeat–containing) family member, the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD), and caspase-1 (17). A variety of molecules can result in activation of NLR pathways. These include molecules from dying mammalian cells causing activation of the inflammasome via NACHT, LRR, and pyrin domain–containing protein 3 (Nalp3) and molecules from Gram-negative organisms causing activation via ICE protease–activating factor (Ipaf) (17). The Nalp3 inflammasome was of interest to us as a candidate molecule responsible for providing the second signal required for IL-1β activity in APAP hepatotoxicity, and this was tested using mice deficient in caspase-1, ASC, or Nalp3. We further aimed to identify clinically applicable strategies for downregulating the caspase-1 inflammasome pathway and test whether they provide protection from APAP-induced hepatotoxicity. Our studies show that there is a requirement for Tlr9 and components of the Nalp3 inflammasome for APAP-induced inflammation and full hepatotoxicity. The Tlr9 requirement is due to the activation of Tlr9 on liver sinusoidal endothelial cells by endogenous DNA from apoptotic hepatocytes. Tlr9 antagonists and the antiinflammatory drug aspirin significantly reduce sterile inflammation, and liver injury, by decreasing the activity of the Tlr9 pathway.
Results
Reduced mortality and liver injury in Tlr9–/– mice in response to APAP.
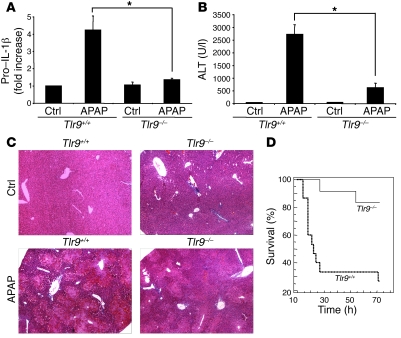
To test whether Tlr9 has a role in the upregulation of IL-1β, we quantified pro–IL-1β transcripts in the livers of Tlr9+/+ and Tlr9–/– mice 12 hours after a single toxic dose of APAP (i.p., 500 mg/kg). There was a significant increase in pro–IL-1β transcripts in the livers of Tlr9+/+ mice 12 hours after APAP injection, which was markedly smaller in Tlr9–/– mice (Figure 1A). To establish that the reduction in pro–IL-1β expression was associated with decreased liver injury, we assayed serum alanine transaminase (ALT) and examined liver histology 12 hours after APAP injection. In Tlr9–/– mice, serum ALT levels were significantly lower, and there was less hepatic hemorrhage and necroinflammation (Figure 1, B and C). To test whether the reduced pro–IL-1β expression and hepatotoxicity were associated with improved survival, we monitored Tlr9+/+ and Tlr9–/– mice over 72 hours after APAP. There was dramatically reduced mortality in the Tlr9–/– mice after APAP, compared with Tlr9+/+ mice (Figure 1D).
Figure 1. APAP-mediated hepatotoxicity is dependent on Tlr9.
(A) Increase in total liver pro–IL-1β transcript in Tlr9+/+ mice 12 hours after APAP (500 mg/kg), which is significantly smaller in Tlr9–/– compared with Tlr9+/+ mice (*P < 0.01). (B) Significantly lower serum transaminase levels in Tlr9–/– compared with Tlr9+/+ mice 12 hours after a single toxic dose of APAP (*P < 0.01). (C) Less liver hemorrhage and necroinflammation in Tlr9–/– compared with Tlr9+/+ mice 12 hours after APAP (H&E staining; original magnification, ×20). (D) Kaplan-Meier survival curves for Tlr9+/+ and Tlr9–/– mice over 72 hours after a single toxic dose of APAP (Tlr9+/+: n = 15, Tlr9–/–: n = 17, P < 0.04). Error bars indicate 1 SD. Ctrl, control.
TLR antagonists reduce APAP-induced liver injury.
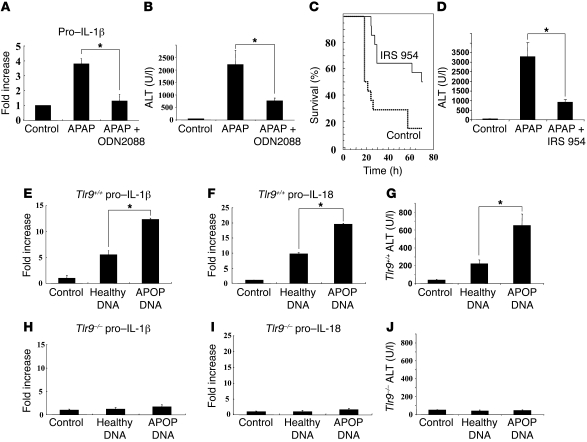
Having demonstrated reduced liver injury and improved survival in Tlr9–/– mice, we tested whether liver injury could be reduced in Tlr9+/+ mice with the administration of a Tlr9 antagonist. The Tlr9 antagonist ODN2088 (50 μg × 2) or PBS was injected i.p. into Tlr9+/+ mice immediately after and at 6 hours after APAP injection, and serum and total mouse liver were obtained at 12 hours after APAP injection for quantitative real-time PCR (Q-PCR) and ALT assay. Injection of ODN2088 significantly reduced pro–IL-1 β transcript and serum ALT levels (Figure 2, A and B). We next wanted to determine whether there was an improvement in survival from APAP hepatotoxicity using a TLR antagonist in clinical development. The immunoregulatory sequence (IRS) 954 can inhibit Tlr9 and Tlr7 and has been shown to ameliorate disease in models of systemic lupus erythematosus (18, 19). We administered IRS 954 (150 μg/mouse i.p.) immediately after a toxic dose APAP and at 14 and finally 28 hours. Administration of IRS 954 resulted in a significant decrease in serum transaminases at 12 hours and improved survival (Figure 2, C and D) (control: n = 14, IRS 954: n = 14, P < 0.006). This further confirms the importance of Tlr9 in APAP hepatotoxicity and also identifies a viable new therapeutic strategy that may be applicable in other diseases caused by a sterile inflammatory response.
Figure 2. Reduction in liver injury by Tlr9 antagonist and induction of liver injury with apoptotic DNA.
(A and B) Treatment of Tlr9+/+ mice with the Tlr9 antagonist ODN2088 significantly reversed the APAP-induced rise in liver pro–IL-1β transcript and serum transaminase levels (*P < 0.01). (C and D) The Tlr7 and Tlr9 antagonist IRS 954 significantly decreased mortality from APAP over 72 hours and also reduced elevations in serum ALT at 12 hours after APAP (control: n = 14, IRS 954: n = 14, P < 0.006; *P < 0.01). (E–G) Direct administration of DNA from apoptotic hepatocytes into the blood supplying the liver resulted in an increase in hepatic transcripts of pro–IL-1β and pro–IL-18 and serum transaminases in Tlr9+/+ mice. Both serum transaminase and pro–IL-1β and pro–IL-18 transcript levels were lower after injection of DNA from healthy hepatocytes (*P < 0.01). (H–J) In control Tlr9–/– mice, there were no significant changes in serum transaminases and hepatic transcripts of IL-1β and IL-18 after direct administration of DNA from apoptotic or healthy hepatocytes. Error bars indicate 1 SD.
DNA from apoptotic cells upregulates liver pro–IL-1β and pro–IL-18 in a Tlr9-dependent manner.
To directly test whether apoptotic DNA can upregulate pro–IL-1β and induce liver injury, we injected DNA (200 μg/mouse) from healthy and apoptotic hepatocytes directly into the portal vein of wild-type mice and examined upregulation of pro–IL-1β. Twelve hours after injection of DNA, there was significant upregulation of pro–IL-1β transcript and an increase in serum ALT levels (Figure 2, E and G). Pro–IL-1β upregulation and ALT elevations were significantly greater in response to DNA from apoptotic as compared with healthy cells. IL-18 is important in many types of liver injury and is also dependent on caspase-1 for cleavage and activation (20). There is substantial basal level of pro–IL-18 mRNA in many cell types, and this can be further upregulated by viral infection and bacterial products (21, 22). As was the case for pro–IL-1β, there was significant upregulation of pro–IL-18 in response to mammalian DNA, and this increase was greatest in response to DNA from apoptotic hepatocytes (Figure 2F). To confirm that upregulation of pro–IL-1β and pro–IL-18 and increase in serum ALT were due to actions of hepatocyte DNA via Tlr9, the experiments were performed in parallel in Tlr9–/– mice. There were no significant changes in either pro–IL-1β or pro–IL-18 and no increase in serum ALT (Figure 2, H–J).
DNA from apoptotic cells upregulates pro–IL-1β and pro–IL-18 in sinusoidal endothelium in a Tlr9-dependent manner.
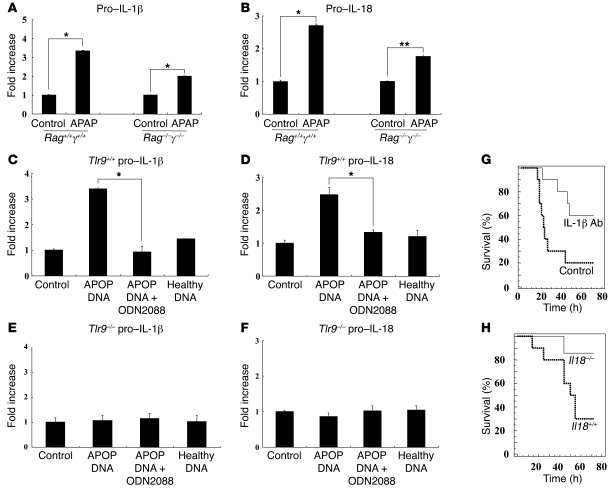
Having demonstrated an important role for Tlr9 in APAP- and DNA-induced liver injury, we were interested in identifying the liver cell type responding to Tlr9. A number of cell types in the liver have the ability to respond to Tlr9, including stellate cells and NKT cells (23). In the liver, however, Tlr9 is expressed primarily on sinusoidal endothelium, and we therefore focused on this cell type as a candidate for Tlr9 activation and upregulation of pro–IL-1β and pro–IL-18 (24). To initially test whether classic nonimmune cells such as endothelium are important in the upregulation of pro–IL-1β and pro–IL-18 after APAP-induced hepatotoxicity, we gave a toxic dose of APAP to genetically altered mice lacking the genes encoding Rag1 and common gamma chain (Rag1–/–γ–/–) and compared them with Rag1+/+γ+/+ mice. Rag1–/–γ–/– mice lack T, B, NK, and NKT cells, with other lineages reduced, and their livers still showed significant upregulation of pro–IL-1β and pro–IL-18, suggesting that nonimmune cells play a major role (Figure 3, A and B) (25).
Figure 3. DNA from apoptotic hepatocytes increases pro–IL-1β and pro–IL-18 transcript levels in primary liver endothelial cells, and this is inhibited by Tlr9 antagonist.
(A and B) To determine whether APAP-induced upregulation of pro–IL-1β and pro–IL-18 was dependent on immune cells, we examined the livers of Rag1–/–γ–/– mice, which lack most immune cell populations. There was significant upregulation of the transcripts of both cytokines in the livers of Rag1–/–γ–/– mice (*P < 0.001 and **P < 0.01). (C and D) Culture of primary mouse endothelial cells from Tlr9+/+ mice with DNA from apoptotic (APOP) but not healthy hepatocytes results in upregulation of pro–IL-1β and pro–IL-18, and this is downregulated by Tlr9 antagonist ODN2088 (*P < 0.001). (E and F) Culture of mouse endothelial cells from Tlr9–/– mice with DNA from apoptotic and healthy hepatocytes does not result in upregulation of pro–IL-1β and pro–IL-18. (G) To establish the importance of IL-1β in APAP hepatotoxicity, an anti–IL-1β antibody (0.2 mg per mouse) was used for in vivo neutralization. This demonstrates a significant increase in survival of wild-type mice in the presence of IL-1β neutralization compared with control antibody after APAP (control antibody: n = 10, anti–IL-1β: n = 10, P < 0.02). (H) To establish the importance of IL-18 in APAP hepatotoxicity, we treated Il18–/– and Il18+/+ mice with APAP. There was significantly better survival in Il18–/– compared with Il18+/+ mice (Il18+/+: n = 10, Il18–/–: n = 7, P < 0.036). Error bars indicate 1 SD.
We then directly tested whether DNA from apoptotic hepatocytes (50 μg/ml) can upregulate pro–IL-1β and pro–IL-18 in liver sinusoidal endothelial cells (LSECs) from wild-type mice and found significant upregulation 24 hours after culture (Figure 3, C and D). This upregulation of pro–IL-1β and pro–IL-18 was inhibited by the Tlr9 antagonist ODN2088 and did not occur in LSECs from Tlr9–/– mice (Figure 3, E and F). To confirm in vivo the importance of IL-1β and IL-18 in APAP-induced hepatotoxicity, we gave a single toxic dose of APAP to wild-type mice in which IL-1β had been neutralized and also to Il18–/– mice. In the absence of either IL-1β or IL-18, there was significantly reduced mortality compared with wild-type mice in response to APAP (Figure 3, G and H).
Reduced mortality and liver injury in mice lacking components of the Nalp3 inflammasome.
Pro–IL-1β and pro–IL-18 require cleavage to become biologically active, and this occurs predominantly via capsase-1 (10). This regulatory importance of caspase-1 is demonstrated by the fact that many cells constitutively synthesize pro–IL-18, but there is no functional IL-18 until cleavage and activation (26). The importance of caspase-1 in pro–IL-1β and pro–IL-18 processing has been known for some time, and recently the molecular components responsible for caspase-1 activation have been identified. These consist of a family of cytosolic proteins that form a complex called the inflammasome consisting of a NALP family member, the adaptor protein ASC, and caspase-1 (17). The best characterized of the NALP molecules that can activate caspase-1 is Nalp3, which itself can be activated by monosodium urate (MSU) and ATP. Another NLR family member, Ipaf, can also activate caspase-1 in response to Gram-negative bacteria.
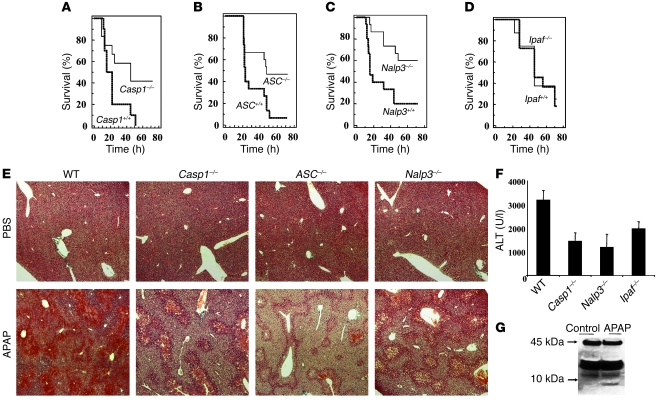
The important role of IL-1β and IL-18 in APAP-induced hepatotoxicity and their dependence on casapse-1 for activation directed us to investigate the role of the caspase-1 pathway in APAP hepatotoxicity. We tested the requirement for components of the inflammasome using mice deficient in caspase-1, ASC, Nalp3, or Ipaf (Casp1–/–, ASC–/–, Nalp3–/–, and Ipaf–/–). We found that Casp1–/–, ASC–/–, and Nalp3–/– mice were significantly less susceptible to APAP-induced injury than controls (Figure 4, A–C), but Ipaf –/– mice were not protected (Figure 4D). Histological analysis showed that there was less liver injury in the absence of caspase-1, ASC, or Nalp3 (Figure 4E). Measurement of serum ALT levels confirmed this by showing significantly reduced serum ALT in Casp1–/– and Nalp3–/– mice (P < 0.03; Figure 4F). This demonstrates a critical role for the Nalp3 inflammasome pathway and confirms the important role for IL-1β and IL-18 in APAP-induced liver injury. Total liver pro–IL-1β levels increased in Nalp3–/– mice to a degree comparable to that in Nalp3+/+ mice; however, there was no increase in serum IL-1β in Nalp3–/– mice, consistent with a role of Nalp3 in caspase-1 activation (Supplemental Figure 1, A and B; supplemental material available online with this article; doi: 10.1172/JCI35958DS1). In the liver, Tlr9 is predominantly expressed on sinusoidal endothelial cells, and it was therefore important to establish whether there is caspase-1 cleavage in these cells during APAP-induced hepatotoxicity. Indeed, 12 hours after administration of APAP, we isolated LSECs, and Western blot analysis showed cleavage of caspase-1 in these cells (Figure 4G).
Figure 4. APAP-mediated hepatotoxicity is dependent on the Nalp3, but not the Ipaf, inflammasome.
(A) Survival of Casp1–/– and control mice after i.p. injection of 500 mg/kg APAP (Casp1+/+: n = 12, Casp1–/–: n = 12, P < 0.04) (B) Survival of ASC–/– and control mice after APAP (ASC+/+: n = 15, ASC–/–: n = 15, P < 0.03). (C) Survival of Nalp3–/– and control mice after APAP (Nalp3+/+: n = 15, Nalp3–/–: n = 15, P < 0.006). (D) Survival of Ipaf–/– and control mice after APAP (Ipaf+/+: n = 12, Ipaf–/–: n = 8, P = NS). (E) H&E staining of livers (original magnification, ×20) from wild-type, Casp1–/–, ASC–/–, and Nalp3–/– mice 12 hours after i.p. injection of PBS or APAP showing reduced necroinflammation and hemorrhage in all the mice lacking components of the Nalp3 inflammasome. (F) Serum ALT from wild-type, Casp1–/–, Nalp3–/–, and Ipaf–/– mice 12 hours after APAP. In Casp1–/– and Nalp3–/– serum, ALT levels were significantly lower than those in wild-type serum after APAP (P < 0.03) (G) To confirm in vivo Casp1 activation in endothelial cells, 24 hours after administration of APAP or control PBS, liver sinusoidal cells were isolated and cleavage of caspase-1 detected by Western blotting. Error bars indicate 1 SD.
Aspirin inhibits the caspase-1 pathway and protects from APAP-induced mortality.
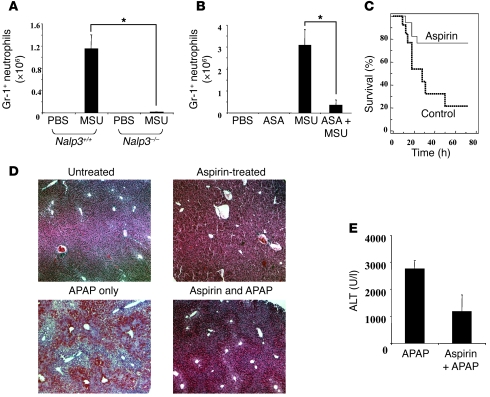
The above data have identified pathways critical for APAP hepatotoxicity that converge on caspase-1. One value of demonstrating new pathways in a disease process is that it can lead to novel therapies. In this context, our goal was to identify a safe, antiinflammatory agent that would inhibit the Nalp3/ASC/caspase-1 pathway. This agent could have therapeutic potential in APAP-induced hepatotoxicity and possibly other types of liver injury. To test candidate drugs, we utilized an established model of Nalp3 inflammasome activation in which i.p. injection of MSU crystals induces a neutrophilic peritonitis (27). First we confirmed that Nalp3 was required in this model by testing in mice deficient in Nalp3. As expected, Nalp3–/– mice had markedly less neutrophilic infiltrate at 3 hours compared with Nalp3+/+ mice challenged with MSU crystals (Figure 5A). Then we tested whether low-dose aspirin (acetylsalicylic acid [ASA]), a widely available, inexpensive, and safe drug could inhibit this pathway. We found an 8-fold reduction in neutrophil exudates when mice were pretreated with low-dose aspirin in drinking water for 3 days prior to induction of MSU peritonitis (Figure 5B). We next investigated whether low-dose aspirin would protect against APAP-induced liver injury. We found that low-dose aspirin administration protected against APAP-induced liver injury, as demonstrated by markedly improved survival and histology (Figure 5, C–E). When aspirin was given concordantly with APAP, it still offered significant, though reduced, protection (control: 22% ± 19% survival, aspirin at 6 mg/kg: 43% ± 11% survival, P < 0.04).
Figure 5. Aspirin inhibits the Nalp3 pathway and reduces APAP-induced liver injury.
(A) Nalp3+/+ or Nalp3–/– mice were injected with MSU crystals i.p. (3 mg/mouse). After 3 hours, peritoneal lavage was performed and the number of GR-1–positive neutrophils quantified (*P < 0.0001). (B) Wild-type mice were treated with or without aspirin (60 mg/l) in the drinking water for 3 days and then injected i.p. with MSU crystals or PBS. After 3 hours, peritoneal lavage was performed and the number of Gr-1–positive neutrophils quantified (*P < 0.0001). (C) Survival analysis of mice treated with and without aspirin (60 mg/l) in the drinking water for 3 days and then injected i.p. with APAP (500 mg/kg) (control drinking water: n = 13, aspirin drinking water: n = 17, P < 0.02). (D) H&E-stained liver tissue sections (original magnification, ×20) from wild-type mice 12 hours after i.p. injection with APAP or PBS. Mice were on aspirin or regular drinking water for 3 days prior to APAP injection. There is substantial reduction in APAP-induced liver injury and hemorrhage in mice receiving aspirin. (E) ALT levels in serum from wild-type mice 12 hours after APAP injection with and without pretreatment with aspirin. The aspirin-treated group had significantly lower serum ALT levels (P < 0.04). Error bars indicate 1 SD.
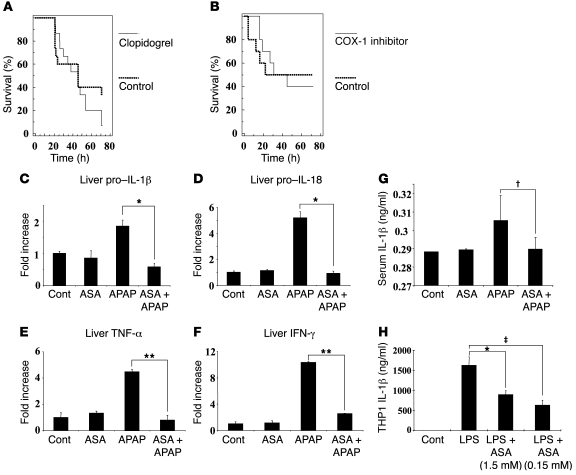
Aspirin has a number of well-characterized dose-dependent effects. At low dose (1–6 mg/kg/d), aspirin inhibits COX-1 and platelet degranulation and recently has been found to regulate gene transcription (28). At higher doses, there is inhibition of COX-2 and NF-κB. The dose we used (4–6 mg/kg) was lower than that required for COX-2 and NF-κB inhibition. COX-2 inhibition is also known to increase rather than decrease APAP-induced hepatotoxicity and was therefore unlikely to be a mechanism for the protective effects of aspirin (29). We therefore tested whether the protective effect of aspirin could be due to inhibition of COX-1 or platelet degranulation by administering the anti-platelet agent clopidogrel or COX-1 inhibitor SC-560. Inhibition of platelet degranulation or COX-1 prior to APAP exposure did not protect mice against APAP toxicity (Figure 6, A and B), suggesting that a novel mechanism accounted for the protective effects of low-dose aspirin.
Figure 6. Aspirin downregulates pro–IL-1β and pro–IL-18 transcripts.
(A) Survival after i.p. injection of APAP with and without clopidogrel by gavage (30 mg/kg every 24 hours) (PBS gavage: n = 15, clopidogrel gavage: n = 15, P = 0.31). Clopidogrel or PBS was gavaged every 24 hours beginning 48 hours prior and ending 24 hours after APAP injection. (B) Survival after i.p. injection of APAP with and without the COX-1 inhibitor SC-560. SC-560 (5 mg/kg) or control PBS was gavaged twice daily beginning 60 hours prior to and ending 48 hours after APAP injection (PBS gavage: n = 10, SC-560 gavage: n = 10, P = 0.97). (C–F) Q-PCR for pro–IL-1β, pro–IL-18, TNF-α, and IFN-γ from whole livers of mice treated as describe above. Shown are results from 1 representative experiment of 4; each group represents 3 mice (*P < 0.03, **P < 0.005). (G) ELISA for IL-1β from serum of mice given APAP with and without aspirin in drinking water (†P < 0.02). (H) ELISA for IL-1β from THP1 cells that were incubated overnight with control vehicle or various doses of aspirin and then for 8 hours with or without LPS. Data shown are from 1 representative experiment of 3 in which each treatment was performed in triplicate (‡P < 0.05, #P < 0.05). Error bars indicate 1 SD.
Due to the dependence of APAP toxicity on IL-1β and IL-18, and the recent demonstration of transcriptional downregulation of a number of genes by low-dose aspirin, we next examined whether aspirin reduced the upregulation of pro–IL-1β and pro–IL-18 transcripts induced by APAP (30). Using whole liver extracts, we found the level of pro–IL-1β and pro–IL-18 message by APAP hepatoxicity was reduced to normal with aspirin pretreatment (Figure 6, C and D). IL-1β and IL-18 are known to be potent stimulators of TNF-α and IFN-γ, respectively, and we confirmed that expression of TNF-α and IFN-γ was also reduced by aspirin treatment (Figure 6, E and F). To confirm changes in IL-1β levels in vivo, we assayed serum IL-1β levels from mice treated with APAP, with and without aspirin. As shown in Figure 6G, aspirin resulted in a significant reduction in the elevated IL-1β levels induced by APAP hepatotoxicity. Aspirin directly reduced IL-1β levels, as demonstrated by reduction in IL-1β from LPS-stimulated THP1 cells (human acute monocytic leukemia cell line) in response to aspirin (Figure 6H). Our data show that aspirin reduced IL-1β and IL-18 by decreasing transcript levels and identifies what we believe to be a novel antiinflammatory mechanism for aspirin.
Discussion
We have identified a 2-signal requirement for amplification of APAP-induced liver toxicity. Tlr9 provides a signal for the transcription of pro–IL-1β and pro–IL-18, and the Nalp3 inflammasome provides the signal for cleavage and activation of these pro-cytokines. In addition we have demonstrated the biological significance of mammalian DNA from apoptotic cells in activating Tlr9, expanding its known role as a stimulus for the development of autoimmunity to include induction of sterile inflammation (19, 23, 31).
Activation of Tlr9 results in upregulation of IL-1β and IL-18, and we have shown the importance of each of these cytokines by using neutralizing antibodies and genetically altered mice, respectively. Our findings support the recent report of the importance of IL-1R in the sterile inflammatory response and demonstrate the importance of IL-1β as an upstream signal (7). The requirement for IL-18 in APAP-induced liver injury is consistent with its known roles in immune-activation and infectious models of liver injury, including concanavalin A and LPS injury after Propionibacterium acnes priming (32). This study further expands the role of IL-18, demonstrating that it has an important and nonredundant function in the sterile inflammatory response to cellular death in the liver. In a model of sterile inflammation induced by i.p. injection of necrotic cells, Chen et al. (7) demonstrated that IL-1α was more important than IL-1β and that IL-18 had a minimal role. The importance of IL-1β and IL-18 in the liver, but not the peritoneum, highlights that pathways involved in the sterile inflammatory response have organ specificity, and this is likely due to the unique immune cellular composition of each organ. This conclusion is supported by the observed reduction in injury after myocardial infarction in the absence of caspase-1 activity (33).
Identification of DNA from apoptotic cells as an agonist for Tlr9 in APAP hepatotoxicity has important therapeutic implications in the near future, and we have demonstrated that a Tlr7 and -9 antagonist currently in clinical development can improve survival from APAP toxicity (Figure 2C). The signals required for activation of the Nalp3 inflammasome are not as well defined as those for Tlr9, with uric acid and ATP from dying hepatocytes being candidates (34, 35). The relative importance of these for Nalp3 activation in APAP hepatotoxicity needs to be established.
Our model builds on the known mechanism of APAP-induced toxic injury to hepatocytes and identifies DNA from apoptotic cells as a signal for immune activation (Figure 7). This model requires the presence of a sensing cell that detects and responds to the DNA from apoptosing cells. Such a cell would have to demonstrate upregulation of pro–IL-β and pro–IL-18 in response to apoptotic DNA and also caspase-1 activation in vivo. In the liver, Tlr9 is expressed predominantly on sinusoidal endothelial cells, and these were therefore prime candidates for the sensing cell population (24). We have shown that LSECs can be stimulated by mammalian apoptotic DNA in a Tlr9-dependent manner to upregulate pro–IL-1β and pro–IL-18 and that caspase-1 activation occurs in LSECs after APAP hepatotoxicity (Figure 3, C–F). The liver is, however, known to contain a very complex population of nonparenchymal cells, including Kupffer cells, NK cells, NKT cells, dendritic cells, and stellate cells. There is very limited information on the response of these populations to Tlr9 activation, although some are known to express Tlr9 (23, 36). The role of these populations in sensing DNA from apoptotic cells remains to be established. There are, however, substantial data demonstrating that IL-1β and IL-18 stimulate activation of liver nonparenchymal cells. Taken in association with the model shown in Figure 7, activation of liver nonparenchymal cells is predicted to occur downstream of the production of IL-1β and IL-18 by the sensing cell.
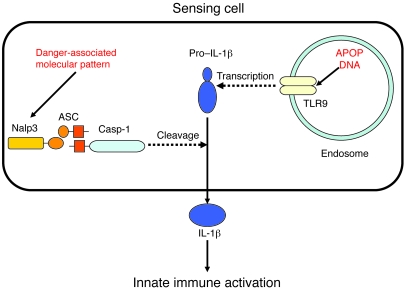
Figure 7. Production of mature IL-1β in APAP hepatotoxicity.
Release of mature IL-1β requires transcription of pro–IL-1β and subsequent cleavage and secretion by activated caspase-1. In APAP hepatotoxicity, apoptotic mammalian DNA has been shown to increase transcription of pro–IL-1β via a Tlr9-dependent pathway, and caspase-1 has been shown to be activated via a Nalp3/ASC pathway. The identity of the presumed danger-associated molecules responsible for activating the Nalp3 inflammasome in APAP hepatotoxicity remains to be determined.
This study also demonstrated that aspirin inhibits Nalp3 inflammasome–mediated inflammatory responses at a low dose (4–6 mg/kg) (Figure 5B). Previously, aspirin has been demonstrated to reduce liver injury but not improve survival from APAP in mice and rats when given at a dose range toxic to humans (200–600 mg/kg) (37, 38). Such high doses are known to inhibit COX-2, and the subsequent demonstration that COX-2 is protective in APAP toxicity may be the reason for the inability of aspirin at these doses to improve mortality (29). Due to the lack of affect on mortality and the toxic doses of aspirin, there were no clinical implications for these earlier findings.
Our findings represent the first demonstration to our knowledge of inhibition of the inflammasome-mediated pathway and reduction in transcription of inflammatory cytokines by aspirin (28). This is of value because the known ability of aspirin to inhibit COX-1 and COX-2 does not explain its antiinflammatory effects. Consistent with this, inhibition of COX-1 and COX-2 and platelet degranulation do not protect against APAP hepatotoxicity. The requirement of Nalp3 inflammasome–mediated inflammation in APAP-induced hepatotoxicity and the ability of aspirin to inhibit this pathway to the degree that it reduces liver injury and improves survival have significant clinical implications. If these findings are confirmed in humans, coformulation of aspirin with APAP may reduce hepatoxicity from APAP overdoses. Furthermore the Nalp3 inflammasome may have an important role in other forms of sterile inflammation, such as ischemic and nonalcoholic steatohepatitis.
Methods
Animals.
C57BL/6 mice were purchased from the National Cancer Institute. Nalp3–/–, Ipaf–/–, and ASC–/– mice were provided by A. Coyle, J. Bertin, and E. Grant (Millennium Pharmaceuticals) (39–41). All mice were backcrossed 9 generations onto the C57BL/6 background, except for Casp1–/– and Ipaf–/– mice, which were backcrossed 5–6 generations. Il18–/– and Tlr9–/– mice have been described previously and were a gift of S. Akira (Osaka University, Osaka, Japan) (42, 43). IL-1β was neutralized using the anti–IL-1β antibody from clone B122 (a gift of R. Schreiber, Washington University, St. Louis, Missouri, USA) at a dose of 0.2 mg/mouse i.v. twice a day for a total of 48 hours after administration of APAP. Control mice received Armenian hamster isotype control antibody. For survival experiments, animals were euthanized when they became moribund according to the criteria of lack of response to stimuli or lack of righting reflex. Animal protocols were approved by the Yale University IACUC.
APA-induced hepatotoxicity.
APAP (Sigma-Aldrich) solution was made fresh for each experiment in PBS at 20 mg/ml and heated in a water bath to 55°C to dissolve. APAP was dosed at 500 mg/kg and injected i.p. after 15 hours of starvation. Animals were euthanized by ketamine/xylazine injection at 12 hours for collection of serum, isolation of liver lymphocytes, or collection of liver tissue for histology, or they were observed every 4 hours for 72 hours until they became moribund.
Aspirin, clopidogrel, SC-560.
Aspirin (Sigma-Aldrich) was made fresh for each experiment. For dosing prior to APAP, aspirin was dissolved in single deionized water at 60 mg/l and heated to 42°C with rapid stirring to dissolve, then rapidly placed in an ice water bath to cool. Aspirin in water was given to the mice 60–72 hours prior to APAP injection. Assuming water consumption of 3–5 ml per day, this dose would be equivalent to a 300–500 mg dose in an 80 kg adult human. For coadministration of APAP and aspirin, aspirin was gavaged at a dose of 6 mg/kg in a volume of 100 μl of water immediately after i.p. injection of APAP. Clopidogrel (Gilead) was dissolved in PBS at 6 mg/ml and administered by gavage of 100 μl, 30 mg/kg, every 24 hours beginning 48 hours prior to and ending 24 hours after APAP injection. COX-1 inhibitor (SC-560; Cayman Chemical) was dissolved at 50 mg/ml in DMSO and further dissolved in PBS in order to gavage a dose of 5 mg/kg in 100 μl. It was administered twice per day beginning 60 hours prior to APAP and continued for 48 hours after APAP injection as previously established (44).
Uric acid peritonitis.
Peritonitis was induced with uric acid crystals as previously described by injecting 3 mg of MSU i.p. per mouse (27). Three hours after injection, peritoneal lavage was performed on mice euthanized by isoflurane inhalation. Neutrophil infiltration was evaluated by flow cytometry. The percentage of Gr-1–positive (BD Biosciences — Pharmingen) cells was multiplied by the total cell counts.
LSEC isolation.
After in situ pronase digestion, the nonparenchymal cell suspension was centrifuged for 5 minutes at 100 g to remove most of the parenchymal cells. This process was repeated until no pellet was observed. The supernatant, enriched in LSECs, was centrifuged for 10 minutes at 350 g. The pellet was resuspended in PBS and centrifuged for 10 minutes at 350 g. The cells were resuspended in PBS and layered (3.3 ml) on the top of a 2-step Percoll gradient (5 ml of 50% Percoll in the bottom and 6.6 ml of 25% Percoll in the top). The gradients were centrifuged at 900 g for 20 minutes, and the intermediate layer including LSECs was collected and cultured in the medium (EGM-2 MV Microvascular Endothelial Cell Medium-2; cc-4147; Lonza).
ODN2088 and IRS 954 injection.
Tlr9 antagonist (ODN2088, 50 μg × 2,) (Invivogen), or PBS, were injected i.p. into Tlr9+/+ mice immediately after and at 6 hours after APAP injection, and total mouse liver was obtained 12 hours after APAP injection for quantitative Q-PCR for IL-1β. Tlr9 antagonist IRS 954 was provided by A.F. Barrat (Dynavax Technologies). IRS 954 was injected i.p. at a dose of 150 μg per mouse immediately after APAP and at 14 and 28 hours.
Apoptotic DNA portal vein injection.
DNA from healthy and apoptotic hepatocytes (200 μg) was isolated using QIAGEN DNeasy Tissue Kit according to the manufacturer’s directions. Hepatocytes were cultured in 15 mm dishes and when near confluent were exposed to 600 mJ of ultraviolet irradiation using a UV Stratalinker 1800 (Stratagene). Cell apoptosis was evident 6 hours after irradiation in typical morphological changes. At this time, DNA was extracted and run on a standard ethidium bromide–stained gel to confirm DNA degradation consistent with apoptosis. DNA was injected via the portal vein in wild-type and Tlr9–/– mice. After 12 hours, total mouse liver was obtained for histology and Q-PCR for IL-1β and IL-18.
LSECs.
Primary mouse LSECs from the Tlr9+/+ and Tlr9–/– mice were cultured in the presence of apoptotic DNA (50 μg/ml) or apoptotic DNA plus Tlr9 antagonist (ODN2088; Invivogen). Twenty-four hours after culture, complementary DNA was prepared.
Q-PCR.
Q-PCR was performed for IL-1β and IL-18 using commercial primer-probe sets (Applied Biosystems Inc.) and the Applied Biosystems 7500 real-time PCR system. Expression of GAPDH was used to standardize the samples, and the results were expressed as a ratio relative to untreated HSCs. Quantitative real-time PCR was performed for mRNA expression of IL-18, IL-1β, TNF-α, and IFN-γ. Total mouse liver was obtained 12 hours after APAP injection, and cDNA was prepared. Q-PCR was performed for IL-18 and IL-1β using commercial primer-probe sets (Applied Biosystems Inc.) and the Applied Biosystems 7500 real-time PCR system. Expression of GAPDH was used to standardize the samples, and the results were expressed as a ratio relative to control.
Western blot analysis.
Caspase-1 Western blot analysis carried out according to standard protocols using cell lysate from 2 × 105 LSECs and anti–caspase-1 antibody (Santa Cruz Biotechnology Inc.). ELISA for serum IL-1β was carried out using antibody pairs from R&D Systems.
Cell line.
The human monocytic cell line THP1 was maintained in RPMI with 10% FBS. Stimulation with LPS (Sigma-Aldrich) was performed by plating cells at 5 × 105 per 24 well, incubating them overnight with aspirin or control media, and then adding LPS at 10 μg/ml for 8 hours.
Statistics.
Kaplan-Meier plots and statistical analysis were performed using MedCalc software version 9.2.0.1. Unpaired 2-tailed Student’s t test was used to compare groups. A P value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We are grateful to R. Schreiber (Washington University) for the generous gift of neutralizing anti–IL-1β antibody and to Kenneth Rock and Yan Shi (University of Massachusetts) for advice on preparing uric acid crystals. This work was supported by an Ellison Medical Foundation Grant (to R.A. Flavell) and NIH grants K08 AI065517 (to F.S. Sutterwala), R01DK076674-01A2 (to W.Z. Mehal), T32 DK7356 (A.B. Imaeda), and P30 DK34989.
Footnotes
Authorship note: Avlin B. Imaeda, Azuma Watanabe, and Muhammad A. Sohail contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: ALT, alanine transaminase; APAP, acetaminophen; ASC, apoptosis-associated speck-like protein containing a CARD; Ipaf, ICE protease–activating factor; IRS, immunoregulatory sequence; LSEC, liver sinusoidal endothelial cell; MSU, monosodium urate; Nalp3, NACHT, LRR, and pyrin domain–containing protein 3; NLR, nucleotide-binding domain, leucine-rich repeat–containing ; Q-PCR, quantitative real-time PCR.
Citation for this article: J. Clin. Invest. 119:305–314 (2009). doi:10.1172/JCI35958
See the related Commentary beginning on page 246.
References
- 1.Lee W.M. Acetaminophen toxicity: changing perceptions on a social/medical issue. Hepatology. 2007;46:966–970. doi: 10.1002/hep.21926. [DOI] [PubMed] [Google Scholar]
- 2.Kaplowitz N. Acetaminophen hepatoxicity: what do we know, what don’t we know, and what do we do next? Hepatology. 2004;40:23–26. doi: 10.1002/hep.20312. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z.X., Han D., Gunawan B., Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z.X., Govindarajan S., Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 5.Cover C., et al. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Fiorucci S., et al. A NO-releasing derivative of acetaminophen spares the liver by acting at several checkpoints in the Fas pathway. Br. J. Pharmacol. 2002;135:589–599. doi: 10.1038/sj.bjp.0704500. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Chen C.J., et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 8.Mariathasan S., et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 9.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 10.Ogura Y., Sutterwala F.S., Flavell R.A. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Vollmer J. TLR9 in health and disease. Int. Rev. Immunol. 2006;25:155–181. doi: 10.1080/08830180600743107. [DOI] [PubMed] [Google Scholar]
- 12.Lamphier M.S., Sirois C.M., Verma A., Golenbock D.T., Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann. N. Y. Acad. Sci. 2006;1082:31–43. doi: 10.1196/annals.1348.005. [DOI] [PubMed] [Google Scholar]
- 13.Enari M., et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 14.Huck S., Deveaud E., Namane A., Zouali M. Abnormal DNA methylation and deoxycytosine-deoxyguanine content in nucleosomes from lymphocytes undergoing apoptosis. FASEB J. 1999;13:1415–1422. doi: 10.1096/fasebj.13.11.1415. [DOI] [PubMed] [Google Scholar]
- 15.Lunec J., Herbert K., Blount S., Griffiths H.R., Emery P. 8-Hydroxydeoxyguanosine. A marker of oxidative DNA damage in systemic lupus erythematosus. FEBS Lett. 1994;348:131–138. doi: 10.1016/0014-5793(94)00583-4. [DOI] [PubMed] [Google Scholar]
- 16.Rifkin I.R., Leadbetter E.A., Busconi L., Viglianti G., Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol. Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 17.Mariathasan S., Monack D.M. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 18.Barrat F.J., Meeker T., Chan J.H., Guiducci C., Coffman R.L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 2007;37:3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 19.Barrat F.J., et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello C.A. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin. Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Pirhonen J., Sareneva T., Kurimoto M., Julkunen I., Matikainen S. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J. Immunol. 1999;162:7322–7329. [PubMed] [Google Scholar]
- 22.Kalina U., et al. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur. J. Immunol. 2002;32:2635–2643. doi: 10.1002/1521-4141(200209)32:9<2635::AID-IMMU2635>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe A., et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Armas M., et al. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J. Hepatol. 2006;44:939–946. doi: 10.1016/j.jhep.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Traggiai E., et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 26.Puren A.J., Fantuzzi G., Dinarello C.A. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 28.Wu K.K., Liou J.Y., Cieslik K. Transcriptional Control of COX-2 via C/EBPbeta. Arterioscler. Thromb. Vasc. Biol. 2005;25:679–685. doi: 10.1161/01.ATV.0000157899.35660.61. [DOI] [PubMed] [Google Scholar]
- 29.Reilly T.P., et al. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem. Res. Toxicol. 2001;14:1620–1628. doi: 10.1021/tx0155505. [DOI] [PubMed] [Google Scholar]
- 30.Wu K.K. Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Semin. Vasc. Med. 2003;3:107–112. doi: 10.1055/s-2003-40668. [DOI] [PubMed] [Google Scholar]
- 31.Viglianti G.A., et al. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/S1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsui H., Matsui K., Okamura H., Nakanishi K. Pathophysiological roles of interleukin-18 in inflammatory liver diseases. Immunol. Rev. 2000;174:192–209. doi: 10.1034/j.1600-0528.2002.017418.x. [DOI] [PubMed] [Google Scholar]
- 33.Pomerantz B.J., Reznikov L.L., Harken A.H., Dinarello C.A. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2871–2876. doi: 10.1073/pnas.041611398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akahoshi T., Murakami Y., Kitasato H. Recent advances in crystal-induced acute inflammation. Curr. Opin. Rheumatol. 2007;19:146–150. doi: 10.1097/BOR.0b013e328014529a. [DOI] [PubMed] [Google Scholar]
- 35.Duncan J.A., et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8041–8046. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsujimoto H., et al. A critical role of CpG motifs in a murine peritonitis model by their binding to highly expressed toll-like receptor-9 on liver NKT cells. J. Hepatol. 2006;45:836–843. doi: 10.1016/j.jhep.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Whitehouse L.W., Paul C.J., Thomas B.H. Effect of acetylsalicylic acid on a toxic dose of acetaminophen in the mouse. Toxicol. Appl. Pharmacol. 1976;38:571–582. doi: 10.1016/0041-008X(76)90188-5. [DOI] [PubMed] [Google Scholar]
- 38.De Vries J., et al. Protection against paracetamol-induced hepatotoxicity by acetylsalicylic acid in rats. Toxicology. 1984;30:297–304. doi: 10.1016/0300-483X(84)90140-9. [DOI] [PubMed] [Google Scholar]
- 39.Lara-Tejero M., et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuida K., et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 41.Sutterwala F.S., et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Takeda K., et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/S1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 43.Hemmi H., et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 44.Schmassmann A., et al. Role of the different isoforms of cyclooxygenase and nitric oxide synthase during gastric ulcer healing in cyclooxygenase-1 and -2 knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G747–G756. doi: 10.1152/ajpgi.00416.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.