Abstract
The clinical syndrome of acetaminophen-induced liver injury represents the combined result of drug toxicity and a potent innate immune response that follows drug-induced cell death. In this issue of the JCI, Imaeda and colleagues report that DNA released from dying hepatocytes is a key stimulus of innate immune activation in the acetaminophen-treated mouse liver (see the related article beginning on page 305). They present evidence indicating that hepatocyte DNA promotes immune activation by acting as a danger-associated molecular pattern (DAMP) that stimulates cytokine production in neighboring sinusoidal endothelial cells via Tlr9 and the Nalp3 inflammasome.
The analgesic acetaminophen is widely known for its potential to cause severe and sometimes lethal liver injury. When ingested in large amounts, acetaminophen overwhelms the normal metabolic pathways of glucuronidation and sulfation and undergoes oxidation to form the highly reactive intermediate N-acetyl-p-benzoquinone-imine (NAPQI). NAPQI is not harmful if it combines rapidly with glutathione; however, when hepatic glutathione stores are depleted, NAPQI escapes detoxification, resulting in liver cell death (1). An important but underappreciated aspect of acetaminophen toxicity is that direct, drug-induced harm accounts for only part of the overall syndrome of acetaminophen-induced liver injury. The reason for this is that the initial wave of drug-induced hepatocellular destruction is followed by a robust innate immune response, in which invading inflammatory cells release toxic oxidants and cause a second wave of destruction. The collateral damage inflicted by inflammatory cells can be so severe as to double the degree of tissue injury caused by acetaminophen alone (2).
Innate immunity is the result of danger signaling
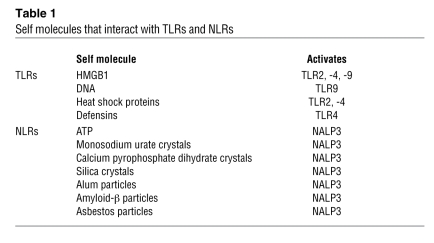
Activation of the innate immune system following a noninfectious insult such as drug toxicity arises when dying cells release molecules that serve as “danger signals.” Danger molecules trigger inflammation by engaging pattern recognition receptors such as TLRs (3) and nucleotide-binding domain, leucine-rich repeat–containing proteins (NLRs) (4) and are thus referred to as danger-associated molecular patterns (DAMPs) (5). Through TLRs, DAMPs signal cytokine and chemokine production and upregulate the expression of cell-adhesion molecules. When DAMPs interact with NLRs, they stimulate NLRs to complex with ASC (apoptosis-associated speck-like protein containing a CARD) to form macromolecular complexes called inflammasomes, which activate caspase-1 and stimulate cleavage of the proinflammatory cytokines pro–IL-1β and pro–IL-18 to their mature forms, IL-1β and IL-18 (6). Self molecules that act as DAMPs interact primarily with TLR2, -4, and -9 and an NLR with an N-terminal pyrin domain designated NACHT, LRR, and pyrin domain–containing protein 3 (NALP3; also known as NLRP3). The list of these molecules is rapidly growing (Table 1), emphasizing the importance of endogenous danger signaling to a broad array of medical disorders ranging from gout to systemic lupus erythematosus to Alzheimer disease (7–9). A danger molecule that is believed to play a central role in inflammatory diseases of the liver is the chromatin-binding protein high-mobility group box 1 (HMGB1). Upon cellular necrosis, HMGB1 is released into the extracellular milieu, where it activates TLR2 and TLR4 and, when complexed with DNA, TLR9 (10, 11). Studies to date indicate that HMGB1 plays an important role in liver injury due to hemorrhagic shock and ischemia/reperfusion (12), but, interestingly, it does not strongly influence acetaminophen toxicity (13).
Table 1 .
Self molecules that interact with TLRs and NLRs
Danger signaling in acetaminophen hepatotoxicity
Although the precise nature of the danger signal that activates innate immunity in the acetaminophen-exposed liver is uncertain, there is no question that danger signaling is involved in the process. This is evident from studies in a mouse strain with a mutation in Tlr4, in which liver disease is significantly attenuated following an acetaminophen challenge (14). Recently, acetaminophen toxicity was shown to be reduced in mice that lack the ability to respond to IL-1 (15). This observation is of interest because IL-1 secretion typically requires the combined activity of TLRs and NLRs, the former to stimulate expression of the IL-1 propeptide and the latter to process the propeptide into a mature cytokine. It also suggests a need for two, possibly unique, danger signals in the liver: one to activate a TLR and another to signal NLR assembly into an inflammasome (6).
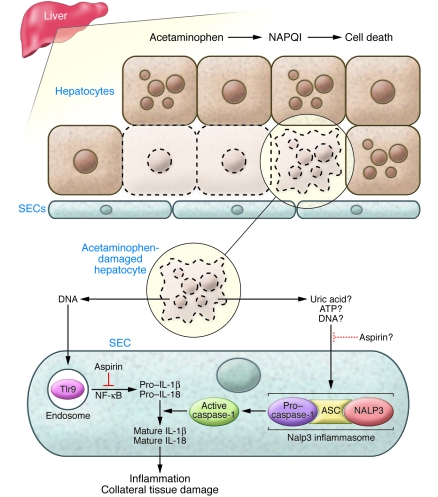
If the danger signal that augments acetaminophen-induced liver injury is not HMGB1, then another molecule that could accomplish this task is DNA from dying hepatocytes. DNA interacts specifically with TLR9, which, like all nucleic acid–sensing TLRs, is sequestered intracellularly within endosomes. TLR9 was once considered incapable of binding mammalian DNA because of its affinity for unmethylated CpG motifs characteristic of microbial DNA. DNA from injured mammalian cells, however, has the capacity to activate TLR9 (8, 16), and recently even normal mammalian DNA has been shown to engage this receptor and stimulate an immune response (17). In this issue of the JCI, Imaeda and colleagues (18) demonstrate that DNA is indeed a trigger of the innate immune response that amplifies acetaminophen toxicity. They showed, in a mouse model, that acetaminophen-induced liver injury is dependent upon not only IL-1β, but also IL-18, the two cytokines classically processed by caspase-1 (Figure 1). They determined that acetaminophen-mediated induction of pro–IL-1β and pro–IL-18 mRNA in the liver is Tlr9 dependent; in addition, they showed that cleavage of the IL-1β propeptide to the mature cytokine in the acetaminophen-treated liver requires the presence of the Nalp3 inflammasome. Inhibition of either Tlr9 signaling or Nalp3 activity by genetic or pharmacologic means markedly attenuated liver injury and improved survival in acetaminophen-treated mice. These results indicate that Tlr9 and Nalp3 must both be functional to activate the innate immune system following acetaminophen exposure, and they underscore the degree to which immune-mediated collateral damage intensifies drug-induced liver injury.
Figure 1. DNA-mediated danger signaling in acetaminophen toxicity.
Acetaminophen is directly cytotoxic to hepatocytes through its conversion to the reactive intermediate NAPQI (1). In this issue of the JCI, Imaeda and colleagues provide evidence that DNA released from acetaminophen-damaged hepatocytes induces an innate immune response in the liver that augments the injury caused by the drug alone (18). They show in a mouse model that acetaminophen, which damages hepatocytes, as well as damaged DNA per se, activates Tlr9 within SECs, thereby stimulating the production of pro–IL-1β and pro–IL-18. Acetaminophen treatment also promotes activation of the Nalp3 inflammasome in SECs, whose function is to activate caspase-1 and promote the cleavage of pro–IL-1β and pro–IL-18 to their mature, active forms (6). (The specific stimulus to Nalp3 activation in the acetaminophen-treated liver is unknown, but potential candidates include uric acid, ATP, and DNA.) IL-1β and IL-18 in turn enhance acetaminophen-induced liver injury by promoting hepatic inflammation and secondary tissue damage. Imaeda et al. further demonstrate that this immune-mediated amplification of acetaminophen-induced liver injury can be blocked by aspirin. Aspirin prevents hepatic induction of pro–IL-1β and pro–IL-18 and may have an independent inhibitory effect on the Nalp3 inflammasome.
Imaeda et al. (18) did not demonstrate that DNA released specifically from dying hepatocytes is the stimulus for Tlr9 activation in the liver following acetaminophen treatment. They did, however, demonstrate that DNA from dead (UV-irradiated) hepatocytes upregulates IL-1β in a Tlr9-dependent manner when infused into a normal liver in vivo. They also made the unique observation that much of the innate immune response to acetaminophen poisoning is mediated by sinusoidal endothelial cells (SECs), which are actively endocytic cells. TLR9 is expressed by several resident liver cells, including hepatocytes, SECs, Kupffer cells, and stellate cells, but among these, SECs exhibit the greatest potential for taking up extracellular DNA (19). Purified SECs displayed DNA-mediated activation of Tlr9 and acetaminophen-mediated activation of caspase-1 (18), which pinpoints these cells as immune effectors in acetaminophen toxicity and places them in a category with bone marrow–derived cells as sites of the molecular machinery of the inflammasome.
Viewing immune amplification of acetaminophen-induced liver injury as a therapeutic target, Imaeda and colleagues subsequently investigated whether the immune-mediated collateral damage in an acetaminophen-treated liver was preventable by administration of an antiinflammatory agent (18). Aspirin, when given before acetaminophen, significantly protected mice against acetaminophen-induced liver injury. Indeed, aspirin-pretreated mice fared as well or better after acetaminophen challenge than mice lacking Tlr9 or components of the Nalp3 inflammasome. The protective effect of aspirin coincided with reduced induction of hepatic pro–IL-1β and pro–IL-18 mRNA after acetaminophen administration. This suggests that aspirin is exerting its protective effect on the Tlr9 arm of the danger pathway during acetaminophen toxicity, perhaps by inhibiting IκB kinase β (20). Aspirin also inhibited experimental inflammation induced by monosodium urate crystals, which activate Nalp3, but it remains unclear whether this is interpretable as an independent effect of aspirin on caspase-1 activation by the Nalp3 inflammasome (Figure 1).
Novel concepts and open questions
Overall, the work of Imaeda and colleagues (18) sheds important light on the role of the inflammasome in drug-induced liver injury. It highlights potential differences between innate immune responses to different stimuli (e.g., drugs versus ischemia/reperfusion) and places SECs in a category with bone marrow–derived cells as orchestrators of innate immune responses to DNA. Still, there remain some unanswered questions. One is whether bone marrow–derived cells, which also contribute to innate immune activation in response to acetaminophen, sense DNA as their danger signal in the same fashion as SECs. Another is whether DNA from hepatocytes treated with acetaminophen has any unique characteristics with respect to Tlr9 activation. Third, one wonders whether immune-mediated collateral damage in acetaminophen toxicity is due entirely to leukocyte invasion, or whether inflammasome-mediated cell death is also involved. Under certain circumstances, NALP3, ASC, and caspase-1 can interact to cause cell death (21). Such a pathway could be operative in SECs during acetaminophen toxicity. If so, this may explain why innate immune activation in the liver is so often accompanied by sinusoidal cell breakdown and parenchymal hemorrhage, which in many animal models of acute liver injury is the fatal event.
Imaeda and coworkers (18) posited that two separate danger signals are required to activate IL-1β and IL-18 in the acetaminophen-treated liver: hepatocyte DNA to induce cytokine gene expression via Tlr9 and another molecule, possibly uric acid or ATP, to activate the Nalp3 inflammasome and promote cytokine cleavage. This theory was logical, based on evidence that few if any compounds stimulate both TLRs and NLRs. Recent work by Muruve et al. (22), however, indicates that mammalian DNA can promote inflammasome formation, albeit in the absence of NALP3. Further research in this area may ultimately lead to a unified theory according to which self DNA activates not only TLR9 but also the NALP3 inflammasome following a cytotoxic insult in vivo, culminating in immune-mediated collateral damage to the affected organ.
Acknowledgments
This work is supported by grants R01 DK061510, R01 DK068450, and P30 DK026743 (UCSF Liver Center).
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: DAMP, danger-associated molecular pattern; HMGB1, high-mobility group box 1; NALP3, NACHT, LRR, and pyrin domain–containing protein 3; NAPQI, N-acetyl-p-benzoquinone-imine; NLR, nucleotide-binding domain, leucine-rich repeat–containing protein; SEC, sinusoidal endothelial cell.
Citation for this article: J. Clin. Invest. 119:246–249 (2009). doi:10.1172/JCI38178.
See the related article beginning on page 305.
References
- 1.Gunawan B.K., Kaplowitz N. Mechanisms of drug-induced liver disease. Clin. Liver Dis. 2007;11:459–475. doi: 10.1016/j.cld.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z.X., Han D., Gunawan B., Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T., Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 4.Ye Z., Ting J.P. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr. Opin. Immunol. 2008;20:3–9. doi: 10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi M.E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 6.Mariathasan S., Monack D.M. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 7.Halle A., et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamphier M.S., Sirois C.M., Verma A., Golenbock D.T., Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann. N. Y. Acad. Sci. 2006;1082:31–43. doi: 10.1196/annals.1348.005. [DOI] [PubMed] [Google Scholar]
- 9.Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 10.Park J.S., et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 11.Tian J., et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 12.Tsung A., et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 14.Yohe H.C., et al. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G1269–G1279. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]
- 15.Chen C.J., et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 16.Ishii K.J., et al. Genomic DNA released by dying cells induces the maturation of APCs. . J. Immunol. 2001;167:2602–2607. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 17.Haas T., et al. The DNA sugar backbone 2’ deoxyribose determines toll-like receptor 9 activation. Immunity. 2008;28:315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Imaeda A.B., et al. Acetaminophen-induced hepatotoxicity in mice is dependent on TLR9 and the Nalp3 inflammasome. J. Clin. Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Armas M., et al. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J. Hepatol. 2006;44:939–946. doi: 10.1016/j.jhep.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Yin M.J., Yamamoto Y., Gaynor R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 21.Ting J.P., Willingham S.B., Bergstralh D.T. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 22.Muruve D.A., et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]