Abstract
Glucocorticoids (GCs) play a critical role in neural development; however, their prenatal or neonatal therapeutic use can have detrimental effects on the developing brain. In this issue of the JCI, Heine and Rowitch report that the molecular mechanisms underlying these detrimental effects involve the sonic hedgehog (Shh) signaling pathway, a crucial regulator of brain development and neural stem/progenitor cells (see the related study beginning on page 267). They show that GCs suppress Shh-induced proliferation of cerebellar progenitor cells in postnatal mice and that, conversely, Shh signaling is protective against GC-induced neonatal cerebellar injury by inducing the enzyme 11βHSD2, which inactivates the GCs corticosterone and prednisolone, but not dexamethasone. The data provide a rationale for the therapeutic use of 11βHSD2-sensitive GCs, but not dexamethasone, or for the exploitation of the neuroprotective effect of Shh agonists to prevent GC-induced pre- or neonatal brain injury.
Corticosteroids and the brain
The corticosteroid glucocorticoids (GCs), together with the sex steroids estrogen, progesterone, and testosterone, belong to the large family of steroid hormones. GCs are secreted by the adrenal cortex and exert their effects in target cells via binding to specific intracellular receptors (e.g., mineralocorticoid receptor and GC receptor [GR]) that activate or repress target gene transcription, although nongenomic signaling activity has also been reported (1). GRs mediate the effects of high levels of endogenous GCs, such as corticosterone (Cort) and cortisol, and also bind synthetic steroids, such as prednisolone (Pred), dexamethasone (Dex), or β-methasone. GCs exert a wide spectrum of influences on developing organs, including lung and brain. The brain sensitivity to GCs begins in embryonic life, as GR is expressed in fetal neurons (2) and is maintained in several adult brain regions, including the hippocampus and cerebellar cortex (3). Exposure to supraphysiologic GC levels during embryonic life leads to alterations in neuronal proliferation, growth, and differentiation. In animal models, perinatal GC administration inhibits brain neuron division and reduces cerebellum and brain size and cell number, but may also cause hippocampal neuron death (2).
In the adult, GC excess may suppress neurogenesis in the subgranular layer of the dentate gyrus, a brain stem cell district in which new granular neurons are generated (4). Accordingly, adrenalectomy greatly increases neurogenesis in the dentate gyrus (5). It should be noted that GCs can also protect against neurodegeneration (5), suggesting that GCs are capable of exerting adaptive effects that prevent neural injury caused by overaggressive cellular defense mechanisms.
Neural side effects of prenatal or postnatal therapy with synthetic GCs
A variety of synthetic GCs, including Pred, Dex, β-methasone, and hydrocortisone, have been created for therapeutic use, and the therapeutic benefits versus adverse effects of each must be considered when choosing which GC to administer. Prenatal treatment with synthetic GCs is commonly used for women at risk of preterm birth in order to prevent respiratory distress syndrome and neonatal death caused by inadequate surfactant production in fetal lungs (6). However, this therapy has potential adverse effects on neurodevelopment (7). Postnatal GC therapy is used in the prevention and/or treatment of chronic lung disease in preterm babies. This therapy is effective in improving lung function and reducing neonatal mortality but is associated with long-term side effects, including altered neurodevelopment with increased risk of cerebral palsy and neurosensory disability (8). Delaying GC therapy could reduce, but not eliminate, the incidence of GC-mediated adverse neurological outcomes (9).
Molecular mechanisms underlying GC-induced neurodevelopmental injury: role of the hedgehog pathway
Previously, the molecular mechanisms underlying GC-induced brain injury have been unclear. Heine and Rowitch now report, for what is believed to be the first time, the identification of antagonistic crosstalk between GCs and the sonic hedgehog (Shh) signaling pathway in proliferating cerebellar granule neuron precursors (CGNPs) in postnatal mice (10).
The hedgehog (Hh) signaling pathway is crucial for the regulation of developmental processes and is activated by the binding of the secreted Hh proteins Shh, desert hedgehog (Dhh), and indian hedgehog (Ihh) to the transmembrane receptor Patched (Ptch) in target cells. Secreted Hh proteins, upon binding to Ptch, abolish a repression effect on a second transmembrane protein, Smoothened (Smo), which activates the downstream signaling cascade and the glioma-associated oncogene (Gli) family of transcription factors that regulate a number of target genes controlling cell proliferation and differentiation, such as those encoding N-myc and D-type cyclins (11).
Hh signaling plays a paradigmatic role during cerebellar development, during which this pathway is a master promoter of the expansion of the CGNP population; it also plays a critical role in whole-brain morphogenesis (reviewed in refs. 12, 13). A specific emerging function of Hh signaling is the maintenance and self renewal of neural progenitors in stem cell niches located in several regions of the embryonic, postnatal, and adult brain, thereby sustaining developmental and tissue repair processes (13).
Using mouse models, Heine and Rowitch show that chronic GC treatment of mouse pups from P0 through P7 inhibits cerebellar growth and proliferation of external granule layer CGNPs by promoting cell cycle exit and premature differentiation, whereas acute GC administration at P7 causes transient CGNP apoptosis (Figure 1 and ref. 10). While they did not observe substantial modulation of GR expression or Shh and Gli1 mRNA levels in vivo (albeit evaluated by nonquantitative immunohistochemical or in situ hybridization assays), in vitro analysis showed that the GC-induced antiproliferative effect on CGNPs may be caused by counteraction of the Shh-mediated proliferative pathway. Indeed, the authors observed a GC-driven reduction in protein levels of Shh targets Gli1, N-myc, and D-type cyclins in CGNPs (Figure 1). The mechanism of action of GCs on these proteins was not identified, although GCs did not appear to act by modulating the mRNA levels of these target proteins. Nevertheless, it seems reasonable that modulation of Gli1 protein translation or stability would be the initial event, although a direct effect on N-myc and D-type cyclins cannot be ruled out. More importantly, the authors have unveiled the existence of a cross-antagonism between GCs and Shh signaling, based on their finding that both Shh treatment in vitro and overexpression of the constitutively activated form of Smo in vivo counteract the antiproliferative effects of GC treatment in CGNP cultures (10).
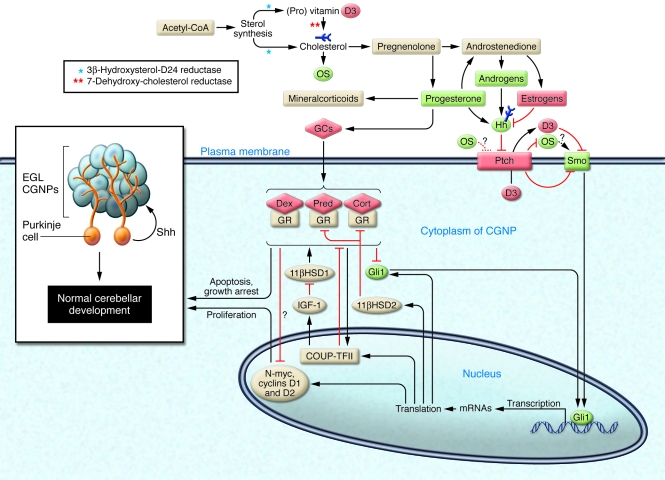
Figure 1. Crosstalk between Hh and steroid hormones during neuronal development and injury.
Steroid hormone synthesis starts with acetyl-CoA and leads to the formation of (pro) vitamin D3 and cholesterol. Cholesterol-derived pregnenolone is synthesized from and is the origin of all steroid hormones. Cholesterol also gives rise to oxysterols (OS), important positive mediators of Hh pathway activation. Inhibition or mutation of enzymes involved at early steps of steroid hormone biosynthesis (asterisks) blocks cholesterol and (pro) vitamin D3 production, leading to defects in Hh signaling. Progesterone induces Ihh in the uterus, androgens upregulate Shh in the developing prostate, and estrogens downregulate Shh in the prostate and Ihh and Dhh in the uterus. In this issue of the JCI, Heine and Rowitch report that GCs can modulate the Hh pathway in postnatal mice (10). Proliferation of CGNPs in the cerebellar external granule layer (EGL) is physiologically promoted by Shh secreted by Purkinje cells. This process is antagonized by binding of GCs to the GR, which also leads to CGNP apoptosis. GC antagonism of Hh signaling leads to neuronal injury and aberrant cerebellar development. Shh protects CGNPs via 11βHSD2, which inactivates cortisol, Cort, and Pred, but not Dex, suggesting that it is better to use 11βHSD2-sensitive GCs to avoid neuronal injury in neonates. GCs may exert their effects via interaction with COUP-TFII: the GC-bound GR stimulates COUP-TFII–induced transactivation, while COUP-TFII represses GR transcriptional activity (27). COUP-TFII is also an Hh target and modulates the expression of IGF-1, which regulates GC metabolism through 11βHSD1. Positive and negative regulators of the Hh pathway are shown in green and red, respectively.
The authors also investigated the mechanism through which Shh may antagonize GCs (10). Both 11β-hydroxysterol dehydrogenase 1 (11βHSD1) and 11βHSD2, 2 isozymes of 11βHSD, catalyze the conversion of hormonally active GCs to inactive cortisone, and vice versa. While 11βHSD2 converts endogenous GCs (e.g., Cort and cortisol) and some synthetic GCs (e.g., Pred, but not Dex) into inactive 11-ketoderivatives (cortisone), 11βHSD1 mainly converts cortisone to active GCs (14).
The authors show that Shh signaling potently induces 11βHSD2 upregulation and by this mechanism reduces the activity of GCs in CGNPs (Figure 1 and ref. 10). The protective effects exerted by Shh through 11βHSD2 upregulation were further documented by the increase in GC toxicity on CGNPs following administration of the 11βHSD2 inhibitor carbenoxolone.
In summary, the findings described in the current study reveal another important mechanism of action of the Hh pathway during the critical phases of cerebellar development and most likely other organ morphogenesis, improving our consciousness of the complex interplay between hormones and developmental pathways.
Crosstalk between steroids and the Hh pathway
The findings of Heine and Rowitch (10) add another piece of information regarding the importance, for proper Hh pathway function and regulation, of steroid hormones and their biosynthetic pathway, which includes intermediates such as (pro) vitamin D3, cholesterol, and its derivatives, the oxysterols. Covalent binding of cholesterol at the C-terminal domain of Hh protein is required for proper Hh extracellular diffusion (15), and in animal models, disruption of cholesterol synthesis processes leads to developmental malformation (e.g., holoprosencephaly) that mimics malformations observed in Hh mutant mice (15).
Hh signaling is instead regulated by cholesterol derivatives. For instance, oxysterols may activate the Smo receptor via direct interaction (16), or indirectly by binding to Ptch and inhibiting its activity (17), while (pro) vitamin D3, which is secreted through Ptch, can bind to and inhibit Smo (18) (Figure 1). Steroid hormones have previously been described as being involved in interplay with the Hh signaling pathway, either as regulators of the pathway or as targets. Ihh is positively modulated by progesterone in the uterus during progesterone-induced processes of proliferation, angiogenesis, and decidualization, which are critical for embryo implantation (19). In contrast, Ihh and Dhh are downregulated by estrogens in the uterus (20). Finally, estrogens and androgens have previously been implicated in the negative and positive regulation, respectively, of Shh expression during prostate gland development (21, 22). Therefore, the Hh pathway is emerging as an important mediator of the effects of steroid hormones (Figure 1).
In contrast, the reverse ability of Hh to control steroid hormone function is still poorly understood. In addition to the candidates described in the current report (10), the Hh target chicken ovalbumin upstream promoter–transcription factor II (COUP-TFII; ref. 23), a member of the nuclear orphan receptor superfamily, is a good candidate for GC control (Figure 1). COUP-TFII plays a critical role in the development of CNS and cerebellum (24) and may modulate GCs through IGF-1 and 11βHSD1 (Figure 1 and refs. 24, 25).
Clinical relevance and perspectives
Heine and Rowitch have shed light on the complex interplay existing between steroid hormones and pathways involved in development and cell stemness (10). Their observations regarding the cross-regulatory mechanisms at play between Hh and GCs raise new questions and have several molecular and clinical implications.
From the molecular point of view, 2 open questions remain, regarding (a) the mechanism through which GCs modulate Hh target genes and (b) how Hh is able to upregulate 11βHSD2. Knowledge of the underlying molecular events in detail will allow the development of new molecules and more targeted treatments, which could render therapeutic intervention with GCs more effective and less neurotoxic.
From the clinical point of view, the authors point out that 11βHSD2, which inactivates a subset of steroid hormones (e.g., Cort, hydrocortisone, and Pred), is not able to efficiently inactivate the synthetic GCs Dex or β-methasone; they propose a rationale for the use of 11βHSD2-sensitive GCs instead of Dex in neonatal infants, especially in the most vulnerable, critical first postnatal week of life (10). Regarding the proposed use of small-molecule Hh agonists to activate the neuroprotective role of this pathway, caution should be used because of the well-documented role of Hh in cerebellar neuronal tumorigenesis (12, 13).
Finally, there are possible implications regarding mechanisms of neurogenesis and neurodegeneration, which have been shown to be modulated by both Hh signaling and GCs. New neurons developing from neural stem cells or multipotent progenitor cells populate the adult hippocampus throughout life and contribute to cognitive functions, including learning and memory (26). Discovery of natural molecular regulators by which GCs and Hh regulate each other during adult neurogenesis is important for the development of pharmacological drugs and of successful neuron replacement strategies to treat neurodegenerative diseases.
Acknowledgments
The authors are supported by Telethon Grant GGP07118, Associazione Italiana per la Ricerca sul Cancro, the Italian Ministry of University and Research, the Italian Ministry of Health, and the Pasteur Institute–Cenci Bolognetti Foundation. The authors thank Andrea Dotta for helpful comments.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: CGNP, cerebellar granule neuron precursor; Cort, corticosterone; COUP-TFII, chicken ovalbumin upstream promoter–transcription factor II; Dex, dexamethasone; Dhh, desert hedgehog; GC, glucocorticoid; Gli, glioma-associated oncogene; GR, GC receptor; Hh, hedgehog; 11βHSD, 11β-hydroxysterol dehydrogenase; Ihh, indian hedgehog; Pred, prednisolone; Ptch, Patched; Shh, sonic hedgehog; Smo, Smoothened.
Citation for this article: J. Clin. Invest. 119:243–246 (2009). doi:10.1172/JCI38387.
See the related article beginning on page 267.
References
- 1.Stahn C., Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008;4:525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B.S. Steroid hormones and brain development: some guidelines for understanding actions of pseudohormones and other toxic agents. Environ. Health Perspect. 1987;74:177–184. doi: 10.2307/3430447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozawa H. Steroid hormones, their receptors and neuroendocrine system. J. Nippon Med. Sch. 2005;72:316–325. doi: 10.1272/jnms.72.316. [DOI] [PubMed] [Google Scholar]
- 4.McEwen B.S. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Nichols N.R., Agolley D., Zieba M., Bye N. Glucocorticoid regulation of glial responses during hippocampal neurodegeneration and regeneration. Brain Res. Brain Res. Rev. 2005;48:287–301. doi: 10.1016/j.brainresrev.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Miracle X., Di Renzo G.C., Stark A., Fanaroff A., Carbonell-Estrany X., Saling E. Guideline for the use of antenatal corticosteroids for fetal maturation. J. Perinat. Med. 2008;36:191–196. doi: 10.1515/JPM.2008.032. [DOI] [PubMed] [Google Scholar]
- 7.Crowther C.A., Doyle L.W., Haslam R.R., Hiller J.E., Harding J.E., Robinson J.S. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N. Engl. J. Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 8. Halliday, H.L., Ehrenkranz, R.A., and Doyle, L.W. 2003. Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants.Cochrane Database Syst. Rev. CD001146. [DOI] [PubMed] [Google Scholar]
- 9. Halliday, H.L., Ehrenkranz, R.A., and Doyle, L.W. 2003. Delayed (>3 weeks) postnatal corticosteroids for chronic lung disease in preterm infants.Cochrane Database Syst. Rev. CD001145. [DOI] [PubMed] [Google Scholar]
- 10.Heine V.M., Rowitch D.H. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11βHSD2-dependent mechanism. J. Clin. Invest. 2009;119:315–322. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varjosalo M., Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 12.Gulino A., Di Marcotullio L., Ferretti E., De Smaele E., Screpanti I. Hedgehog signaling pathway in neural development and disease. Psychoneuroendocrinology. 2007;32(Suppl 1):S52–S56. doi: 10.1016/j.psyneuen.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 13. Ruiz i Altaba, A. 2006.Hedgehog-Gli signaling in human disease. Landes Bioscience. Georgetown, Texas, USA. 228 pp. [Google Scholar]
- 14.Holmes M.C., Seckl J.R. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Mol. Cell. Endocrinol. 2006;248:9–14. doi: 10.1016/j.mce.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Lewis P.M., et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/S0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 16.Corcoran R.B., Scott M.P. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwyer J.R., et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 18.Bijlsma M.F., et al. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K., et al. Molecular mechanisms involved in progesterone receptor regulation of uterine function. J. Steroid Biochem. Mol. Biol. 2006;102:41–50. doi: 10.1016/j.jsbmb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katayama S., et al. The expression of Hedgehog genes (Ihh, Dhh) and Hedgehog target genes (Ptc1, Gli1, Coup-TfII) is affected by estrogenic stimuli in the uterus of immature female rats. Toxicol. Appl. Pharmacol. 2006;217:375–383. doi: 10.1016/j.taap.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Prins G.S., Huang L., Birch L., Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Ann. N. Y. Acad. Sci. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pu Y., Huang L., Birch L., Prins G.S. Androgen regulation of prostate morphoregulatory gene expression: Fgf10-dependent and -independent pathways. Endocrinology. 2007;148:1697–1706. doi: 10.1210/en.2006-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan V., et al. Mediation of Sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science. 1997;278:1947–1950. doi: 10.1126/science.278.5345.1947. [DOI] [PubMed] [Google Scholar]
- 24.Kim B.J., Takamoto N., Yan J., Tsai S.Y., Tsai M.J. Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev. Biol. 2008 doi: 10.1016/j.ydbio.2008.11.001. Online publication ahead of print. doi:10.1016/j.ydbio.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agha A., Monson J.P. Modulation of glucocorticoid metabolism by the growth hormone — IGF-1 axis. Clin. Endocrinol. (Oxf). 2007;66:459–465. doi: 10.1111/j.1365-2265.2007.02763.x. [DOI] [PubMed] [Google Scholar]
- 26.Hagg T. Endogenous regulators of adult CNS neurogenesis. Curr. Pharm. Des. 2007;13:1829–1840. doi: 10.2174/138161207780858393. [DOI] [PubMed] [Google Scholar]
- 27.De Martino M.U., Alesci S., Chrousos G.P., Kino T. Interaction of the glucocorticoid receptor and the chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII): implications for the actions of glucocorticoids on glucose, lipoprotein, and xenobiotic metabolism. Ann. N. Y. Acad. Sci. 2004;1024:72–85. doi: 10.1196/annals.1321.006. [DOI] [PubMed] [Google Scholar]