Abstract
Asthma is a type-I allergic airway disease characterized by Th2 cells and IgE. Episodes of bronchial inflammation, eosinophilic in nature and promoting bronchoconstriction, may become chronic and lead to persistent respiratory symptoms and irreversible structural airway changes. Representative mostly of mild to moderate asthma, this clinical definition fails to account for the atypical and often more severe phenotype found in a considerable proportion of asthmatics who have increased neutrophil cell counts in the airways as a distinguishing trait. Neutrophilic inflammation is a hallmark of another type of allergic airway pathology, hypersensitivity pneumonitis. Considered as an immune counterpart of asthma, hypersensitivity pneumonitis is a prototypical type-III allergic inflammatory reaction involving the alveoli and lung interstitium, steered by Th1 cells and IgG and, in its chronic form, accompanied by fibrosis. Although pathologically very different and commonly approached as separate disorders, as discussed in this review, clinical studies as well as data from animal models reveal undeniable parallels between both airway diseases. Danger signaling elicited by the allergenic agent or by accompanying microbial patterns emerges as critical in enabling immune sensitization and in determining the type of sensitization and ensuing allergic disease. On this basis, we propose that asthma allergens cause severe noneosinophilic asthma because of sensitization in the presence of hypersensitivity pneumonitis-promoting danger signaling.
Conventionally, asthma is defined as a type-I allergic airway disease mediated by Th2 cells and IgE and characterized by bronchial inflammation that is eosinophilic in nature. In a considerable number of patients, the chronic inflammation and ensuing airway remodeling can result in persistence of symptoms and decreased lung function. However, the conventional definition of asthma and its emphasis on eosinophilia in the context of a Th2-biased immune response does not explain all clinical observations.1,2 For example, neutrophilic infiltration is observed during severe acute asthma attacks and in severe persistent asthma. Furthermore, severe chronic asthma frequently also includes an additional Th1 component and even alveolitis. The etiology underlying severe asthma is not well understood and treatment of severe asthmatics is often resistant to conventional asthma anti-inflammatory treatment. This renders noneosinophilic or mixed neutrophilic/eosinophilic severe asthma enigmatic as well as an important challenge to the medical and immunological community.
Allergic alveolitis and allergen-specific CD4+ T-cell responsiveness polarized toward Th1 are features also observed in a dissimilar type of allergic disease, namely hypersensitivity pneumonitis (HP). Similarly to asthma, HP is a pathological response of the airways to airborne antigen that, however, is driven by Th1 cells and IgG. Chronic HP can ultimately lead to lung fibrosis and respiratory insufficiency.
This review starts from the proposition that the identification of shared and inflammation type-specific mechanisms at work in the onset and pathology of either allergic disease, (severe) asthma or HP, might help to better comprehend at least some aspects of severe asthma. We review the main pathological features observed in mild to moderate asthmatics and commonly associated with conventional asthma phenotypes. From here, we discuss how mouse models have contributed to unravel the immunological basis and pathogenesis of mild asthma. Special emphasis is put on the nature of asthma-eliciting allergens and the dependence of their experimental counterparts on accompanying adjuvants to generate the danger signals necessary for raising Th2-biased sensitization. Reminding us that mouse asthma as such does not exist, the shortcomings of mouse models to mimic characteristic features of especially chronic and severe asthma are discussed in the last part of this section. In the next section devoted to HP, comparison with asthma illustrates prominent differences in pathology and immunology and highlights the crucial role of the origin of the sensitizing antigen, the nature of the danger signaling elicited at the time of antigen encounter, and genetic predisposition. From these differences and similarities we propose in the final section of the review that noneosinophilic or mixed neutrophilic/eosinophilic severe asthma may represent a separate pathology that results from an accidental HP-like sensitization by asthma-characteristic allergens that are generally associated with mild to moderate eosinophilic asthma. Furthermore, we discuss experimental data from mouse models that support this proposition.
Immunological and Pathological Features of Mild Asthma
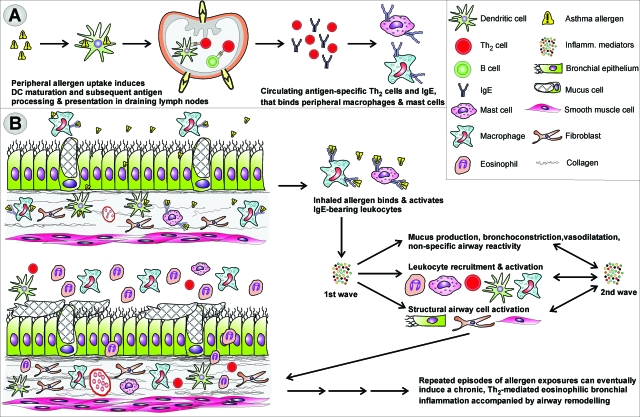
Persistent mild asthma is characterized by chronic inflammation of the airways that is mostly eosinophilic in nature. The airways of patients with mild asthma have an increased sensitivity and responsiveness to inhaled allergen and often to nonspecific irritants such as cold air, cigarette smoke, perfume, and others. This results in variable and episodic bronchoconstriction with increased mucus production, cough, wheezing, and dyspnea3 [see also the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2007. Available from http://www.ginasthma.com/download.asp?intId=309, p 2, last accessed September 15, 2008]. Genetic factors such as predisposition toward the development of atopy, and environmental factors such as viral infection, intensity, and frequency of exposure to airborne allergens, occupational exposures, and overall hygiene, seem to cooperate through still ill-defined mechanisms to initiate allergic sensitization and to control the further evolution to asthma as well as its severity.1,4,5 As illustrated in Figure 1, eosinophils along with mast cells, Th2 lymphocytes, dendritic cells (DCs), and macrophages as well as structural cells such as airway smooth muscle, mucous glands, and lung epithelium are the main cellular protagonists in the inflamed airways of patients with mild to moderate asthma.6 From an immunological viewpoint, the allergic sensitization and resulting inflammation are interpreted as a breakdown of immune tolerance toward environmental antigens. Although these antigens as such are not associated with infectious microbial organisms, they do evoke a futile immunological response in sensitive individuals. As a result, allergen-specific Th2 cells and IgE are generated and on encountering the allergen initiate the complex cascade illustrated in Figure 1 that ultimately leads to the type-I allergic reaction.
Figure 1.
Development of mild asthma. A: On primary allergen exposure, DAMPs and/or PAMPs intrinsic to or accompanying the allergen activate DCs to become APCs biased toward the induction of Th2 cells. As a result, immune sensitization featuring allergen-specific IgE and Th2 cells is generated. B: Activation of IgE-bearing innate cells by inhaled allergen triggers the release of pro-inflammatory mediators such as histamine, leukotrienes, Th2-associated cytokines and chemokines, and reactive oxygen species. The resulting early-phase allergic reaction is manifested by smooth muscle contraction, mucus hypersecretion, and increased airway responsiveness. Inflammatory cell recruitment and activation leads to the production of a second wave of particularly Th2-associated inflammatory mediators such as IL-4, IL-5, IL-13, eotaxins, RANTES, and others. This late-phase reaction occurs ∼6 to 9 hours after allergen inhalation and usually lasts for not more than a day.
Role of Danger-Associated Signaling in Allergic Sensitization
Although everyone is exposed to numerous indoor and outdoor allergens such as house dust mites, pollen, and pets, the normal outcome of such exposures in nonatopic individuals is immunological tolerance.7,8 Regulatory T cell (Treg) and DC subsets are central in controlling tolerance versus sensitization and in determining the nature of subsequent immune responses. Specifically, myeloid DCs have been shown to be responsible for Th2-skewed sensitization against inhaled allergen. In contrast, plasmacytoid DCs promote tolerogenic responses and protect the airways against allergic inflammation.9,10 Both DC subsets are highly flexible, and co-existing environmental danger-associated molecular patterns (DAMPs) may trigger danger signaling that shifts this balance from immune tolerance toward Th2-skewed immunity.
In mice, contact with allergen alone is not sufficient to circumvent inherent tolerance mechanisms without the support of immune potentiators. Most often a biphasic protocol is applied in which systemic administration of antigen in the presence of a Th2-skewing adjuvant (generally aluminum hydroxide salts; alum) is followed by secondary exposure(s) to aerosolized antigen. Alum, the archetypical adjuvant, is used not only in most mouse asthma models but also in human vaccines. Lacking conserved pathogen-associated molecular patterns (PAMPs), alum does not require Toll-like receptor (TLR) signaling for its adjuvant activity.11 Instead, intraperitoneal injection of alum has been shown to induce the release of uric acid.12 Acting as endogenous DAMP, uric acid proved to be responsible for the recruitment of monocytes into the peritoneal cavity and their subsequent conversion into inflammatory DCs that drive Th2 cell responses. Also, the Nalp3 inflammasome has been identified as a crucial mediator of the Th2-stimulatory activity of alum.13 Nalp3 is an intracellular protein component belonging to the Nod-like receptor arm of the innate immune system that, similar to TLRs, senses both nonself and endogenous DAMPs. Thus, the induction of uric acid by alum and a Nalp3-dependent activation of innate inflammatory pathways seem to be at the basis of the Th2-skewed adjuvant activity of the inorganic alum salt.
Low-level contamination of experimental allergens such as ovalbumin (OA) with lipopolysaccharide14 or other PAMPs may also elicit signals that promote allergic sensitization. This is supported by the fact that inhalation of endotoxin-free OA induces tolerance instead of sensitization, and the fact that sensitization by conventional, endotoxin-contaminated, OA preparations requires the functional lipopolysaccharide-cognitive receptor, TLR-4.8 Interestingly, other allergens used for mouse models such as pollens, house dust mite, ragweed, molds, and cockroach proteins do not require adjuvant (alum) support for inducing sensitization. These real-life allergens differ from the inert model allergen, OA, by their intrinsic enzymatic activity, which triggers danger signaling. Likewise in humans, exogenous enzymes such as proteases from molds and mites as well as industry-related proteases, cellulases, and lipases have allergenic characteristics.15,16,17,18,19 Environmental pollutants such as cigarette smoke may further increase the allergenic properties of antigens: mice inhaling OA together with cigarette smoke exhibit high OA-specific IgE levels (representative of atopy) and distinct eosinophil- and mucus cell-enriched airway inflammation on airway challenge with nebulized OA.20 This observation could provide a mechanistic basis for the notion that smoking is a risk factor for asthma development.21
Although signals leading to Th2 reactivity can be endogenous in nature, induced by chemical or enzymatically active substances, their role in the development of atopy and asthma is complex and dependent on both the genetic background and immune conditioning of the individual by environmental PAMPs. Thus, the timing of allergen exposure during one’s lifetime, along with the frequency and intensity of exposure, play a crucial role in the establishment of tolerance or sensitization and the development of asthma.22,23 The presumed impact on atopy of immune conditioning by prior microbial exposures is translated in the hygiene hypothesis postulating that reduced exposure to bacteria, viruses, and parasites in early childhood facilitates atopic sensitization,1,24 probably because of a diminished induction of regulatory T cells.25 Inversely, frequent stimulation of the innate immune system by environmental PAMPs such as lipopolysaccharide or by contact with livestock may diminish the risk of developing allergic sensitization. This so-called farming effect is suggested by several population studies in rural areas of Europe.26 Also studies in mice demonstrated a protection against systemic OA sensitization by prior lipopolysaccharide inhalation.27 Additionally, gene linkage studies showed a correlation between gene polymorphisms related to the innate immune system, the response to endotoxin exposure and infections, and allergic disease.28,29,30,31 Here, the effects may be age-specific as was reported for the influence on atopy of a specific CD14 polymorphism that was apparent during mid childhood but no longer at early adulthood.32
Prolonged Exposure Protocols to Study Asthma: A Difficult Road
Repeated episodes of allergen exposure and subsequent inflammatory responses can eventually lead to a worsening of the asthmatic phenotype because of a state of chronic inflammation. This condition may result in persistent respiratory symptoms and a permanent decrease in lung function, with the airways becoming increasingly sensitive and reactive not only to specific allergens, but also to environmental stimuli such as cigarette smoke, cold air, or fog. This nonspecific airway hyperreactivity may be at least partly attributed to structural alterations in the airways observed in chronic or severe asthma: mucus gland hyperplasia, airway smooth muscle hypertrophy, epithelial shedding, and subepithelial thickening of the basement membrane (subepithelial fibrosis), together called airway remodeling.33,34,35 In contrast to the long-term allergen exposure of patients, most mouse models of asthma involve relatively short-term allergen exposures of up to 10 days. Although experimentally convenient, these short-term models are likely to be driven by immune and inflammatory mechanisms quite distinct from those involved in mild and severe persistent asthma that have chronic inflammation as a hallmark. In mice, prolonged exposure protocols lead to highly divergent outcomes. Generally, down-regulation of inflammatory responses is observed along with the establishment of a long-lasting tolerant state,36,37 in fact quite similar to the response of nonasthmatic individuals. Interestingly, other studies on prolonged OA aerosol exposure, given three times a week for 6 weeks, showed consistent evidence for airway remodeling but with inflammation varying from low level38 to moderate sustained eosinophilic airway inflammation and hyperreactivity.39 This dissociation of remodeling from inflammation in chronicity models indicates that once initiated, these pathological features are not necessarily intertwined, as also observed in paucigranulocytic asthma patients.40
Genetic factors are also determinants in the establishment of chronic asthma-like features in mouse models. Using a protocol of tightly controlled, low-level exposure to OA aerosol throughout a period of 8 weeks, BALB/c mice developed inflammatory features much like chronic asthma: intraepithelial presence of eosinophils, chronic inflammation in the lamina propria, airway remodeling and hyperreactivity, and no alveolitis. Strikingly, no significant airway hyperreactivity or airway lesions were observed when this protocol was applied to C57BL/6 mice,41 although these mice are often used in regular, short-term models of asthma. Other studies too have underscored the importance of strain specificity and route of exposure in the outcome of chronic asthma models. One study showed that in A/J, BALB/c, C57BL/6, and C3H/HeJ mice, repeated inhalational exposure to OA initially promotes a characteristic eosinophilic airway inflammation in the first weeks, but ultimately leads in all strains to antigenic tolerance42 as was also reported by others.36,37 In contrast, intranasal antigen exposure in A/J mice resulted in continuous eosinophilic airway inflammation and, after 12 weeks of antigen exposure, in airway remodeling. Strikingly, both eosinophilic inflammation and remodeling were less prominent in BALB/c mice and absent in C57BL/6 and C3H/HeJ mice. These observations illustrate how genetic predisposition and route of exposure may co-operate to overcome inherent anti-inflammatory and tolerance mechanisms, thus leading to a condition much like that observed in chronic asthma. The observed strain differences clearly do not follow the Th1-Th2 paradigm because both Th1-biased C57BL/6 mice and Th2-biased BALB/c mice developed tolerance to the instilled antigen after an initial inflammatory episode. It seems that other factors besides genetic factors dictating Th2 reactivity and atopy determine the propensity for progression to chronic disease and irreversible histological changes in the lungs. One such factor may be granulocyte-macrophage colony-stimulating factor (GM-CSF). In chronically exposed antigen-tolerant BALB/c mice, eosinophilic inflammation was fully restored after instillation of recombinant GM-CSF.43 Inversely, GM-CSF-deficient mice showed reduced eosinophil numbers despite unaltered atopy, Th2 reactivity, and airway hyperreactivity.44
Understanding Asthma through Its Counterpart—Hypersensitivity Pneumonitis
When considering asthma, at least in its early stages, as a Th2 cell-mediated eosinophilic inflammation of the airways, HP (extrinsic allergic alveolitis) may be considered as its counterpart both from an immunological and pathological point of view. The inflammatory process in the acute phase of HP characteristically features a nonatopic neutrophilic inflammation of the respiratory bronchioles, alveoli, and interstitial tissue of the lungs.45,46,47,48 Similarly to asthma, the pathology is induced by repeated exposure to airborne agents in individuals previously sensitized to specific agents via the pulmonary mucosa, and manifests itself in acute, subacute, or chronic forms.48,49 However, in contrast to asthma, the causative agents are small organic particles, often of microbial origin, or volatile reactive chemicals, and the resulting pathology is clearly of a different nature. The majority of patients with acute HP show severe symptoms characterized by alveolitis with infiltration of lymphocytes, macrophages, and neutrophils. Moreover, unlike asthma, nonspecific airway hyperreactivity and excessive mucus production are generally not observed. Repeated exposures can lead to subacute and chronic HP with symptoms such as dyspnea and cough becoming progressively more intense.45,46,49 In the chronic phase, a characteristic triad of alterations becomes apparent: interstitial pneumonitis predominantly around the small airways, lymphocytic bronchiolitis, and poorly-formed granulomas. Intraluminal granulation tissue and fibrosis can be observed in alveoli (organizing pneumonia) or bronchioles (bronchiolitis obliterans). The lymphocytic infiltrates show a predominance of CD8+ T cells over CD4+ T cells. Pulmonary fibrosis may result in respiratory insufficiency caused by lung volume restriction and impaired gas exchange capacity.
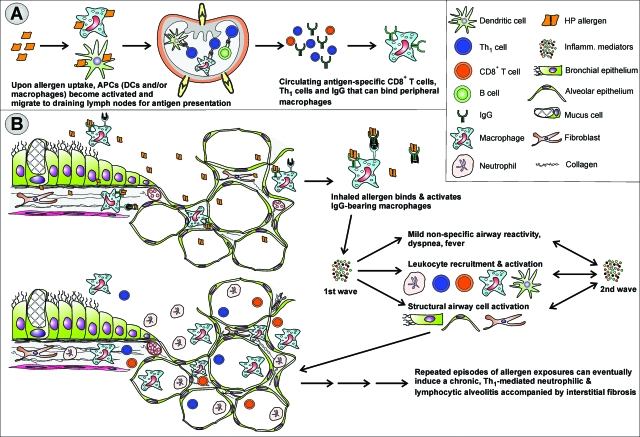
The different airway pathology in HP reflects the different immune basis of HP as compared with asthma, as illustrated in Figure 2. In HP, airway inflammation in susceptible individuals is initiated by the formation of IgG-antigen immune complexes and Th1-cell reactivity. As a consequence, HP and allergic asthma can, certainly in the early stages, be considered as predominantly Th1-mediated50,51,52,53 and Th2-mediated10,54,55,56 immune counterparts. The HP-associated inflammatory cytokine environment along with the released monocyte and neutrophil attractant chemokines results in infiltration of macrophages, neutrophils, and large numbers of Th1 and Tc cells into the distal airway walls, alveoli, and interstitium. Considered mainly as a type-III allergic response, the presence of CD8+ Tc cells and their suspected contribution to tissue injury indicate that a type-IV reaction is also present in HP. Whereas DCs have been identified as the main type of antigen-presenting cell (APC) eliciting the Th2-mediated second wave of bronchial inflammation in mouse models of asthma, alveolar macrophages are considered to play a crucial role in HP by the local stimulation of T cells and the further steering of inflammation.57 Evidence hereof is, however, rather scarce and mainly based on the increased expression of the T-cell co-stimulatory molecules, CD80 and CD86, by alveolar macrophages in patients with HP, a feature we also observed in mouse models (unpublished data). Concluding that DCs do not take part in disease onset and in later stages of the disease seems presumptuous because no functional studies on the role of APCs, either DCs or alveolar macrophages, have been reported in the literature. Detailed (mouse) studies on the nature and functional characteristics of the APC(s) involved and their steering of the T-cell response will undoubtedly be vital for further unraveling the immunological basis of HP.
Figure 2.
Development of HP. A: Primary allergen exposure and subsequent naïve CD4+ T-cell stimulation by activated APCs—alveolar macrophages and/or lung DCs—results in the generation of allergen-specific Th1 cells and IgG. Danger signals elicited by PAMPs intrinsic to the HP allergen and/or present at the time of exposure are crucial in generating the Th1-bias of sensitization. B: Airway inflammation is initiated on secondary allergen exposure in the alveoli. IgG-allergen immune complexes trigger the complement cascade and/or directly activate alveolar macrophages to produce reactive oxygen species and inflammatory cytokines such as tumor necrosis factor-α and IL-12. These in turn trigger the production of IFN-γ by Th1 cells, resulting in a highly inflammatory cytokine mix that along with various chemokines such as IL-8, IP-10, I-TAC, and MIG, is at the basis of the neutrophilic nature of the inflammatory cell infiltrate, characteristic of HP.
The Type of Danger Signals Influences the Type of Sensitization
Because of the small size of HP-inducing agents, the aerosolized particles can easily reach the alveoli. This is an important difference from allergic asthma, in which the responsible allergens are usually larger and, in consequence, are deposited more proximally in the bronchi. This physical characteristic is often used to explain the absence of alveolitis in asthma and the confinement of inflammation to the airways. Another important difference between asthma and HP may be the origin of the causal agents. Whereas in asthma allergens are mainly nonmicrobial in nature, the etiological agents of HP are mostly proteins from bacteria, fungi, and other organisms. However, similar to allergens associated with asthma, the allergenicity of these proteins has been attributed to intrinsic adjuvant activity58 and/or the presence of substances within the inhaled organic particles that act as adjuvants by eliciting innate immune activation.45,48 The most frequent forms of HP are caused by exposure to microorganisms growing on stored hay or corn (farmer’s lung), by inhaling proteins present on feather dust and in bird droppings (bird fancier’s lung), or by exposure to contaminated water from air-conditioning systems (humidifier fever).45
Reflecting the different natures of the causal agents, the majority of HP-related research in mice relies on Saccharopolyspora rectivergula (also known as Micropolyspora faeni or Faenia rectivergula) as the inducing antigen. This thermophilic actinomycete causes farmer’s lung in humans, presumably cooperating with a yet unidentified co-factor present in the grain dust.45 As a real-life HP-eliciting antigen, S. rectivergula possesses endogenous immunogenic characteristics, and HP can be established in mice by repeated intranasal instillation without adjuvant support.58,59 However, most mouse protocols to induce HP rely on a systemic sensitization against S. rectivergula in the presence of complete Freund’s adjuvant (CFA) as the immunogenic co-factor, followed by intratracheal administration of S. rectivergula,60 thus leading to a HP-like neutrophilic, Th1-driven pulmonary inflammation. Interestingly, systemic sensitization to OA, the prototypical allergen used in mouse asthma models, using CFA (instead of alum as in experimental asthma induction), followed by inhalational OA challenge also induced Th1-driven responses with neutrophilic airway inflammation in the perivascular and peribronchiolar areas and little to no airway hyperreactivity or mucus production.61 The use of alum versus CFA to induce allergic sensitization in mouse models of asthma and HP, respectively, directly reflects the highly divergent nature of the allergens involved and of the danger signals elicited, promoting sensitization. Similarly to alum, emulsification of antigen with the mineral oil from CFA provides a depot for slow antigen release. Furthermore, the oily solution and especially the heat-killed Mycobacterium tuberculosis bacilli dissolved in the oil supply a wide range of danger signals, such as interferon (IFN)-γ, interleukin (IL)-6, and IL-12, that elicit Th1-oriented cellular immunity. Various studies have implicated a role for M. tuberculosis-associated TLR-ligands in co-stimulating the adjuvant effect of CFA.13,62 However, CFA-supported early antibody responses are not hampered in mice deficient in TLR signaling,11 suggesting that TLRs are an essential component in the maintenance rather than the initiation of CFA-facilitated immune responses. In 1974, it was shown that muramyl dipeptide is the minimal mycobacterial cell wall component required for adjuvant activity of CFA.63 Because muramyl dipeptide is now recognized as an important activator of the Nod2 and Nalp3 Nod-like receptors,64,65 this suggests a determining role for Nod-like receptor rather than TLR triggering by CFA in establishing HP-like sensitization. Intriguingly, as mentioned before, Nalp3 is also crucial for the immunostimulatory properties of alum.13 The identification of the cellular and molecular pathways triggered by CFA or alum in establishing and maintaining HP- or asthma-like immune responses will be of importance to better understand the mechanisms of sensitization for HP and asthma in humans, which are just as poorly understood.
Acute-to-Chronic HP: Similar Features but Dissimilar Mechanisms from Asthma
Population studies and S. rectivergula-based mouse models of HP point toward genetic predisposition as a critical factor in the development of sensitization and inflammation and in the further progression of the disease to chronicity. Only 5 to 15% of the people who are exposed to HP-related antigens develop the disease66 although both symptomatic patients and individuals without clinical symptoms can exhibit antigen-specific IgG responses.67,68 In mouse models it was shown that Th1 but not Th2 cells are required for sensitization and are involved in the pathogenesis.50 In addition, Th1-biased C3H/HeJ or C57BL/6 mice are more susceptible to HP than Th2-biased BALB/c or DBA/2 mice.69,70 Whereas in asthma models the Th2 cytokines IL-4, IL-5, and IL-13 are crucial mediators, the prototypic Th1-promoting cytokine, IL-12, is a critical factor in inducing S. rectivergula-mediated HP in naïve mice.52 Endogenous resistance to S. rectivergula-induced HP in DBA/2 mice can be overcome by IL-12 treatment. In addition, inflammatory cytokines downstream of IL-12 such as IFN-γ, IL-1, and tumor necrosis factor are elevated in HP-prone C57BL/6 mice,70 thus identifying the IL-12 pathway as predisposing toward the development of HP. Not surprisingly, in these models of acute HP, endogenous production of the anti-inflammatory cytokine IL-10 has an important modulatory effect on the intensity of inflammation and granuloma formation.71
Although few mouse models are available to study the pathogenesis of HP, even fewer reports address chronic features. Intranasal instillation of S. rectivergula for 3 consecutive days per week for 3 weeks does not only induce the HP-characteristic diffuse bronchoalveolitis and lung granulomas but also elevated levels of hydroxyproline, indicative of fibrosis, in C57BL/6 mice.70 Yet, similar to models of asthma, it was found that continued exposure to S. rectivergula for more than 6 weeks results in a waning of inflammation and fibrosis,72 a process that may be attributable to increased infiltration of Treg.73 Therefore in HP as well as in asthma, at least in the mouse, progression to chronicity seems to require a crossing of additional thresholds to those involved in control of sensitization and acute inflammation. Whereas in asthma models, increments in GM-CSF levels may constitute such a threshold, a decline in IL-4 levels may be the signal for progression to chronic HP. This is supported by the suppression of IFN-γ-producing neutrophils by IL-4- and IL-4-producing NKT cells and the resulting regression of inflammation and fibrosis.74,75
Where Mouse Models of Asthma and Hypersensitivity Pneumonitis Meet—Noneosinophilic Severe Asthma
In many contemporary definitions of asthma, chronic inflammation that is eosinophilic in nature is put forward as the most distinctive pathological hallmark of the disease, thus reflecting the presence of large numbers of eosinophils not only in the airway wall but also in the sputum and bronchoalveolar lavage fluid.76,77 However, studies that show a proportion of asthmatics, mostly suffering from severe asthma, have increased neutrophil cell counts in the airway lumen78,79,80,81,82 and the more recent observation of a clear correlation between sputum neutrophil numbers and cough frequency in less severely affected asthmatic children83 illustrate that asthma as clinically defined covers distinct pathological phenotypes. In fact, noneosinophilic inflammation is present in the airway lumen of ∼50% of asthmatic patients.81 These and other observations have fuelled the ongoing debate on whether the neutrophil rather than or in addition to the eosinophil is the key effector cell in severe asthma84 and whether severe asthma might represent a different form of asthma rather than an increase in asthma symptoms.82
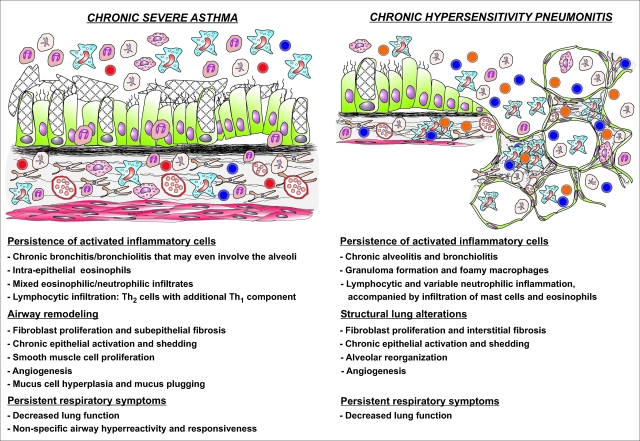
This debate actually illustrates the failure of present day mouse models to implement the neutrophil component from severe asthma and hereby to offer tools to dissect the pathways leading to neutrophilia in severe asthmatics and to analyze the role of the neutrophil in the allergic inflammatory cascade. As discussed before, classical murine models of asthma are derived from a Th2-biased sensitization and eosinophilic inflammation, and are representative mainly of mild to moderate asthma. In contrast, S. rectivergula-based models of HP share the neutrophilic nature of the inflammatory response with severe asthma but depart from a Th1-oriented sensitization. Interestingly, in severe asthma and chronic HP patients the distinction between the inflammatory players involved in either disease is becoming blurred, as illustrated in Figure 3. The inflammatory cell infiltrate includes neutrophils in severe asthma, and eosinophils and mast cells in chronic HP. Furthermore, in severe asthma Th1 cells are also recruited and increased levels of the HP-characteristic inflammatory cytokines, tumor necrosis factor and IFN-γ, are detected in these patients.85,86 In line with the increased neutrophil cell count, Th17 cells have gained increasing attention as potential mediators of neutrophilic inflammation in patients with severe and uncontrolled asthma.87,88 This effector T-cell subset produces IL-17, which causes the release of neutrophil-mobilizing cytokines from airway epithelial cells.89 Intriguingly, with a protocol similar to that mentioned above61 in which C57BL/6 mice are sensitized systemically to OA in the presence of CFA and subsequently exposed to nebulized OA, we found that the resulting neutrophilic pulmonary inflammation was accompanied by a strong polarization of lung CD4+ T cells toward Th1, with a barely detectable Th2 component. However, in this model of acute HP we also observed an important Th17 component (unpublished data), indicating that in clinical HP, Th17 in addition to Th1 cells, may contribute to the neutrophilic inflammation of the airways. Nevertheless, it is clear that there are important pathological differences between these two chronic disorders, as is summarized in Figure 3. Models of acute HP might nonetheless help to study at least some aspects of severe asthma; mediators that orchestrate inflammation in acute HP models might also represent driver mechanisms associated with severe asthma.
Figure 3.
(Dis-)similar features of chronic severe asthma and chronic HP. Although the site, nature, and regulation of the inflammatory response in both diseases differ greatly, the chronic stages of severe asthma and HP show some remarkable similarities. In both conditions the chronic inflammatory state is induced by repeated episodes of allergen exposures and subsequent inflammatory responses. The resulting sustained production of pro-inflammatory mediators and growth factors generates a self-perpetuating process of inflammatory cell survival and accumulation, chronic epithelial activation, and deregulation of normal healing processes, accompanied by persistent respiratory symptoms and decrease in lung function. Moreover, the distinction between Th2-driven airway eosinophilia versus Th1-driven neutrophilic alveolitis becomes blurred: the inflammatory cell infiltrate contains a significant fraction of neutrophils in chronic severe asthma and eosinophils and mast cells in chronic HP.
The clinical relevance of such an approach is illustrated by the remarkable efficacy that macrolide antibiotics can have as an add-on treatment for patients with severe asthma that do not achieve control with high-dose corticosteroids plus long-acting β-agonists.90,91,92 Macrolides have previously been proven to be efficient in a variety of other (neutrophil-driven) airway diseases including a mouse model of HP.93 The explanation for their efficacy is that these molecules not only are effective as antibiotics, but also interfere at low doses with neutrophil chemotaxis and function, rendering them anti-inflammatory agents.94,95,96
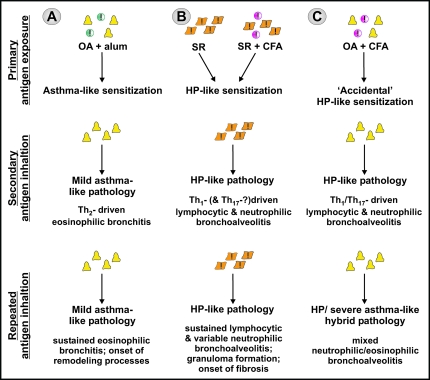
As previously described, the inert antigen OA is frequently used in combination with alum to predispose mice to Th2-driven airway eosinophilia, whereas the substitution of alum with CFA elicits Th1-driven airway neutrophilia on secondary OA inhalation. This finding again emphasizes the critical role that accompanying danger signals exert not only in promoting immunogenicity versus tolerance, but equally in determining the nature of the induced sensitization, independent of the antigen used. CFA-supported systemic OA-sensitization, followed by OA aerosol inhalation in fact constitutes a hybrid model that combines the Th1-biased sensitization in HP with the inert, nonmicrobial nature of model asthma allergens. We have now characterized a similar model in which C57BL/6 mice are sensitized subcutaneously to OA-CFA, followed by daily exposures to OA aerosols. After two exposures, pathology resembling HP emerged with pulmonary inflammation characterized by alveolitis with numerous neutrophils, macrophages, and CD4+ and CD8+ T cells, and marginal numbers of eosinophils present. In agreement with other models, distinct Th1-associated cytokines that are crucial for the development of HP, such as IFN-γ and IL-12, and CXC chemokines were found (unpublished data).51,53,59,97 Unexpectedly, with further exposures to OA aerosol, the inflammation not only intensified but also changed in nature, evolving from a predominantly neutrophilic airway response to a mixed neutrophilic and eosinophilic inflammation. This inflammatory pattern closely mimics the nonatopic, eosinophilic and neutrophilic inflammation observed in severe asthma. Although speculative and requiring further mechanistic studies, these data indicate that at least in the mouse, a severe asthma-like inflammatory phenotype may be raised as the result of an accidental Th1/Th17-biased sensitization by antigens that intrinsically do not elicit the danger signals necessary to sustain this type of response. Extension to the human situation leads to the hypothesis, illustrated in Figure 4, that the etiology of severe asthma, especially of the nonatopic form, may be distinct from that of mild to moderate asthma and closer to that of HP.
Figure 4.
Experimental mouse models illustrate the critical role of accompanying adjuvant activity in determining the type of immune sensitization and ensuing allergic disease. A: Systemic sensitization against inert OA in the presence of alum predisposes to a Th2-driven eosinophilic bronchoalveolitis. Repeated OA exposures in the absence of further immunogenic stimuli sustain the eosinophilic inflammatory airway response, much like mild asthma. B: Intranasal instillation of S. rectivergula or systemic immunization in the presence of CFA predisposes for a Th1- and possibly Th17-driven neutrophilic bronchoalveolitis. Like HP, repeated S. rectivergula inhalations further sustain this inflammatory response, presumably through the intrinsic adjuvant property of S. rectivergula. C: Systemic sensitization against OA in the presence of CFA similarly predisposes to a Th1- and Th17-driven neutrophilic bronchoalveolitis. On further exposures, the intrinsically inert nature of the antigen along with the absence of Th1-promoting stimuli supposedly is at the basis of the inflammatory pattern evolving to a mixed neutrophilic and eosinophilic inflammation, much like severe noneosinophilic asthma.
Concluding Remarks
This overview of the extensive body of literature on asthma and HP illustrates the complexity and still incompletely understood nature of both inflammatory diseases. Even though asthma and HP intrinsically represent allergic immune disorders, both diseases are commonly approached as separate pathologies having few or no shared features. Yet, as discussed here, clinical studies as well as data from experimental models reveal a number of important parallels that may help to better understand the etiology and pathogenesis of both diseases. In particular, the strong entanglement in both diseases of danger signaling with sensitization and pathology is of special relevance; DAMP/PAMP-elicited innate immune (danger) signals are crucial in enabling sensitization and in determining the type of sensitization, and hence the type of ensuing pathology. This entwinement of danger signals, sensitization, and pathology may be relevant also in explaining the substantial neutrophil inflammatory infiltrate frequently observed in severe asthma. An HP-like sensitization against antigens that intrinsically do not elicit nor sustain this type of cellular immune response may very well be at the basis of at least some forms of severe asthma. This mechanism, in agreement with the importance of gene-by-environment interactions in the development of asthma, would imply a therapeutic approach to severe asthma patients that is different from the approach taken when the symptoms are perceived simply as a worsening of mild asthma. Of clinical relevance, hybrid mouse models manifesting elements of experimental asthma and HP may help to resolve the still elusive immune and pathogenic processes underlying severe asthma.
Acknowledgments
We thank Dr. Marleen Praet from the N. Goormaghtigh Institute for Pathology at the Ghent University Hospital for fruitful discussions, and Freddy Heirman for reviewing the manuscript.
Footnotes
Address reprint requests to Dr. Johan Grooten, Lab Molecular Immunology, Department of Molecular Biology, Ghent University, Technologiepark 927, B-9052 Ghent, Belgium. E-mail: johan.grooten@ugent.be.
Supported by the Research Foundation-Flanders (grant G.0149.05) and the Belgian government (Interuniversity Attraction Poles program grant IAP6/18).
References
- Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE, Knox AJ, Britton JR, Hall IP. Asthma. Lancet. 2002;360:1313–1322. doi: 10.1016/s0140-6736(02)11312-2. [DOI] [PubMed] [Google Scholar]
- Kleeberger SR, Peden D. Gene-environment interactions in asthma and other respiratory diseases. Annu Rev Med. 2005;56:383–400. doi: 10.1146/annurev.med.56.062904.144908. [DOI] [PubMed] [Google Scholar]
- Bossé Y, Hudson TJ. Toward a comprehensive set of asthma susceptibility genes. Annu Rev Med. 2007;58:171–184. doi: 10.1146/annurev.med.58.071105.111738. [DOI] [PubMed] [Google Scholar]
- Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- Hoyne GF, Tan K, Corsin-Jimenez M, Wahl K, Stewart M, Howie SE, Lamb JR. Immunological tolerance to inhaled antigen. Am J Respir Crit Care Med. 2000;162:S169–S174. doi: 10.1164/ajrccm.162.supplement_3.15tac6. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijt LS, Lambrecht BN. Dendritic cells in asthma: a function beyond sensitization. Clin Exp Allergy. 2005;35:1125–1134. doi: 10.1111/j.1365-2222.2005.02321.x. [DOI] [PubMed] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminum adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Miyazaki Y, Zimmerman GA, Albertine KH, McIntyre TM. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J Biol Chem. 2003;278:42361–42368. doi: 10.1074/jbc.M307752200. [DOI] [PubMed] [Google Scholar]
- Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Brant A, Hole A, Cannon J, Helm J, Swales C, Welch J, Taylor AN, Cullinan P. Occupational asthma caused by cellulase and lipase in the detergent industry. Occup Environ Med. 2004;61:793–795. doi: 10.1136/oem.2003.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur X. Enzymes as occupational and environmental respiratory sensitisers. Int Arch Occup Environ Health. 2005;78:279–286. doi: 10.1007/s00420-004-0590-6. [DOI] [PubMed] [Google Scholar]
- Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol. 2008;121:847–852. doi: 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Moerloose KB, Robays LJ, Maes T, Brusselle GG, Tournoy KG, Joos GF. Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res. 2006;7:49. doi: 10.1186/1465-9921-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroux V, Pin I, Oryszczyn MP, Le Moual N, Kauffmann F. Relationships of active smoking to asthma and asthma severity in the EGEA study. Epidemiological study on the genetics and environment of asthma. Eur Respir J. 2000;15:470–477. doi: 10.1034/j.1399-3003.2000.15.08.x. [DOI] [PubMed] [Google Scholar]
- Steerenberg PA, Van Amsterdam JG, Vandebriel RJ, Vos JG, Van Bree L, Van Loveren H. Environmental and lifestyle factors may act in concert to increase the prevalence of respiratory allergy including asthma. Clin Exp Allergy. 1999;29:1303–1308. doi: 10.1046/j.1365-2222.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA. Asthma severity and prevalence: an ongoing interaction between exposure, hygiene, and lifestyle. PLoS Med. 2005;2:e34. doi: 10.1371/journal.pmed.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mutius E. Allergies, infections and the hygiene hypothesis—the epidemiological evidence. Immunobiology. 2007;212:433–439. doi: 10.1016/j.imbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007;148:53–63. doi: 10.1111/j.1365-2249.2007.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mutius E. Asthma and allergies in rural areas of Europe. Proc Am Thorac Soc. 2007;4:212–216. doi: 10.1513/pats.200701-028AW. [DOI] [PubMed] [Google Scholar]
- Tulić MK, Wale JL, Holt PG, Sly PD. Modification of the inflammatory response to allergen challenge after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2000;22:604–612. doi: 10.1165/ajrcmb.22.5.3710. [DOI] [PubMed] [Google Scholar]
- Koppelman GH. Gene by environment interaction in asthma. Curr Allergy Asthma Rep. 2006;6:103–111. doi: 10.1007/s11882-006-0047-y. [DOI] [PubMed] [Google Scholar]
- Simpson A, John SL, Jury F, Niven R, Woodcock A, Ollier WE, Custovic A. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174:386–392. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Yang Y, Haitchi HM, Powell RM, Holloway JW, Yoshisue H, Pang YY, Cakebread J, Davies DE. The genetics of asthma: ADAM33 as an example of a susceptibility gene. Proc Am Thorac Soc. 2006;3:440–443. doi: 10.1513/pats.200603-026AW. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Davies DE, Powell RM, Howarth PH, Haitchi HM, Holloway JW. Local genetic and environmental factors in asthma disease pathogenesis: chronicity and persistence mechanisms. Eur Respir J. 2007;29:793–803. doi: 10.1183/09031936.00087506. [DOI] [PubMed] [Google Scholar]
- O'Donnell AR, Toelle BG, Marks GB, Hayden CM, Laing IA, Peat JK, Goldblatt J, Le Souef PN. Age-specific relationship between CD14 and atopy in a cohort assessed from age 8 to 25 years. Am J Respir Crit Care Med. 2004;169:615–622. doi: 10.1164/rccm.200302-278OC. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- Mauad T, Bel EH, Sterk PJ. Asthma therapy and airway remodeling. J Allergy Clin Immunol. 2007;120:997–1009. doi: 10.1016/j.jaci.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Yiamouyiannis CA, Schramm CM, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec WW, Whiteley HE, Thrall RS. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911–1921. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove CL, Maes T, Joos GF, Tournoy KG. Prolonged inhaled allergen exposure can induce persistent tolerance. Am J Respir Cell Mol Biol. 2007;36:573–584. doi: 10.1165/rcmb.2006-0385OC. [DOI] [PubMed] [Google Scholar]
- Sakai K, Yokoyama A, Kohno N, Hamada H, Hiwada K. Prolonged antigen exposure ameliorates airway inflammation but not remodeling in a mouse model of bronchial asthma. Int Arch Allergy Immunol. 2001;126:126–134. doi: 10.1159/000049503. [DOI] [PubMed] [Google Scholar]
- McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy. 2004;34:497–507. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Foster PS. Modeling allergic asthma in mice: pitfalls and opportunities. Am J Respir Cell Mol Biol. 2002;27:267–272. doi: 10.1165/rcmb.F248. [DOI] [PubMed] [Google Scholar]
- Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med. 2003;168:959–967. doi: 10.1164/rccm.200210-1188OC. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J Immunol. 2002;169:3499–3506. doi: 10.4049/jimmunol.169.7.3499. [DOI] [PubMed] [Google Scholar]
- Su YC, Rolph MS, Hansbro NG, Mackay CR, Sewell WA. Granulocyte-macrophage colony-stimulating factor is required for bronchial eosinophilia in a murine model of allergic airway inflammation. J Immunol. 2008;180:2600–2607. doi: 10.4049/jimmunol.180.4.2600. [DOI] [PubMed] [Google Scholar]
- Yi ES. Hypersensitivity pneumonitis. Crit Rev Clin Lab Sci. 2002;39:581–629. doi: 10.1080/10408360290795583. [DOI] [PubMed] [Google Scholar]
- McSharry C, Anderson K, Bourke SJ, Boyd G. Takes your breath away—the immunology of allergic alveolitis. Clin Exp Immunol. 2002;128:3–9. doi: 10.1046/j.1365-2249.2002.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasse Y, Selman M, Costabel U, Dalphin JC, Ando M, Morell F, Erkinjuntti-Pekkanen R, Muller N, Colby TV, Schuyler M, Cormier Y. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003;168:952–958. doi: 10.1164/rccm.200301-137OC. [DOI] [PubMed] [Google Scholar]
- Mohr LC. Hypersensitivity pneumonitis. Curr Opin Pulm Med. 2004;10:401–411. doi: 10.1097/01.mcp.0000135675.95674.29. [DOI] [PubMed] [Google Scholar]
- Ismail T, McSharry C, Boyd G. Extrinsic allergic alveolitis. Respirology. 2006;11:262–268. doi: 10.1111/j.1440-1843.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- Schuyler M, Gott K, Cherne A, Edwards B. Th1 CD4+ cells adoptively transfer experimental hypersensitivity pneumonitis. Cell Immunol. 1997;177:169–175. doi: 10.1006/cimm.1997.1107. [DOI] [PubMed] [Google Scholar]
- Gudmundsson G, Hunninghake GW. Interferon-gamma is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest. 1997;99:2386–2390. doi: 10.1172/JCI119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler M, Gott K, Cherne A. Is IL12 necessary in experimental hypersensitivity pneumonitis? Int J Exp Pathol. 2002;83:87–98. doi: 10.1046/j.1365-2613.2002.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance S, Cross R, Yi AK, Fitzpatrick EA. IFN-gamma production by innate immune cells is sufficient for development of hypersensitivity pneumonitis. Eur J Immunol. 2005;35:1928–1938. doi: 10.1002/eji.200425762. [DOI] [PubMed] [Google Scholar]
- Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825–857. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–573. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108–116. doi: 10.1038/35100503. [DOI] [PubMed] [Google Scholar]
- Israël-Assayag E, Dakhama A, Lavigne S, Laviolette M, Cormier Y. Expression of costimulatory molecules on alveolar macrophages in hypersensitivity pneumonitis. Am J Respir Crit Care Med. 1999;159:1830–1834. doi: 10.1164/ajrccm.159.6.9810087. [DOI] [PubMed] [Google Scholar]
- Bice DE, McCarron K, Hoffman EO, Salvaggio J. Adjuvant properties of Micropolyspora faeni. Int Arch Allergy Appl Immunol. 1977;55:267–274. doi: 10.1159/000231935. [DOI] [PubMed] [Google Scholar]
- Nance S, Cross R, Fitzpatrick E. Chemokine production during hypersensitivity pneumonitis. Eur J Immunol. 2004;34:677–685. doi: 10.1002/eji.200324634. [DOI] [PubMed] [Google Scholar]
- Schuyler M, Gott K, Haley P. Experimental murine hypersensitivity pneumonitis. Cell Immunol. 1991;136:303–317. doi: 10.1016/0008-8749(91)90354-e. [DOI] [PubMed] [Google Scholar]
- Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974;59:1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Martinon F, Gaide O, Petrilli V, Mayor A, Tschopp J. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol. 2007;29:213–229. doi: 10.1007/s00281-007-0079-y. [DOI] [PubMed] [Google Scholar]
- Pan Q, Mathison J, Fearns C, Kravchenko VV, Da Silva Correia J, Hoffman HM, Kobayashi KS, Bertin J, Grant EP, Coyle AJ, Sutterwala FS, Ogura Y, Flavell RA, Ulevitch RJ. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82:177–183. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- Fink JN. Hypersensitivity pneumonitis. Clin Chest Med. 1992;13:303–309. [PubMed] [Google Scholar]
- Camarena A, Juarez A, Mejia M, Estrada A, Carrillo G, Falfan R, Zuniga J, Navarro C, Granados J, Selman M. Major histocompatibility complex and tumor necrosis factor-alpha polymorphisms in pigeon breeder’s disease. Am J Respir Crit Care Med. 2001;163:1528–1533. doi: 10.1164/ajrccm.163.7.2004023. [DOI] [PubMed] [Google Scholar]
- Schuyler M. Are polymorphisms the answer in hypersensitivity pneumonitis? Am J Respir Crit Care Med. 2001;163:1513–1514. doi: 10.1164/ajrccm.163.7.2103055a. [DOI] [PubMed] [Google Scholar]
- Schuyler M, Gott K, Mapel V, Cherne A, Nikula KJ. Experimental hypersensitivity pneumonitis: influence of Th2 bias. Int J Exp Pathol. 1999;80:335–348. doi: 10.1046/j.1365-2613.1999.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson G, Monick MM, Hunninghake GW. IL-12 modulates expression of hypersensitivity pneumonitis. J Immunol. 1998;161:991–999. [PubMed] [Google Scholar]
- Gudmundsson G, Bosch A, Davidson BL, Berg DJ, Hunninghake GW. Interleukin-10 modulates the severity of hypersensitivity pneumonitis in mice. Am J Respir Cell Mol Biol. 1998;19:812–818. doi: 10.1165/ajrcmb.19.5.3153. [DOI] [PubMed] [Google Scholar]
- Denis M, Bisson D, Ghadirian E. Cellular and cytokine profiles in spontaneous regression phase of hypersensitivity pneumonitis. Exp Lung Res. 1993;19:257–271. doi: 10.3109/01902149309031723. [DOI] [PubMed] [Google Scholar]
- Park Y, Chung D. CD4+CD25+ regulatory T cells attenuate hypersensitivity pneumonitis by suppressing IFN-gamma secreting T cells. J Immunol. 2007;178:B25. doi: 10.1189/jlb.0908542. [DOI] [PubMed] [Google Scholar]
- Denis M. Mouse hypersensitivity pneumonitis: depletion of NK cells abrogates the spontaneous regression phase and leads to massive fibrosis. Exp Lung Res. 1992;18:761–773. doi: 10.3109/01902149209031706. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Kim S, Park WS, Chung DH. IL-4-secreting NKT cells prevent hypersensitivity pneumonitis by suppressing IFN-gamma-producing neutrophils. J Immunol. 2006;177:5258–5268. doi: 10.4049/jimmunol.177.8.5258. [DOI] [PubMed] [Google Scholar]
- Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005;11:148–152. doi: 10.1016/j.molmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lemière C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, Martin JG, Hamid Q. Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006;118:1033–1039. doi: 10.1016/j.jaci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–1539. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham B, Antó JM, Barreiro E, Bel EHD, Bousquet J, Castellsagud J, Chanez P, Dahién B, Dahién SE, Dews N, Djukanovic R, Fabbri LM, Folkerts G, Gaga M, Gratziou C, Holgate ST, Howarth PH, Johnston SL, Kanniess F. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. The ENFUMOSA Study Group. Eur Respir J. 2003;22:470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- Li AM, Tsang TW, Chan DF, Lam HS, So HK, Sung RY, Fok TF. Cough frequency in children with mild asthma correlates with sputum neutrophil count. Thorax. 2006;61:747–750. doi: 10.1136/thx.2005.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AV, Pavord ID, Ruparelia PR, Chilvers ER. Is the neutrophil the key effector cell in severe asthma? Thorax. 2005;60:529–530. doi: 10.1136/thx.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- Truyen E, Coteur L, Dilissen E, Overbergh L, Dupont LJ, Ceuppens JL, Bullens DM. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006;61:202–208. doi: 10.1136/thx.2005.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Akiyama K, Kobayashi N, Mori A. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int Arch Allergy Immunol. 2005;137(Suppl 1):S51–S54. doi: 10.1159/000085432. [DOI] [PubMed] [Google Scholar]
- Lindén A. Role of interleukin-17 and the neutrophil in asthma. Int Arch Allergy Immunol. 2001;126:179–184. doi: 10.1159/000049511. [DOI] [PubMed] [Google Scholar]
- Hatipoglu U, Rubinstein I. Low-dose, long-term macrolide therapy in asthma: an overview. Clin Mol Allergy. 2004;2:4. doi: 10.1186/1476-7961-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotfried MH. Macrolides for the treatment of chronic sinusitis, asthma, and COPD. Chest. 2004;125:52S–60S. doi: 10.1378/chest.125.2_suppl.52s. [DOI] [PubMed] [Google Scholar]
- Takizawa H. Novel strategies for the treatment of asthma. Recent Patents Inflammation Allergy Drug Discov. 2007;1:13–19. doi: 10.2174/187221307779815101. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Suga M, Nakagawa K, Ito K, Ando M. Effects of erythromycin on experimental extrinsic allergic alveolitis. Clin Exp Allergy. 1999;29:253–261. doi: 10.1046/j.1365-2222.1999.00430.x. [DOI] [PubMed] [Google Scholar]
- Hoyt JC, Robbins RA. Macrolide antibiotics and pulmonary inflammation. FEMS Microbiol Lett. 2001;205:1–7. doi: 10.1111/j.1574-6968.2001.tb10917.x. [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. Am J Med. 2004;117(Suppl 9A):5S–11S. doi: 10.1016/j.amjmed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol Ther. 2008;117:393–405. doi: 10.1016/j.pharmthera.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Schuyler M, Gott K, Cherne A. Mediators of hypersensitivity pneumonitis. J Lab Clin Med. 2000;136:29–38. doi: 10.1067/mlc.2000.107694. [DOI] [PubMed] [Google Scholar]